Translate this page into:
Anticarcinogenic effect of zinc oxide nanoparticles synthesized from Rhizoma paridis saponins on Molt-4 leukemia cells
⁎Corresponding author. lwf052987@sina.com (Liwei Fang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Leukemia is considered as a multistep progression by progressively genetic modifications that make the conversion of normal human hematopoietic progenitor/stem cells into leukemic cells. In this current investigation, we optimized the reaction of parameters to control the nanoparticle size that was examined via SEM. A various characterization techniques, for example UV–visible spectroscopy, FTIR and EDX were carried out for the synthesized ZnONPs from Rhizoma paridis saponins plant extract. The RPS-ZnONPs were evaluated to inspect their cytotoxic activity against Molt-4 cells via MTT test. Apoptosis was determined through the ROS, MMP and AO/EtBr fluorescent staining techniques. Moreover, pro and anti-apoptotic signaling protein expression was inspected through RT-PCR method. We observed the fabricated RPS-ZnONPs have a crystalline nature, spherical formand various biomolecules were exist. As well, we detected the cytotoxicity effect of synthesized RPS-ZnONPs at a range of 23.5 μg followed to 24hrs treatment. Apoptosis was triggered via the RPS-ZnONPs along with augmented ROS, decreased MMP and altered dual staining. Moreover, the apoptosis were identified through significant up-regulation of Bax, caspase-9 and caspase-3, but the anti-apoptotic protein expression i.e., Bcl-2 was down regulated. Though, this study, we investigated the synthesis, characterization and anticancer activity of ZnONPs of Rhizoma paridis saponins plant extract (RPS-ZnONPs) were useful for treatments of leukemia.
Keywords
Zinc oxide nanoparticle
Rhizoma paridis saponins
Apoptosis
Leukemia
Molt-4 cell lines
1 Introduction
Nanotechnology is a fundamental branch in the main fields of chemistry, biology, material sciences and physics. The NPs have abroad range of functions in the various field’s likes electronics, medicine and therapeutics (Khan et al., 2017). The nanomaterials can be produced by various techniques containing biological, physical and chemical. The improvement of physical or chemical methods has revealed in the environmental contaminations, which produced ahuge amount of dangerous byproducts (Bhumi and Savithramma, 2014). Therefore, there is a demand of green synthesis methods that contains an eco-friendly, safe, clean and nontoxic. Furthermore, in this process, there are none need to utilize more energy, temperature, pressure and toxic chemicals (Savithramma et al., 2011). Metal oxide nanoparticles have been widely used for medicinal functions in the last decades. Zinc oxide nanoparticles (ZnONPs) are a hopeful kind of nanostructures with broad ranges of health related applications (Datta et al., 2017; Raliya and Tarafdar, 2013).
Deregulation of apoptosis disturbs the delicate and complex balance between cell survival, cell proliferation and cell death, which takes an central function on the growth of ailments like cancer, mainly leukemia (Hanahan and Weinberg, 2000). Leukemia is considered as a multistep progression by progressive genetic modifications that make the conversion of normal human hematopoietic progenitor/stem cells into leukemic cells (Plummer et al., 2016). Leukemia initiates from a single cell that has undergone malignant conversion by regular genetic mutations. These actions are clonally selection of mutated cells that exhibits progressively more aggressive actions. There is an obvious indication demonstrating that the molecular and cellular actions resulting to leukemia are organized by leukemic stem cells (Cassier et al., 2017; McBride et al., 2019).
Medicinal plants are utilized conventionally all over the globe for treatments of numerous diseases containing cancer. The WHO reported, 80% of the world’s people are reliant on remedial plants for the cure of various diseases (Javed et al., 2017). Rhizoma paridis saponins (RPS) as important segments of P. polyphylla Smith var. yunnanensis was utilized as anticancer agents in conventional Chinese medicine (Man et al., 2013). Moreover, earlier studies demonstrated that the RPS exhibits antioxidant, anti-lung cancer, anti-hepatocarcinoma and anti-inflammatory properties (Man et al., 2014; Man et al., 2015; Wang et al., 2018). The RPS also possessed the inhibitory effects against liver fibrosis and cirrhosis, suppressed the growth numerous types of cancers in both in vitro and in vivo like lung, ovarian, liver and cervical cancer (Man et al., 2014; Shuli et al., 2011; Yan et al., 2009). For the finest of our understanding, biological methods of using plant extract of RPS the first time as a reducing agent plus surface stabilizing material for the ZnONPs synthesis. A lot of work had been studied on anticancer activities, but synthesis of nanoparticles mainly ZnONPs are insufficient. Therefore, in the current research, we have investigated the biological synthesis of ZnONPs by using the leaves of RPS and distinguished these NPs by FTIR, EDX, UV–vis spectroscopy and SEM. Additionally, the synthesized RPS-ZnONPs were assessed for the anticancer activity against Molt-4 cells.
2 Materials and methods
2.1 Chemicals
DMEM, PBS, MTT, FBS, EtBr and DMSO was bought from Himedia-Lab Ltd., Mumbai, India. Zinc acetate is obtained in Sigma-Aldrich (St. Louis, MO, USA). Entire additional chemicals were utilized as of diagnostic range.
2.2 Cell culture
Molt-4 cells were procured and cultured using DMEM with FBS (10%) and antibiotic antimycotic solution (1%). They were maintained at sterilized condition in a CO2 chamber (5%) at 37 °C. Then cells get 80% of confluence were allowed to sub cultured using trypsin solution and used for further research.
2.3 Preparation of RPS leaf extract
The 30 g of the prepared powder form of RPS leaves were dissolved in distilled water (100 ml) and located in a water bath at 60 °C for 1hrs. After that, the solution and powder layer was divided through the Whatman-No.1 sift paper and funnel. Then filtrated sample was gathered and utilized for the synthesis of RPS-ZnONPs.
2.4 Synthesis of RPS-ZnONPs
The 20 ml of collecting RPS filtrate was mixed with 80 ml of zinc acetate (1 mM). Then, the mixtures were located in a water bath at 60 °C for 3 h. After the suspension completed the fabrication of RPS-ZnONPs, sample was centrifuged at 3000 rpm for 20 min. Centrifugation was done thrice alongwith the addition of deionized water for synthesis of RPS-ZnONPs. After centrifugation, the upper aqueous phase was eliminated and the pellets were kept in a furnace at 60 °C for overnight to get a powdered RPS-ZnONPs.
2.5 Characterization of RPS-ZnONPs
Synthesized RPS-ZnONPs were examined via UV–visible spectroscopy to confirm the bio-reduction of NPs. Bio-fabricated RPS-ZnONPs was amalgamated with KBr to form pellets then measured through FTIR spectroscopy (Shimadzu, Japan). The SEM and HR-TEM were utilized to measure the morphology of the synthesized RPS-ZnONPs (FEI Company, Hillsboro, OR, USA). For the EDX investigations, the drop of RPS-ZnONPs were dehydrated on covered with carbon film and employed in the Hitachi-S-3400 N SEM machine equipped with thermo-EDX attachments.
2.6 Anticancer activity of RPS-ZnONPs
2.6.1 Cell viability by MTT assay
Cytotoxic effectual of RPS-ZnONPs on Molt-4 cells were determined by MTT analyze. The cells (1 × 104) were cultured on 96-well plates then added dissimilar volumes (1, 5, 10, 15, 20 and 25 μg/ml) of RPS-ZnONPs for 24 hrs. Then, the MTT solution(15 μl) was placed to every well then kept at 37 °C for 2hrs, every well was then discarded and add DMSO (100 μl) to all well to liquefy formazan crystals. The results were employed to measure the cell viability.
2.6.2 Measurement of intracellular reactive oxygen species (ROS)
The Molt-4 cells were treated with various quantities of RPS-ZnONPs (15 and 20 μg/ml). Then, cells were treated with DCFH-DA (Hi-media) (10 μM) at 37 °C for 30 min. Later than incubation, cells were cleaned twice with PBS and ROS formation was measured using flow cytometry.
2.6.3 Measurement of mitochondrial membrane potential (MMP)
MMP was studied through JC-1 MMP test kit (Sigma-Aldrich, USA) via adopting the manufacturer’s protocol. Cells were supplemented with various dosages (15 and 20 µg/ml) of RPS-ZnONPs. Then cells were gathered and stained with JC-1 stain for 30 min at 37 °C. The cells were washed and measured by a flow cytometer equipped with Cell-Quest software.
2.6.4 Dual staining study (AO/EtBr)
Dual stained (AO and EtBr) cells were clearly exhibits differentiation among live and dead cells. Initially, cells were supplemented with RPS-ZnONPs (15 and 20 µg/ml) then 0.1% TritonX-100 was added then stained with 10 µl of AO and EtBr (Sigma-Aldrich, USA). Then stained cells were kept for 15 min in a dark stipulation and finally measured beneath the fluorescent microscope. The green fluorescence exhibiting cells were pointed out as viable and red fluorescent meant the dead cells.
2.7 RT-PCR analysis
The RNA were extracted from control as well as RPS-ZnONPs(15 and 20 µg/ml) supplemented Molt-4 cells via utilizing the Trizol-reagent (Sigma-Aldrich, USA). After extraction, the RNA was subjected to spectrophotometric investigation through absorbance taken at 260 nm. Cleansed RNA sample were then reverse transcribed to construct the single-strand cDNA. The cDNA was amplified with the primers (Sigma-Aldrich, USA) of Bax, caspase-3, Bcl-2 and caspase-9.
2.8 Statistical analysis
The statistical investigation were done with the aid of SPSS tool (ver.17) (SPSSInc., Chicago, IL, USA). The one-way ANOVA was employed for expressing significance of the current study. Statistical significance was established at a level of p < 0.05.
3 Results
3.1 Characterization of RPS-ZnONPs
3.1.1 UV–visible spectroscopy study of RPS-ZnONPs
The biosynthesized RPS-ZnONPs were examined via UV–vis spectroscopy at the 200–700 nm range. From the experimental findings, it was noted that the maximum absorbance of of the synthesized RPS-ZnONPs was observed on the 289 nm range, which reveals the changes of the initial material to end product. It confirms the bio-reduction of ZnONPs in the test solution and it was evidently demonstrated in Fig. 1.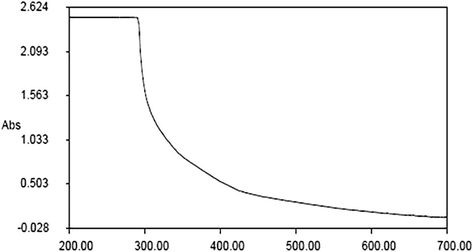
Illustrating the UV–visible spectral pattern of the synthesized ZnONPs from Rhizoma paridis saponins.
3.1.2 SEM and TEM analysis of RPS-ZnONPs
The biosynthesized RPS-ZnONPs were inspected via SEM and TEM analyses to detect the exact magnitude and shapes. From the experimental findings of electron microscopical investigations, it was indicated that the fabricated RPS-ZnONPs possessed a spherical-shaped nano particles and irregular formations. The electron microscopical images of synthesized RPS-ZnONPs were illustrated on Fig. 2.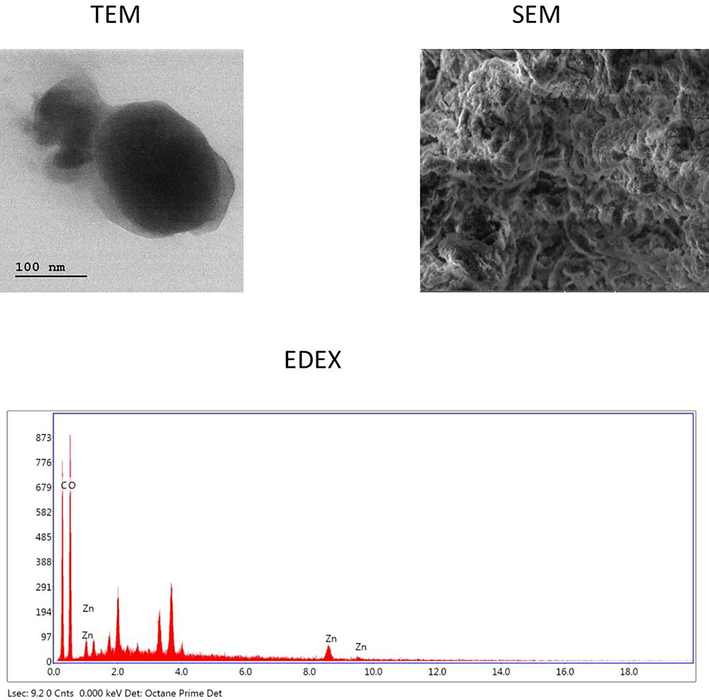
Showing the images of SEM, TEM and EDX analyses of the synthesized ZnONPs from the Rhizoma paridis saponins.
3.1.3 EDX pattern analysis of RPS-ZnONPs
The synthesized RPS-ZnONPs were subjected to the EDX spectral investigations for measuring the combinations of oxides and metallic nature of the synthesized RPS-ZnONPs in a test solution. The spectrum of EDX evidently demonstrated that the 84% of zinc (Zn) were present on the surface area of the synthesized RPS-ZnONPs. The spectrum of EDX analysis was portrayed in the Fig. 2.
3.1.4 FTIR spectroscopic study of RPS-ZnONPs
FTIR examination of ZnONPs from RPS leaves extract demonstrates in Fig. 3. The interaction of nanoparticles with biomolecules of RPS-ZnONPs illustrated intensive peaks at 3199.85, 1736.67, 1564.72, 1408.82, 1241.41, 1082.98, 1006.96, 845.56, 765.93, 677.98, 632.21 and 530.09. The peaks of FTIR was possessed the bonds that is imperative for the existence of alkynes (3199.85 cm−1), aldehyde (1736.67 cm−1), alkanes (1408.82 cm−1), alkyl ketone (1241.41 cm−1), alkyl amine (1082.98 cm−1), esters (1006.96 cm−1), aromatic (765.93 cm−1) and halogen campound (677.98, 632.21 and 530.09 cm−1).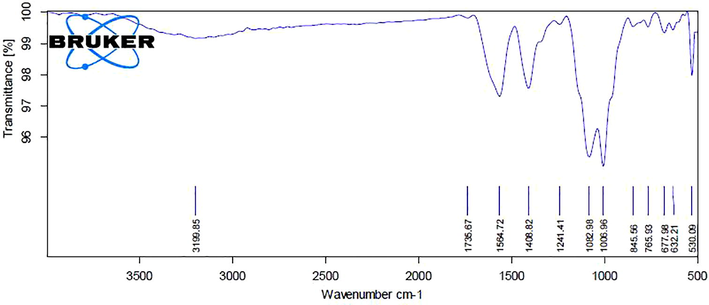
Showing the FT-IR spectral analysis of the synthesized ZnONPs from Rhizoma paridis saponins.
3.2 Anticancer activity of RPS-ZnONPs
3.2.1 Effect of RPS-ZnONPson MTT assay
Fig. 4 illustrating a cytotoxic effectual of RPS-ZnONPs on Molt-4 cells by MTT analyze. The Molt-4 cells were supplemented with various doses of 1, 5, 10, 15, 20 and 25 μg/ml for 24 h with RPS-ZnONPs. Cell viability was determined to be decreased with increasing levels of the biosynthesized RPS-ZnONPs. The inhibitory concentration (IC50) level of the Molt-4 cells was found as 15 µg/ml and we have chosen for 15 and 20 μg concentrations of RPS-ZnONPs to the additional study.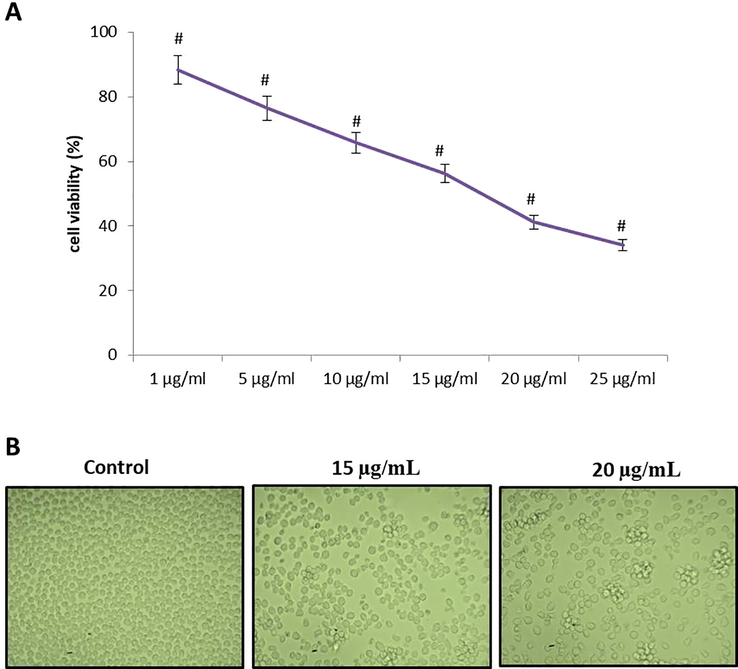
RPS-ZnONPs inhibit the viability of Molt-4 cells by MTT assay. The statistical analysis was carried out using one way ANOVA. Data represent mean ± SD of triplicate, *P < 0.05 as compared with the control group. a)MTT assay b) Cell morphological analysis.
3.2.2 Effect of RPS-ZnONPs in generation of ROS
Fig. 5 displaying the efficiency of RPS-ZnONPs on the production of intracellular ROS level in the Molt-4 cells. The Molt-4 cells were administrated with two concentrations of RPS-ZnONPs (15 and 20 µg/ml) and ROS statuses were examined. The result was revealed the considerable augmentation in the intracellular ROS status on Molt-4 cells. It was observed that the RPS-ZnONPs treated Molt-4 cells elevated ROS when comparing it to normal cells.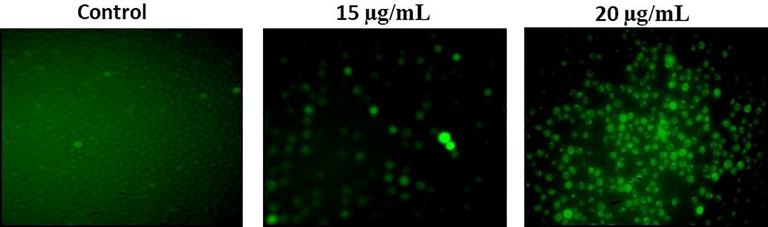
RPS-ZnONPs induce intracellular reactive oxygen species generation in Molt-4 leukemia cells. Data represent mean ± SD of triplicate, *P < 0.05 as compared with the control group.
3.2.3 Effect of RPS-ZnONPson the status of MMP
Fig. 6 evidently showed the potency of RPS-ZnONPs in the status of MMP of Molt-4 cells. The Molt-4 cells were supplemented with different dosages of RPS-ZnONPs (15 and 20 µg/ml) and then the MMP statuses were inspected. The result was evidently exhibited that the considerable diminution of the MMP status was identified in the RPS-ZnONPs supplemented Molt-4 cells which is contrasting to the control cells.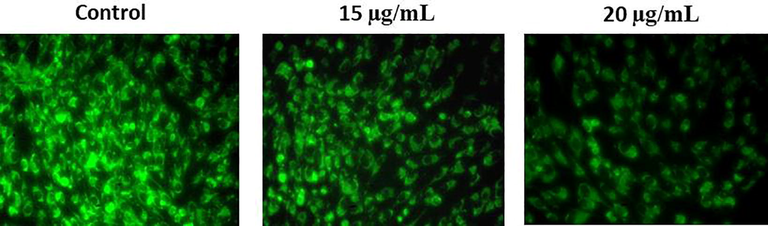
RPS-ZnONPs decreased the mitochondrial membrane potential in Molt-4 leukemia cells. Data represent mean ± SD of triplicate, *P < 0.05 as compared with the control group.
3.2.4 Effect of RPS-ZnONPson status of AO/EtBr
Fig. 7 demonstrates the effectual of RPS-ZnONPs in apoptotic morphological modifications were evaluated via AO/EtBr staining. In current results showed that control cells had a deep green fluorescence nucleus, which point out for live cells. Conversely, different volumes of RPS-ZnONPs (15 and 20 µg/ml) added Molt-4 cells exhibited orange (early apoptosis) and red stained (late apoptosis) apoptotic cells.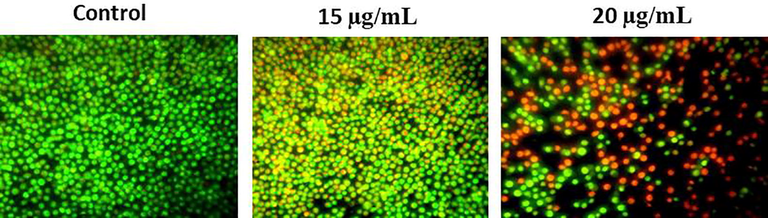
RPS-ZnONPs activate apoptosis through activation of a double staining method by AO/EtBr in Molt-4 leukemia cells. Data represent mean ± SD of triplicate, *P < 0.05 as compared with the control group.
3.3 Effect of RPS-ZnONPson apoptotic protein expressions
Fig. 8 illustrating the expression patterns of apoptotic genes innormal as well as RPS-ZnONPs added Molt-4 cells. The up-lifted expression of Bcl-2 and lowered expression of caspase-9, Bax and caspase-3 were noted in the normal cells. In opposition, RPS-ZnONPs (15 and 20 μg/ml) added cells exhibited decreased expression of the Bcl-2 and enhanced the caspase-3, Bax and caspase-9 expressions were observed.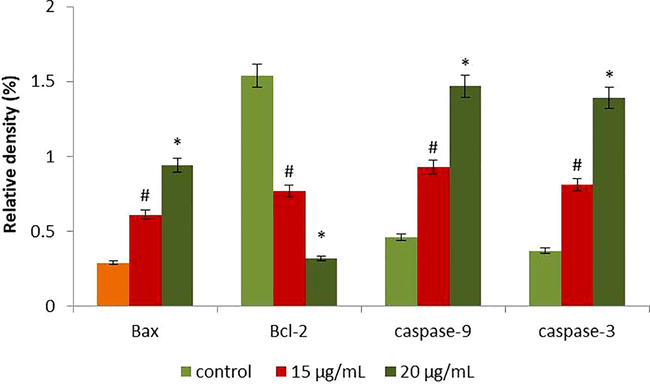
The anticancer effect of RPS-ZnONPs on apoptosis signaling pathway gene expressions in Molt-4 cells were analysed by RT-PCR. The cells were treated with RPS-ZnONPs (15 and 20 µg/ml) for 24 hrs and the gene expressions of Bax, caspase-3, Bcl-2 and caspase-9 were determined. Each bar represents mean ± SD of three independent observations. #p > 0.01, *p > 0.05. The GAPDH was used as an internal control.
4 Discussion
Synthesis of nanoparticles with particular morphologies and properties is one of the mainly significant features of nanoscience, which studies materials whose size lies within the nanometer range (Montasser et al., 2017). The chemical synthetic methods can lead to the production of poisonous chemical by-products or need high temperatures and/or pressure, whereas biosynthesis of nanoparticles using plant extracts offered a simplistic and green approach (Bala et al., 2015). The ZnONPs are presently under exploration owing to its consumption on sarcoma management and diagnostic purposes (Salam et al., 2014). Since the curing of cancer via chemotherapy was inadequate as a result of the difficult outcome of tumor reppressing drugs and drug resisting of cancer cells, herbal plant derivative drug investigates have attention on surmounting those limits. The ZnO nanopowder taken upon optimization was distinguished using different analytical methods to determine their size, shape and functionalization (Jamdagni et al., 2018). The present study, we have been finding that the changing of colour of the fabricated nanoparticles with maximum absorbance, size and shape by SEM, qualitative elemental investigation through EDX (Fig. 2) and existence of various functional groups via testing the FTIR method (Fig. 3) of fabricated RPS-ZnONPs.
Mitochondria are one of the vital organelles that control the cell necrosisas well as pointing the apoptosis. Functional modifications of mitochondria was revealed to took a imperative task on cell apoptosis (Rajeshkumar et al., 2015). ROS mediated intracellular signaling cascades could activate redox signaling pathways, containing cell cycle arrest, apoptosis and oxidative stress (Gupta et al., 2012). According to this latest study, several anticancer agents able to enhance the production of ROS and activated the apoptosis in cancer cells through the mitochondrial-dependent signaling-pathway (Chen et al., 2018). Additionally, the allocation of the MMP is one of the apoptotic developments enthused via anticancer drugs (Liu et al., 2017). The present study shows that the RPS-ZnONPs has caused cell necrosis via destructing the mitochondria (Fig. 6) and rising intracellular ROS generation in Molt-4 cells (Fig. 5). This supports an earlier work that demonstrated the cytotoxic potential of ParisSaponin-I, a functional constituent of Rhizoma paridis in SKOV3 cells (Xiao et al., 2009). The cells undergoing apoptosis reveal following characteristic features such as membrane blebbing, condensation of chromatin, mitochondrial depolarization, shrinkage of the nucleus and DNA degradation by endonucleases into fragments (Jamunakumari and Sakthisekaran, 2014). In this investigation, we found that the RPS-ZnONPs supplementation, considerably modified the morphological alterations linked with Molt-4 cells directing to induction of apoptosis.
Apoptosis occurs mainly during the extrinsic and intrinsic pathways (Li et al., 2012). In extrinsic pathway was activated via caspase-8, while caspase-9 was implicated in the intrinsic pathway (Shimizu et al., 2015; Alabsi et al., 2016). The Bcl-2 protein was an key controllers of cytochrome c release from mitochondria and the diminution of Bcl-2 expressions triggers the releasing of cytochrome c, which leads to the induction of cell necrosis (Hata et al., 2015). The reduced Bcl-2 and augmented Bax escorts to the deliverance of cytochrome c and consequently activate caspase-9 and caspase-3 (Czabotar et al., 2014). In the present research, RPS-ZnONPs treatments promoted the expression of Bax, caspase-3 and caspase-9 and Bcl-2 was considerably reduced in Molt-4 cells (Fig. 8). Previous study also informed that the Rhizoma paridis saponins induce apoptosis in A549 cells, which as recommended in this current research (Zhang et al., 2015).
5 Conclusion
In conclusion, we found that the fabricated ZnONPs from RPS was characterized by various techniques. It was principally identified with the changes of color solution and UV absorption spectra also confirmed the maximum absorbance peak. The SEM and EDX images illustrated the size and morphological structures of nanoparticles. The FTIR results showed synthesized RPS-ZnONPs having different functional groups were observed. Furthermore, RPS-ZnONPs induced cytotoxicity at a concentration range15µg/ml and also triggered apoptosis through augmented ROS formation, decreased MMP, altered AO/EtBr staining and induced pro-apoptotic and suppressed anti-apoptotic protein were observed.
Acknowledgment
The authors would like to thank Beijing Medical And Health Foundation for their financial support to complete research work. This project was supported by Researchers Supporting Project number (RSP-2019/5) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cell Cycle Arrest and Apoptosis Induction via Modulation of Mitochondrial Integrity by Bcl-2 Family Members and Caspase Dependence in Dracaena cinnabari-Treated H400 Human Oral Squamous Cell Carcinoma. Biomed. Res. Int.. 2016;2016:4904016.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv.. 2015;5:4993-5003.
- [Google Scholar]
- Biological synthesis of zinc oxide nanoparticles from Catharanthusroseus (L.) G. Don. Leaf extract and validation for antibacterial activity. Int. J. Drug Dev. Res.. 2014;6:208-214.
- [Google Scholar]
- Isoalantolactone induces apoptosis through ROS-mediated ER stress and inhibition of STAT3 in prostate cancer cells. J. Exp. Clin. Cancer Res.. 2018;37:309.
- [Google Scholar]
- Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol.. 2014;15:49-63.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using partheniumhysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotech. Biomat.. 2017;7:271-275.
- [Google Scholar]
- Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal.. 2012;16:1295-1322.
- [Google Scholar]
- The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov.. 2015;5:475-487.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. JKSUS. 2018;30:168-175.
- [Google Scholar]
- Antiapoptotic Effect of Paclitaxel and Ellagic Acid against Mammary Cancer Induced by 7, 12-Dimethyl Benz (a) Anthracene as Evaluated by Transmission Electron Microscopic Studies. J. Life Sci.. 2014;6:47-51.
- [Google Scholar]
- Plant-derived anticancer agents: a green anticancer approach. Asian Pacific J. Trop. Biomed.. 2017;7:1129-1150.
- [Google Scholar]
- Rhein induces apoptosis of human gastric cancer SGC-7901 cells via an intrinsic mitochondrial pathway. Braz. J. Med. Biol. Res.. 2012;45:1052-1059.
- [Google Scholar]
- Cryptotanshinone induces ROS-mediated apoptosis in human gastric cancer cells. Oncotarget. 2017;8:115398-115412.
- [Google Scholar]
- Curcuma increasing antitumor effect of Rhizomaparidissaponins through absorptive enhancement of paridissaponins. Int. J. Pharm.. 2013;454:296-301.
- [Google Scholar]
- Anti-fibrosis and anti-cirrhosis effects of Rhizoma paridis saponins on diethylnitrosamine induced rats. J. Ethnopharmacol.. 2014;151:407-412.
- [Google Scholar]
- Anti-fibrosis and anti-cirrhosis effects of Rhizomaparidissaponins on diethylnitrosamine induced rats. J. Ethnopharmacol.. 2014;151:407-412.
- [Google Scholar]
- Inhibition of pulmonary adenoma in diethylnitrosamine-induced rats by Rhizomaparidissaponins. J. Steroid Biochem. Mol. Biol.. 2015;154:62-67.
- [Google Scholar]
- The role of inhibition of apoptosis in acute leukemias and myelodysplastic syndrome. Front. Oncol.. 2019;9:192.
- [Google Scholar]
- Effect of gold chloride concentration and volume on size and shape of biological synthesized gold nanoparticles (AuNPs) using red algae (Laurencia papillosa) Sci. Adv. Mat.. 2017;9:1105-1113.
- [Google Scholar]
- Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health.. 2016;4(9):e609-e616.
- [Google Scholar]
- Antiproliferative activity of marine stingray Dasyatissephen venom on human cervical carcinoma cell line. J. Venom Anim. Toxins Incl. Trop. Dis.. 2015;21:41.
- [Google Scholar]
- ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsistetragonoloba L.) Agric. Res.. 2013;2:48-57.
- [Google Scholar]
- Green synthesis and characterization of zinc oxide nanoparticles from Ocimumbasilicum L. var. purpurascensBenth.-Lamiaceae leaf extract. Mater. Lett.. 2014;131:16-18.
- [Google Scholar]
- Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int. J. Chem. Tech. Res.. 2011;3:1394-1402.
- [Google Scholar]
- Nuclear receptor subfamily 4, group A, member 1 inhibits extrinsic apoptosis and reduces caspase-8 activity in H2O2-induced human HUC-F2 fibroblasts. Redox Rep.. 2015;20:81-88.
- [Google Scholar]
- Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch. Pharm. Res.. 2011;34:43-50.
- [Google Scholar]
- Effect of Rhizoma paridis saponin on the pain behavior in a mouse model of cancer pain. RSC Adv.. 2018;8:17060-17072.
- [Google Scholar]
- The antitumoral effect of Paris Saponin I associated with the induction of apoptosis through the mitochondrial pathway. Mol. Cancer Ther.. 2009;8:1179-1188.
- [Google Scholar]
- In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp. Oncol.. 2009;31:27-32.
- [Google Scholar]
- RhizomaParidisSaponins Induces Cell Cycle Arrest and Apoptosis in Non-Small Cell Lung Carcinoma A549 Cells. Med. Sci. Monit.. 2015;21:2535-2541.
- [Google Scholar]







