Translate this page into:
Anticancer effect of herbal and marine products: A systematic review
⁎Corresponding authors. hdmcoutinho@urca.br (Polrat Wilairatana), hdmcoutinho@urca.br (Henrique Douglas Melo Coutinho)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The majority of the world's nations have faced the second-highest cancer mortality rate. The main causes of cancer include an unbalanced diet, genetic factors, and a few specific environmental substances. Recently, a variety of substances have been used to treat cancer, and some are still being studied. It has long been known that the mid of the twentieth century that plant and marine species create a wide range of chemically and physiologically diverse metabolites with a variety of biological effects, including anticancer, anti-inflammatory, antioxidant, antibacterial, antifouling and so on. The focus of this study is on newly found compounds from plant and marine sources that have potent anticancer effects.
Keywords
Plant Source
Marine source
Anti-carcinogenic
Cancer
Phytochemicals
Phyto-constituents
1 Introduction
Cancer is a disorder in which cells in a particular area of the body multiply and develop uncontrolled. The malignant cells have the capacity to penetrate and damage nearby healthy tissue, including organs (Weinberg, 1996). In 2019, there were 23.6 million new instances of cancer each year and 10 million people die worldwide, suggesting rises of 26% and 21% over the previous ten years, respectively (Kocarnik et al., 2022). According to estimates, there will be 1.9 million new cancer diagnoses and 609,360 cancer related deaths are observed in the United States in the time of 2022 (Beger et al., 2008). The growth of cancer registries around the globe has sparked an interest in discovering novel drugs that seem to be toxic against cancer cells but harmless to healthy cells. The anticancer medications that were traditionally used were relatively toxic to both normal body cells and tumor cells in the area of the body where the cancer had first appeared. Right now, both terrestrial plants and marine environments are being used in the search for new anticancer medications (Greenwell and Rahman, 2015). For generations, people have employed plants to treat illnesses. Many plants are consumed around the world for their health advantages as a form of traditional folk remedies. A wide range of anticancer drugs derived from plant materials are purified, and then they are tested in clinical trials on cells (including several cancer cells lines) and experimental animals (Greenwell and Rahman, 2015). In very recent time, the number of recently discovered natural substances has increased dramatically. The use of plants as sources of highly biologically active materials has been around for centuries in traditional medicine (Fridlender et al., 2015). One way to obtain these substances is by extracting them from plant materials. An alternative approach is to use biotechnological tools to produce anticancer compounds derived from plants. Some of the naturally occurring substances found plants and aquatic animals that have antitumor properties include alkaloids, diterpenoquinone, diterpenes, purine-based compounds, peptides,l actonic sesquiterpene, cyclic depsipeptide, macrocyclic polyethers, proteins etc. (Lichota and Gwozdzinski, 2018). Additionally, there is a lot of potential in marine environments to find novel organisms that can help with cancer treatment and prevention. Late in the 19th century, marine first appeared. After 1980, the field of biotechnology emerged as one that gave the study of the oceans direction, focusing on uses like drug development (Newman and Cragg, 2016). There is growing interest in utilizing the diversity and complexity of marine natural product scaffolds due to their tremendous potential for rational drug discovery (Nobili et al., 2009). New anticancer medications are required due to the rise in the prevalence of various types of cancer (Lichota and Gwozdzinski, 2018). This study's objective was to identify compounds with anti-cancer properties that were derived from plant and marine sources.
2 Materials and methods
A search was conducted (till May 2022) in the following databases: PubMed, Science Direct, MedLine, and Google Scholar using the keywords 'plant derivatives' and 'anticancer activity/effect'. There were no language restrictions. The articles were reviewed for information on plant derivatives, marine source, cancer pathophysiology, anticancer activities, test results, and potential mechanisms of action.
3 Results
3.1 Cancer pathophysiology
Cancer is well-known disease that are occurred by the regulation of tissue growth. A normal cell must change its genes to become a cancer cell, which regulates cell development and differentiation. Genetic alterations can take place at a variety of different scales, from the addition or deletion of whole chromosomes to a single DNA nucleotide mutation. These modifications have an impact on two large types of genes. Oncogenes can be either normal genes that are overexpressed or mutated genes that exhibit unique features. In either instance, the expression of these genes promotes cancer cell malignancy. Tumor suppressing genes are those that impede cancer cell division, survival, or other qualities. Tumor suppressing genes are frequently silenced by cancer-promoting genetic mutations. The way of the development of cancer cells are displayed in Fig. 1.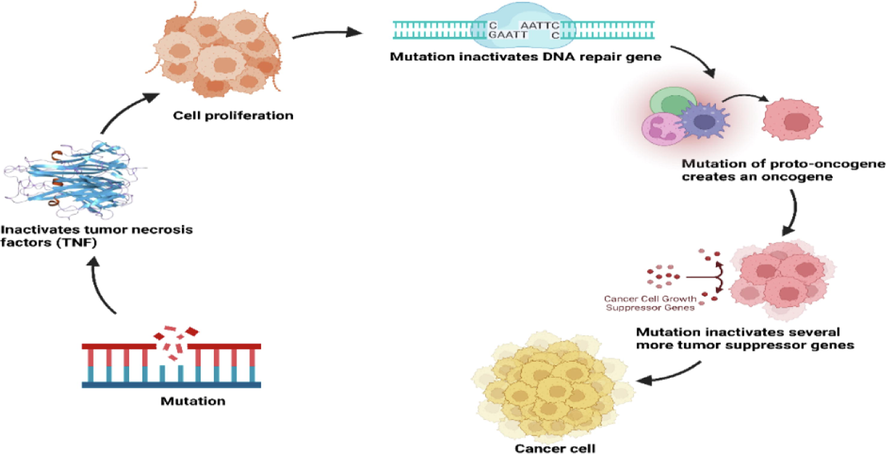
Mutations play a role in the development of cancer. Every mutation modifies how a cell behaves.
The traditional understanding of cancer is that it is a collection of diseases caused by progressive genetic abnormalities such as tumor-suppressor gene mutations, oncogene mutations, and chromosomal abnormalities (Baylin and Ohm, 2006). Epigenetic alterations are those that affect the genome in a way that is relevant to function but do not alter the nucleotide sequence. Changes in DNA methylation (hypermethylation and hypomethylation), histone modification, and chromosomal layout are only a few examples of such modifications (arise through the negative protein expression like HMGA2 or HMGA1) (Kanwal and Gupta, 2012). While epigenetic abnormalities are common in malignancies, epigenetic modifications in DNA repair genes, which result in lower production of DNA repair proteins, may be especially important. Such changes are expected to begin early in cancer growth and are a plausible cause of the genomic instability seen in malignancies (Bernstein et al., 2013). The main role of DNA damage and epigenetic modifications in DNA repair genes in the development of cancer is illustrated in Fig. 2.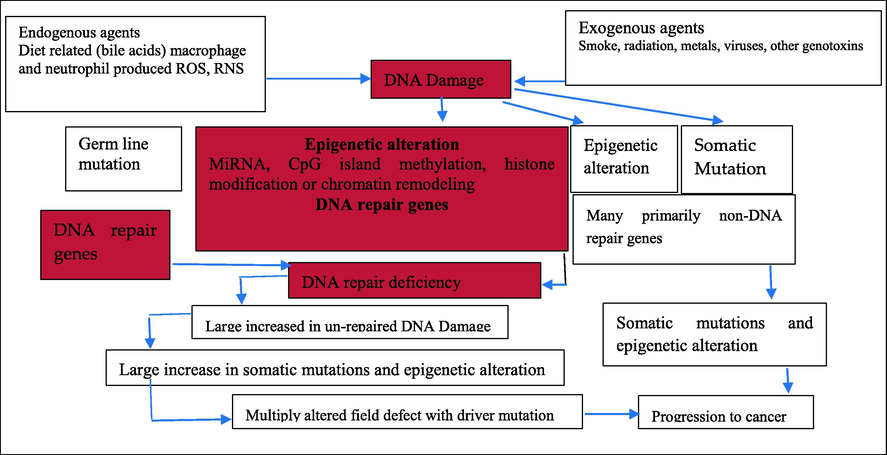
The primary significance of DNA damage and epigenetic changes in DNA repair genes in the development of cancer.
3.2 Plant derived compounds
Plant-derived compounds have shown to be a rich source of different types of novel medicinal molecules applied against several type of human disease. Many anticancer drugs have been isolated from plants, including Catharanthus roseus, Cuphea hyssopifolia, Podophyllum species, Coptis chinensis, Taxus brevifolia, Camptotheca acuminate, Betula alba, Streptococcus peucetius, Cephalotaxus species, Erythroxylum pervillei, Evodiae fructus, Curcuma longa, Ipomoeca batatas, Centaurea schischkinii, Eugenia jambos L., Alnus rubra, Punica granatum L, Phyllanthus niruri L., Hydrastis Canadensis, Sanguinaria canadensis, Stephania tertrandra and others. Scientists are still investigating the bioavailability of anti-cancer substances in heretofore unrecognized plant species. Fig. 3 depicts various plant-derived anticancer medications and their main modes of action.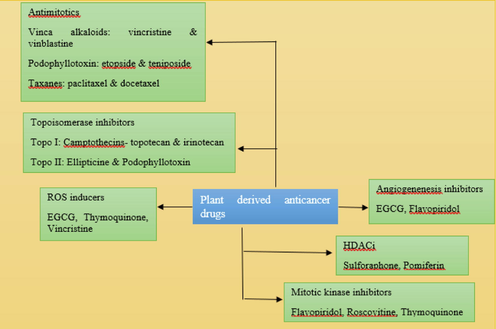
Plant-based anticancer medicines in specific groupings. Some medicines can provide therapeutic and/or chemoprotective actions via various routes. EGCG is well-known for its anti-ROS effect; it may also suppress DNA methylation and angiogenesis. Thymoquinone is both a ROS inducer and a mitotic kinase inhibitor.
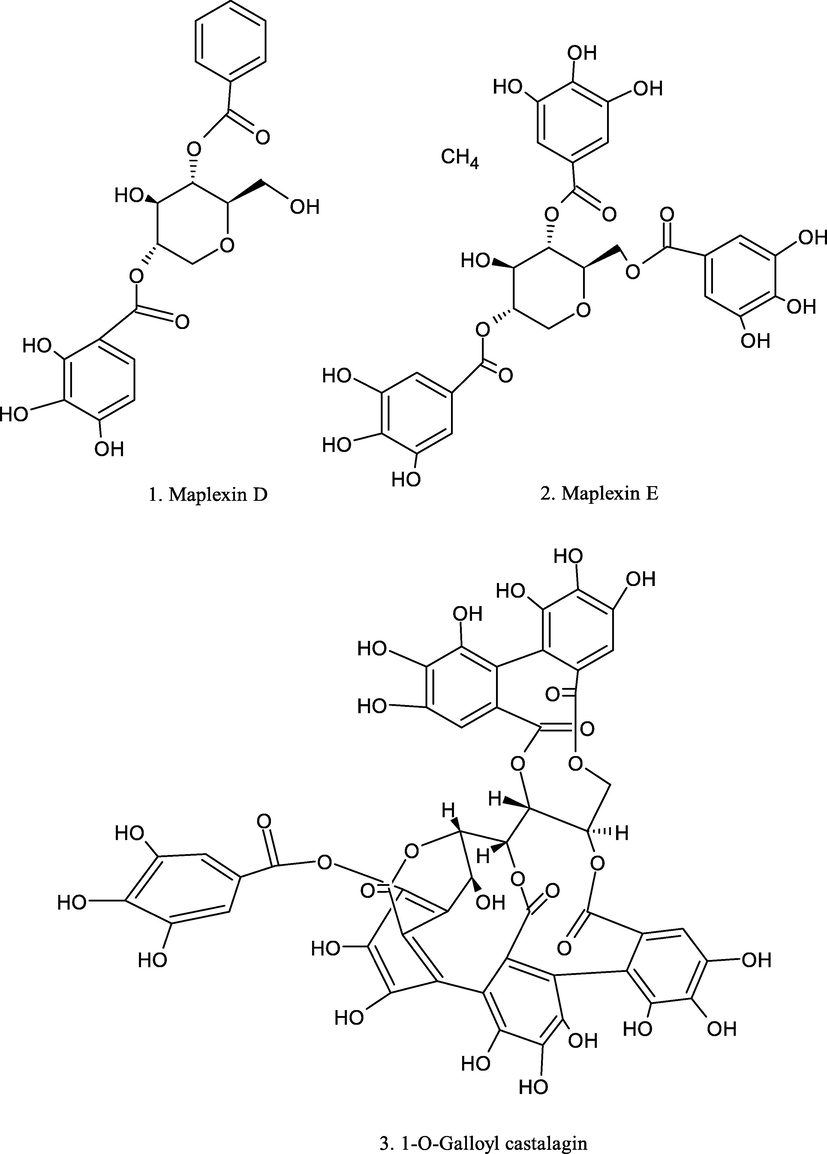
Chemical structure of plant derived compounds.
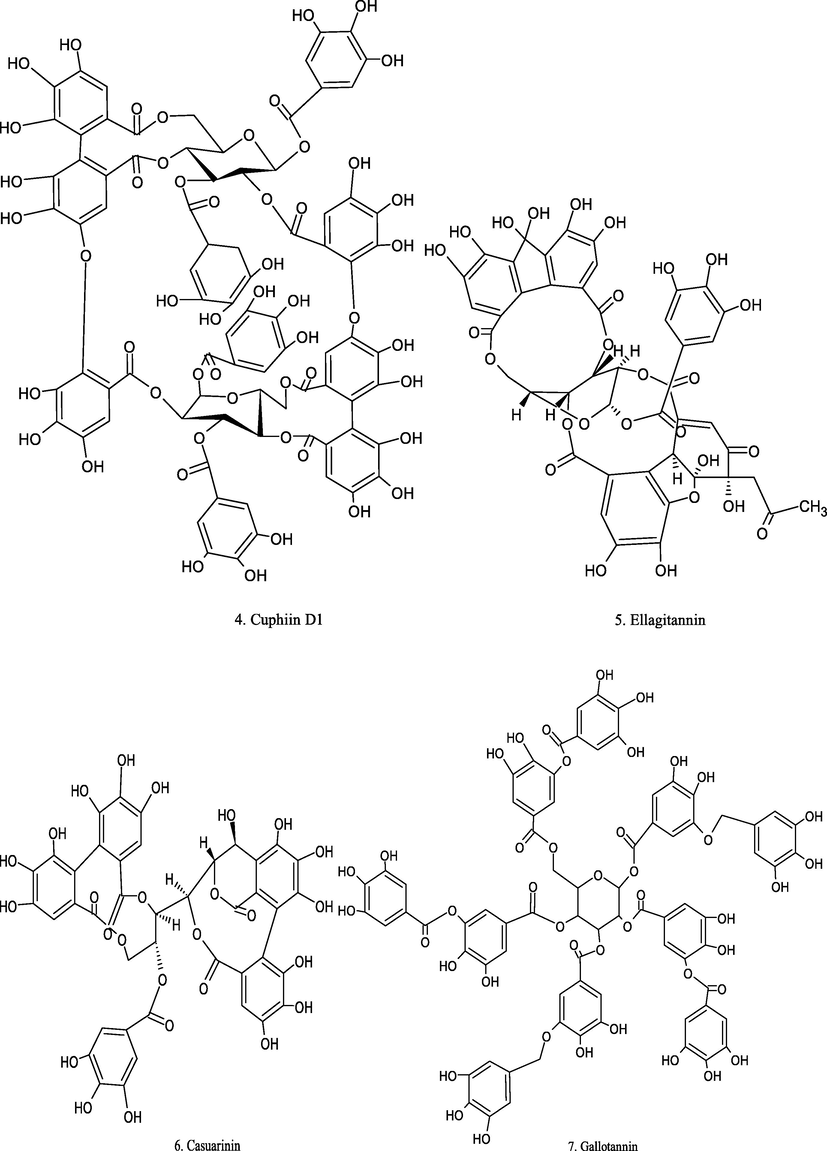
Chemical structure of plant derived compounds.
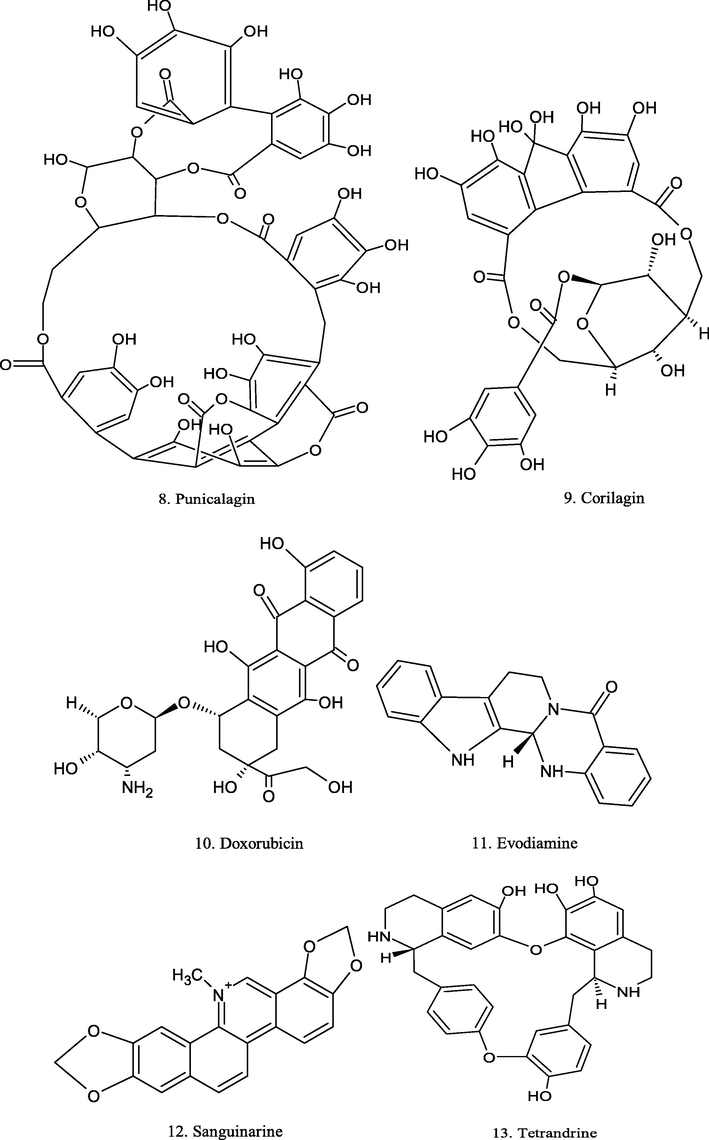
Chemical structure of plant derived compounds.
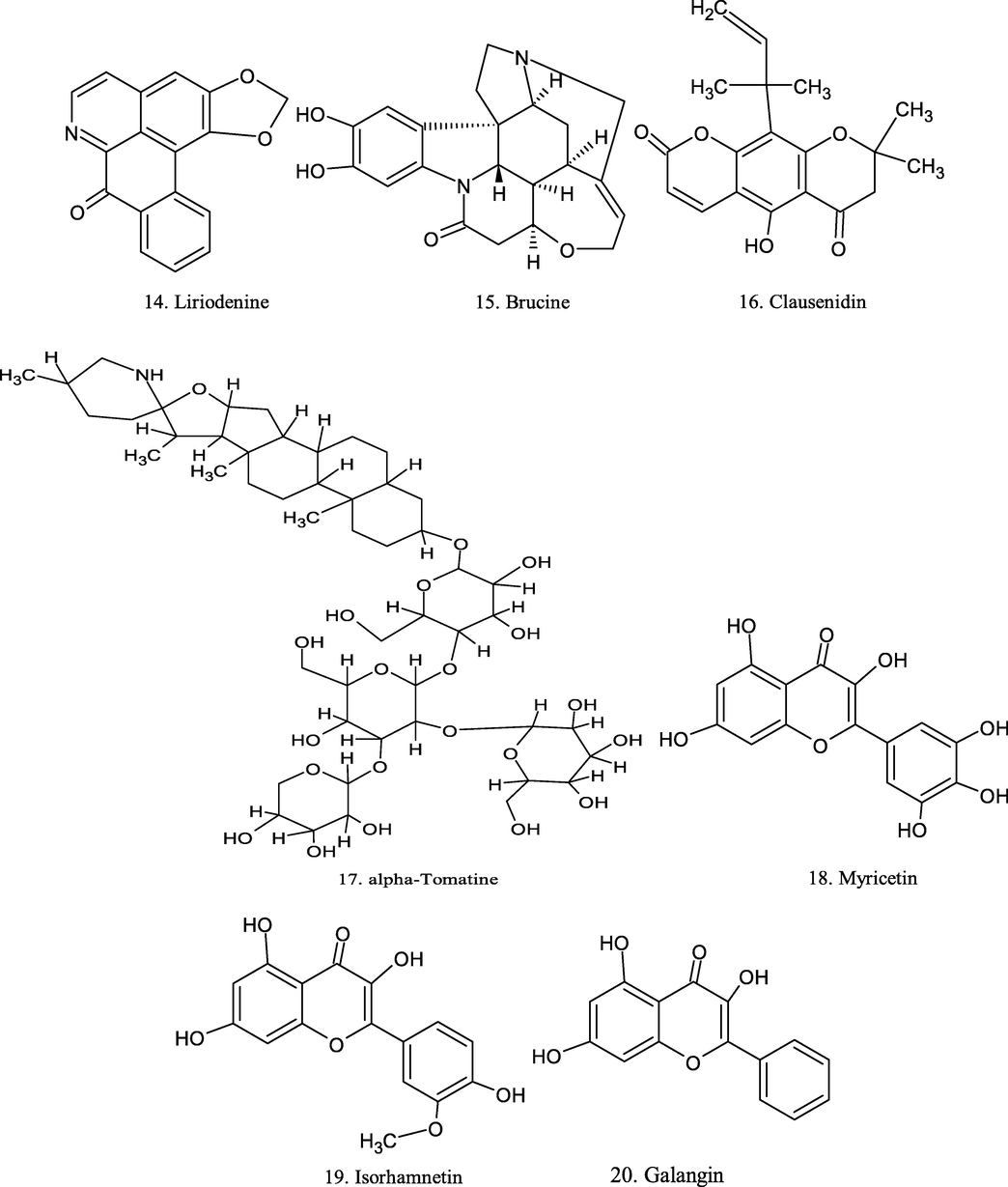
Chemical structure of plant derived compounds.
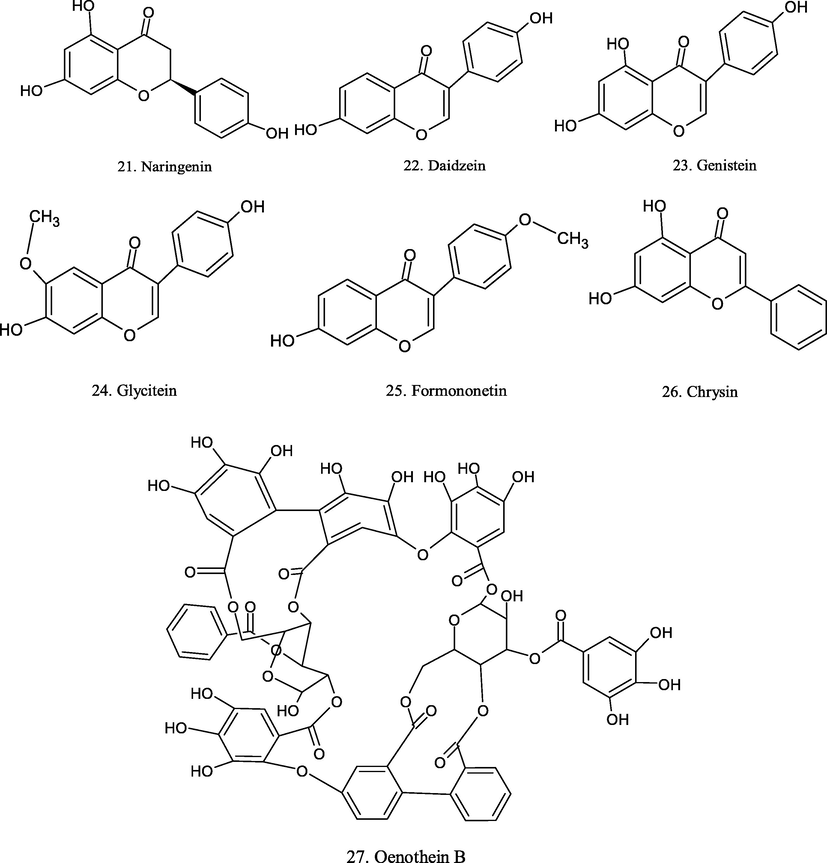
Chemical structure of plant derived compounds.
3.3 Marine source compounds
Based on the previous, numerous research organizations throughout the world have recently focused on the separation and characterization of biologically active components from marine source due to there several application (Fig. 5). The marine environment has developed into a significant source of molecules that have strong anticancer properties and display unusual chemical characteristics and mechanisms of action. Thirty-four of forty compounds in the pipeline for marine pharmaceuticals indicate “cancer therapy,” and twelve of the seventeen marine-derived medications approved by regulatory bodies are used to treat cancer (Mayer et al., 2012). Sea is one of the most abundant habitats, teeming with variety of creatures, where their compounds are stand out because of their distinctive qualities. The development of cancer medicines derived from marine sources is extremely important in the fight against cancer. More than 60% of anti-tumor medications come from natural sources, including pharmaceuticals and compounds that are now being tested in clinical studies. This study is targeted to find out the anticancer activity of marine source compounds.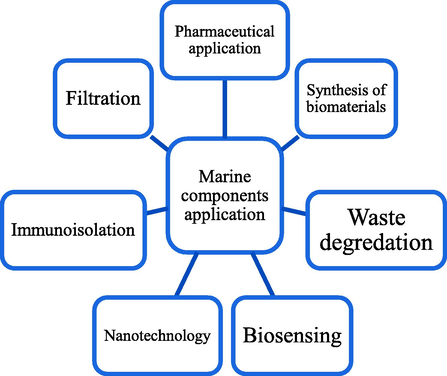
Several applications of marine source components.
4 Discussion
Several research has examined the anticancer potential of compounds derived from plants and marine source. Some of these substances demonstrate efficient anti-cancer activity in one or more cancer types. Based on their activities several compounds have been listed in Table 1/Fig. 4 and Table 2/Fig. 6. For biomedical uses, natural substances are effective therapeutic and chemopreventive agents as well as useful tools for evaluating molecular targets (Orlikova et al., 2014). Numerous studies have shown that phytochemicals found in natural products can prevent the initiation, promotion, and progression of carcinogenesis, and some of their medicinal compounds have the potential to be highly effective chemopreventive and chemotherapeutic approaches against cancer (Gupta et al., 2010). Plants produce a large number of bioactive metabolites, and because of their therapeutic benefits, they are highly sought-after in the field of pharmacology. They play a crucial role in the formation of sophisticated traditional medicine particularly that used to treat cancer diseases (Moghadamtousi et al., 2013). However, marine floras, which make up over 90% of the ocean's biomass, include bacteria, actinobacteria, cyanobacteria, fungus, microalgae, seaweeds, mangroves, and other halophytes. They provide a lot of opportunity for the development of novel anticancer medicines (Sithranga Boopathy and Kathiresan, 2010). Numerous substances derived from plants have cytotoxic properties with a wide range of mechanisms of action, including DNA damage, the inhibition of topoisomerases I and II, the induction of apoptosis, and the inhibition of tumor cell growth. Studies have demonstrated that plant-derived compounds combined with chemotherapy drugs have a significant potential to kill tumor cells without harming healthy cells like lymphocytes and fibroblasts (Lichota and Gwozdzinski, 2018). Marine-derived bioactive molecules have been found to be effective against a variety of tumor cells, including those that cause bone, blood, lung, mammary, melanoma, prostate, bladder, and renal cancers in addition to the recognized mechanisms of action mediated by necrosis, apoptosis, and lysis of tumor cells.
Compounds
Plant Source
Test Medium
Dose/Concentration
Mechanism of action
References
Maplexins C-D and Maplexins E-1
A. rubrum L.
HCT-116 and MCF-7 cells
IC50 = 59.8–67.9 and 95.5–108.5 µM vs 73.7–165.2 and 115.5–182.5 µM
inhibit cancer cell growth
(González-Sarrías et al., 2012)
Cuphiin D1
Cuphea hyssopifolia
HL-60 cells
IC50 = 16 µM
decrease cell population and inhibit Bcl-2 expression
(Wang et al., 2000)
Punicalagin (PUNI) and Ellagic acid (EA)
Pomegranate
Caco-2 and CCD-112CoN cells
PUNI 1; 10; 100 µM/l, EA 1; 10; 30 µM/l
apoptosis induction
(Larrosa et al., 2006)
1-O-galloyl castalagin and casuarinin
Eugenia jambos L.
HL-60
10.8 and 12.5 µM
induced apoptosis
(Yang et al., 2000)
Gallotannin
Alnus rubra
Colon cancer cells from humans (T-84)
10 µg/mL
Induced apoptosis
(Gali-Muhtasib et al., 2001)
Corilagin
Phyllanthus niruri L.
ovarian cancer cells
160 µM
increased apoptosis
(Jia et al., 2013)
Doxorubicin (DXR) + Tannic acid (TA)
–
MDA-MB-231 cells
DXR (2.5 mg/Kg, once weekly), TA (10 mg/Kg)
shows maximum tumor volume reduction
(Tikoo et al., 2011)
Oenothein B, woodfordin C and woodfordin D
–
Human squamous cell carcinoma (HSC-2) and salivary gland tumor (HSG)
CC50 = 0.060 µM
CC50 = 0.026 µM
CC50 = 0.026 µMAccelerated apoptosis
(Sakagami et al., 2000)
8-cetylberberine
Coptis chinensis and Hydrastis Canadensis
A549 and MRC-5 cells
In vivo: 10 mg/Kg
inhibit tumor growth
(Xiao et al., 2018)
In vitro: 2 μg/mL
decreased the survival rate
Evodiamine
Evodiae fructus
MCF-7 and MDA-MB-231cells
–
prevents cells proliferating
(Wang et al., 2013)
Sanguinarine
Sanguinaria canadensis
HeLa and Siha human cervical cancer cells
2.43 µM/L (IC50) in HeLa cells and 3.07 µM/L in SiHa cells
induction of apoptosis
(Xu et al., 2012)
Tetrandrine (TET)
Stephania tertrandra
143B cells
1, 2 and 4 µM
inhibits the proliferation
(Tian et al., 2017)
Liriodenine
natural plant species
A549
20 µM and 50 µM
suppressed proliferation
(Chang et al., 2004)
Brucine
Strychnos nux-vomica L.
MDA-MB-231
1–2 mM
apoptosis induction
(Xu et al., 2019)
Cathachunine
Catharanthus roseus
human leukemia cells
anti-proliferation and pro-apoptosis abilities
(Wang et al., 2016)
Clausenidin
Clausena excavata Burm. f
HepG2
30, 40 and 50 µg/mL
induces apoptosis
(Waziri et al., 2016)
α-tomatine
Solanum lycopersicum
CT-26 colon cancer cells
at 3.5 µM
increased caspase-independent apoptosis
(Kim et al., 2015)
Myricetin
berries, herbs and walnuts
HCT-15 cells
50 and 100 µM
induces apoptosis
(Kim et al., 2014)
Isorhamnetin
Hippophae rhamnoides L
0,10,20,40 and 80 µM /L
reduced cell proliferation
(Li et al., 2014)
Baicalein
Scutellaria baicalensis
cell lung cancer (NSCLS)
0.5% CMC-Na solution, 40 mg/Kg
inhibits tumor growth
(Zhao et al., 2019)
Naringenin
Fruits
A549 cell
0–300 µM
alteration cell proliferation
(Chang et al., 2017)
Daidzein
in nuts, fruits, soybeans, andsoy-based products
JAR and JEG-3
100 µM
induce apoptosis
(Zheng et al., 2018)
Genistein (GEN)
soy isoflavones
HT-29 cells
200 µM /L
induces apoptosi
(Zhou et al., 2017)
Glycitein
Soybean
SKBR-3 cells
10, 30, 60, 100 mg/mL
damaged the cell membranes
(Zhang et al., 2015)
Formononetin
Pongamia pinnata, Astragalus membranaceus, Ononis angustissima and Trifolium pratense
FaDu cell
50 µM
decelerated tumor growth
(Oh et al., 2020)
Chrysin
–
CT26 cells
80 µg/mL
induction of apoptosis
(Bahadori et al., 2016)
Galangin
Alpinia galangal
MCF-7 and T47D
20 µM
inducing apoptosis
(Song et al., 2017)
Class
Natural compound
Chemistry
Test system
Test dose/ concentration
Proposed mechanism
Reference
In Vitro1.Renca renal adenocarcinoma, 2. B16 melanoma3. M5076 reticulum cell sarcoma, the L10A B-cell lymphoma
100 ng/mL
Antiproliferative responses against cancer cell
Marine Bacteria
Bryostatins
Macrolide
In vivo1. mice bearing 8–10-mm s.c. masses of L10A lymphoma (5–10 × 109) 2. Six human B-cell lymphoma cell lines
1 μg/injection/day
B-cell lymphoma growth inhibition
(Hornung et al., 1992)
Taxol/ discodermolide
–
SKOV-3
25 mg/kg i.p. and 5 mg/kg i.v.
induces tumor regressions
(Huang et al., 2006)
Cryptophycins
Depsipeptide
Murine in vivo xenograft models mice model
0.1 mL/10 g body weight of the animals
active antitumor agents against the rat 13,762 mammary carcinoma
(Menon et al., 2000)
Indanone from Lyngbya majuscula
Polyketide
Human hepatocellular carcinoma cell line. Hep3B human liver tumor cells
–
VEGF expression inhibition
(Nagle et al., 2000)
Lyngbyabellin A (Lyngbya majuscula)
Desipeptide
Human nasopharyngeal and colon carcinoma cell line
1.0.03 Ìg/mL2.0.50 Ìg/mL
Disruption of cellular microfilaments
(Luesch et al., 2000)
Apratoxin A from Lyngbya boulloni
Polyketide
Cervical cancerCell line (HeLa)
2.2 nM
Blocking the progression of G1 phase → Cell cycle inhibition → Cytotoxicity
(Ma et al., 2006)
Marine Corals
Cembrane (Alcyonacea, Nephtheidae)
–
Three cancer cell linesSF-268 (CNS), MCF-7 (breast), and H460 (lung)
100 μM
Three primary tumor cell lines were exposed to non-selective anticancer activities
(Januar et al., 2010)
Eleutherobin analogues
Diterpene glycoside
Human breast carcinoma cell line
1–100000 nM
–
(Cinel et al., 2000)
Sterols
Steroids
Dalton's lymphoma ascites cells (DLA)
10 μg/mL, 20 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL
exhibited remarkable apoptosis agonist activity
(Byju et al., 2014)
Marine Algae
Sterol fraction (cholesterol, β-sitosterol, and campesterol)
–
4 T1 cell
10 and 25 mg/Kg
induced apoptosis
Kazłowska et al., 2013)
Fucoidan from Sargassum mcclurei
DLD-1 cells
1–200 µg/mL
colony formation inhibition
(Duc Thinh et al., 2013)
Dioxinodehydroeckol
Isolated from Ecklonia Cava
Phloroglucinol derivatives
MCF-7 and MDA-MB-231 human breast cancer cell line
1, 5, 10, 50 and 100 µM
inhibit the proliferation
(Kong et al., 2009)
Elatol isolated from algae Laurencia microcladia.
Sesquiterpene
Western blot analysis, C57Bl6 mice bearing B16F10 cells
0.1–100 µM
induces apoptosis
(Campos et al., 2012)
Fucoxanthin
Carotenoids
CMT-U27
10, and 20 μM
induced apoptosis
(Jang et al., 2021)
Sargassum oligocystum extract
–
In-vitro testK562 and Daudi human cancer cell lines
0–500 μg/mL.
Most effective concentration 500 μg/mL and 400 μg/mLInhibited G0/G1 stage SGC-7901 from entering to S stage
(Ji et al., 2004)
Violaxanthin from Dunaliella tertiolecta
–
Breast adenocarcinoma (MCF-7)
40 µg/mL (to observe cytostatic activity)
Cancer cell proliferation is inhibited→ ↑Apoptosis
(Pasquet et al., 2011)
Phloroglucinol from Brown seaweed
–
Colorectal cancer Cell lines (HCT116 & HT29)
300 µM
Induce DNA damage → Cytotoxicity→ ↓Cell death
Lopes-Costa et al., 2017)
–
Human leukemia (HL-60) cells
μM
↑Caspase 3 & 7 → ↓Bcl-2→ ↑Apoptosis → Cytotoxicity
Ganesan et al., 2011)
Marine Tunicate
Didemnin B
Depsipeptide
Rabbit reticulocyte lysate and human adenocarcinoma cell line
–
(Ahuja et al., 2000)
10 patients
5.6 mg/m2
competitive inhibition enzyme
(Benvenuto et al., 1992)
Alkaloid
Human and murine leukemia cell lines
μM
Apoptosis induction; no impact on topoisomerases I and II
Dassonneville et al., 2000)
Human colon carcinoma cell line
10–50 nM
Inhibition of transcription of the human P glycoprotein gene (MDR1)
Jin et al., 2000)
Trabectedin (ET-743)
isolated from Ecteinascidia turbinata
Alkylating agent
52 patients with solid tumors (mostly colorectalcancers and sarcomas)
0.05–1.8 mg/m2
impact on a number of transcriptional regulators, cell proliferation, and the nucleotide excision repair system
(Ganjoo and Patel, 2009)
Clam
Spisulosine
––10 μM
Cuadros et al., 2000)
–
Colon and breast, cancers cell lines
–
Vasko et al., 2010)
Sponge
Fascaplysin
Alkaloid
Cell lines from human colon cancer, osteogenic sarcoma, and normal fibroblasts
0.35 μM
Inhibition of Cyclindependent Kinase 4
(Soni et al., 2000)
Aragusterol A
Steroid
Human and murine cancer cell panel and in vivo assays
0.01–1.6 μM
1/S cell cycle phase
(Fukuoka et al., 2000)
Discodermolide
Polyketide
Human and murine tumor cell lines
0–1000 nM
stabilize microtubules and inhibit cells
(Martello et al., 2000)
Sea squirts
Ecteinascidin/ Trabectedin from Ecteinascidia turbinata
Alkaloids
0.6 ng/mL
Cytotoxicity against tumour cell line in vitro.
(Ghielmini et al., 1998)
A549 cell
ng/mL
Diatom
Monoacylglycerides
(MAGs) from Skeletonema marinoi
–
-Haematological cancer cell line (U-937)
µg/mL
↑caspase3/7 activation→ ↑Apoptosis → Cytotoxic activity
(Miceli et al., 2019)
Colon cancer cell line (HCT-116)
MePR-2B normal cells
Polyunsaturated aldehydes
(PUAs2-trans,4-trans-decadienal(DD)) from Skeletonema marinoi
–
A549 cells
2,5 & 10 µM
↑Apoptosis → Cytotoxic effect→↑ on cell death
(Sansone et al., 2014)
Colon adenocarcinoma metastaticascites-deriving (COLO205)
Normal lung/brunch epithelial (BEAS-2B)
Polyunsaturated aldehydes (PUAs) from Thalassiosira rotula, Skeletonema costatum, Pseudo-nitzschia delicatissima
–
Colon adenocarcinoma (Caco-2) cells
(11 ± 17) µg/mL
Arrest cell proliferation→↑Apoptosis
(Miralto et al., 1999)
Chrysolaminaran from Synedra acus
–
Human colon cancer cells (HT-29)
54.5 µg/mL
Inhibition of cancer cell proliferation → Cytotoxic activity
(Kusaikin et al., 2010)
Colon cell line (DLD-1)
47.7 µg/mL
Nonyl 8-acetoxy-6-methyloctanoate (NAMO, fatty alcohol ester) from Phaeodactylum tricornutum
–
Human promyelocytic leukemia (HL-60)
22.3 µg/mL
Cell cycle arrest sub-G1 phase→ ↓damage DNA →↑Apoptosis → Cytotoxicity
(Samarakoon et al., 2014)
Human lung carcinoma (A549)
50 µg/mL
Mouse melanoma (B16F10)
–
–
Monogalactosyl diacylglycerols from Phaeodactylum tricornutum
–
Wild-type W2
64 µM
↑Caspase 3/7 → ↑Apoptosis → Cytotoxicity
(Andrianasolo et al., 2008)
Wild-type D3
1 µM
Fucoxanthin from Phaeodactylum tricornutum
Xanthophyll
Caco-2 (derived from a human colon adenocarcinoma),HepG2, and HeLa (derived from cervical cancer cells)
1 µM
↑Caspase 3/7 → ↑Apoptosis → Cytotoxicity
(Neumann et al., 2019)
from Navicula incerta
Phytosterol
Liver hepatocellular carcinoma (HepG2)
8.25 µg/mL
↑caspase-8, 9 →↓damage DNA → ↑Apoptosis → Cytotoxicity
(Kim et al., 2014)
Chemical structure of marine source compounds.
Chemical structure of marine source compounds.
Chemical structure of marine source compounds.
5 Conclusions
It has been found that a number of plant and marine natural products have anticancer action in vitro on a variety of tumor cell lines, including those originating from kidney, lung, prostate, bladder, melanoma, osteosarcoma, breast, and lymphoid malignancies. Furthermore, the majority of data on just how plant as well as marine products inhibit tumorigenesis both in vitro and in vivo point to the possibility that this is accomplished by inducing apoptosis, necrosis, and lysis in the tumor cells. WHO estimates that more than 80% of people in underdeveloped nations rely on traditional medicines for their most basic medical requirements. A healthy diet rich in fruits and vegetables can help stave against the progression of cancer. As chemoprotective medicines against different forms of cancer, several natural compounds are available. Fruits, vegetables, extracts from plants, herbs, microorganisms, and marine life all contain these chemoprotective compounds. The preventive effect against cancer may be attributed to a variety of natural product ingredients. In this work, we attempted to examine the anticancer properties of a number of organic compounds that were isolated from plant and marine sources.
Funding
Not applicable.
Data availability statement
The data will be available after request to the corresponding authors.
CRediT authorship contribution statement
Md. Mizanur Rahaman: Conceptualization. Polrat Wilairatana: Concepualization, Project Administration. Mehedi Hasan Bappi: Methodology. Tawhida Islam: Methodology. Md. Nayem Mia: Software. Henrique Douglas Melo Coutinho: Project administration. Abolghasem Siyadatpanah: Validation. Muhammad Torequl Islam: Conceptualization, Supervision.
Acknowledgments
This is the Department of Pharmacy, Life science faculty, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj-8100 (Dhaka), Bangladesh.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Inhibition of protein synthesis by didemnins: Cell potency and SAR. J. Med. Chem.. 2000;43:4212-4218.
- [Google Scholar]
- Apoptosis-inducing galactolipids from a cultured marine diatom, Phaeodactylum tricornutum. J. Nat. Prod.. 2008;71:1197-1201.
- [Google Scholar]
- Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of Caspase-3,-9, Bax and Sall4. Iran. J. Biotechnol.. 2016;14:177.
- [Google Scholar]
- Epigenetic gene silencing in cancer–a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer. 2006;6:107-116.
- [Google Scholar]
- Beger, H.G., Rau, B., Gansauge, F., Leder, G., Schwarz, M., Poch, B., 2008. Pancreatic Cancer – Low Survival Rates. Dtsch. Arztebl. Int. Doi: 10.3238/arztebl.2008.0255.
- Phase II clinical and pharmacological study of didemnin B in patients with metastatic breast cancer. Invest. New Drugs. 1992;10:113-117.
- [Google Scholar]
- Epigenetic field defects in progression to cancer. World J. Gastrointest. Oncol.. 2013;5:43.
- [Google Scholar]
- In vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral, subergorgia reticulata. Pharmacogn. Mag.. 2014;10(Suppl 1):S65.
- [Google Scholar]
- Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol.. 2012;64:1146-1154.
- [Google Scholar]
- Anti-cancer effect of liriodenine on human lung cancer cells. Kaohsiung J. Med. Sci.. 2004;20:365-371.
- [Google Scholar]
- Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and-9. Exp. Ther. Med.. 2017;13:739-744.
- [Google Scholar]
- Antimitotic Diterpenes from Erythropodium c aribaeorum Test Pharmacophore Models for Microtubule Stabilization. Org. Lett.. 2000;2:257-260.
- [Google Scholar]
- The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett.. 2000;152:23-29.
- [Google Scholar]
- Inhibition of topoisomerase II by the marine alkaloid ascididemin and induction of apoptosis in leukemia cells. Biochem. Pharmacol.. 2000;60:527-537.
- [Google Scholar]
- Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs. 2013;11:1456-1476.
- [Google Scholar]
- Plant derived substances with anti-cancer activity: from folklore to practice. Front. Plant Sci.. 2015;6:799.
- [Google Scholar]
- Mechanism of action of aragusterol a (YTA0040), a potent anti-tumor marine steroid targeting the G1 phase of the cell cycle. Int. J. Cancer. 2000;88:810-819.
- [Google Scholar]
- Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1, 2-dimethylhydrazine in mice. Nutr. Cancer. 2001;39:108-116.
- [Google Scholar]
- Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta (BBA)-General Subj.. 2011;1810:497-503.
- [Google Scholar]
- Trabectedin: an anticancer drug from the sea. Expert Opin. Pharmacother.. 2009;10:2735-2743.
- [Google Scholar]
- In vitro schedule-dependency of myelotoxicity and cytotoxicity of Ecteinascidin 743 (ET-743) Ann. Oncol.. 1998;9:989-993.
- [Google Scholar]
- Cytotoxicity and structure activity relationship studies of maplexins A-I, gallotannins from red maple (Acer rubrum) Food Chem. Toxicol.. 2012;50:1369-1376.
- [Google Scholar]
- Medicinal plants: their use in anticancer treatment. Int. J. Pharm. Sci. Res.. 2015;6:4103.
- [Google Scholar]
- Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev.. 2010;29:405-434.
- [Google Scholar]
- Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res.. 1992;52:101-107.
- [Google Scholar]
- Potentiation of taxol efficacy by discodermolide in ovarian carcinoma xenograft-bearing mice. Clin. Cancer Res.. 2006;12(1):298-304.
- [Google Scholar]
- Fucoxanthin exerts anti-tumor activity on canine mammary tumor cells via tumor cell apoptosis induction and angiogenesis inhibition. Animals. 2021;11(6):1512.
- [Google Scholar]
- Cytotoxic cembranes from Indonesian specimens of the soft coral Nephthea sp. Mar. Drugs. 2010;8:2142-2152.
- [Google Scholar]
- Influence of Sargassum fusiforme polysaccharide on apoptosis of tumor cells. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China. J. Chinese Mater. Medica. 2004;29:245-247.
- [Google Scholar]
- A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement. Altern. Med.. 2013;13:1-11.
- [Google Scholar]
- Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc. Natl. Acad. Sci.. 2000;97:6775-6779.
- [Google Scholar]
- Kazłowska, K., Lin, H.-T.V., Chang, S.-H., Tsai, G.-J., 2013. In vitro and in vivo anticancer effects of sterol fraction from red algae Porphyra dentata. Evidence-Based Complement. Altern. Med. 2013.
- Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. 2014;34(2):701-706.
- [Google Scholar]
- Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep.. 2014;47:433.
- [Google Scholar]
- The tomato glycoalkaloid α-tomatine induces caspase-independent cell death in mouse colon cancer CT-26 cells and transplanted tumors in mice. J. Agric. Food Chem.. 2015;63:1142-1150.
- [Google Scholar]
- Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol.. 2022;8:420-444.
- [Google Scholar]
- Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol.. 2009;47:1653-1658.
- [Google Scholar]
- Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chem. Nat. Compd.. 2010;46:1-4.
- [Google Scholar]
- The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem.. 2006;17:611-625.
- [Google Scholar]
- Isorhamnetin suppresses colon cancer cell growth through the PI3K-Akt-mTOR pathway. Mol. Med. Rep.. 2014;9:935-940.
- [Google Scholar]
- Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci.. 2018;19:3533.
- [Google Scholar]
- Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Heal. Part A. 2017;80:776-787.
- [Google Scholar]
- Isolation, Structure Determination, and Biological Activity of Lyngbyabellin A from the Marine Cyanobacterium Lyngbya m ajuscula. J. Nat. Prod.. 2000;63:611-615.
- [Google Scholar]
- Total synthesis of the cyclodepsipeptide apratoxin A and its analogues and assessment of their biological activities. Chem Eur J. 2006;12:7615-7626.
- [Google Scholar]
- Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin. Cancer Res.. 2000;6:1978-1987.
- [Google Scholar]
- Mayer, A.M.S., Pierce, M., Glaser, K.B., Newman, J., Jaspars, M., Jimenez, C., Tagliatela-Scafati, O., Yang, J., 2012. The Global Marine Pharmaceuticals Pipeline. Midwest. Univ.
- Antitumor activity of cryptophycins: effect of infusion time and combination studies. Cancer Chemother. Pharmacol.. 2000;46:142-149.
- [Google Scholar]
- Monoacylglycerides from the diatom Skeletonema marinoi induce selective cell death in cancer cells. Mar. Drugs. 2019;17:625.
- [Google Scholar]
- Biological activities and phytochemicals of Swietenia macrophylla King. Molecules. 2013;18:10465-10483.
- [Google Scholar]
- A New Indanone from the Marine Cyanobacterium Lyngbya m ajuscula That Inhibits Hypoxia-Induced Activation of the VEGF Promoter in Hep3B Cells. J. Nat. Prod.. 2000;63:1431-1433.
- [Google Scholar]
- Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants. 2019;8:183.
- [Google Scholar]
- Drugs and drug candidates from marine sources: An assessment of the current “state of play”. Planta Med.. 2016;82:775-789.
- [Google Scholar]
- Natural compounds for cancer treatment and prevention. Pharmacol. Res.. 2009;59:365-378.
- [Google Scholar]
- Formononetin induces apoptotic cell death through the suppression of mitogen-activated protein kinase and nuclear factor-κB phosphorylation in FaDu human head and neck squamous cell carcinoma cells. Oncol. Rep.. 2020;43:700-710.
- [Google Scholar]
- Anti-inflammatory and anticancer drugs from nature. Advances in Nutritioncancer 2014:123-143.
- [Google Scholar]
- Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs. 2011;9:819-831.
- [Google Scholar]
- Cytotoxic activity of hydrolyzable tannins against human oral tumor cell lines—a possible mechanism. Phytomedicine. 2000;7:39-47.
- [Google Scholar]
- Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom. Phaeodactylum tricornutum. J. Funct. Foods. 2014;6:231-240.
- [Google Scholar]
- Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS One. 2014;9:e101220.
- [Google Scholar]
- Sithranga Boopathy, N., Kathiresan, K., 2010. Anticancer drugs from marine flora: An overview. J. Oncol. 2010.
- Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed. Pharmacother.. 2017;89:845-856.
- [Google Scholar]
- Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun.. 2000;275:877-884.
- [Google Scholar]
- Tetrandrine inhibits the proliferation of human osteosarcoma cells by upregulating the PTEN pathway. Oncol. Rep.. 2017;37:2795-2802.
- [Google Scholar]
- Tannic acid ameliorates doxorubicin-induced cardiotoxicity and potentiates its anti-cancer activity: potential role of tannins in cancer chemotherapy. Toxicol. Appl. Pharmacol.. 2011;251:191-200.
- [Google Scholar]
- Mechanistic studies of Sansalvamide A-amide: an allosteric modulator of Hsp90. ACS Med. Chem. Lett.. 2010;1:4-8.
- [Google Scholar]
- Cuphiin D1, the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell line. Cancer Lett.. 2000;149:77-83.
- [Google Scholar]
- Anti-proliferative effects of evodiamine on human breast cancer cells. PLoS One. 2013;8:e67297.
- [Google Scholar]
- Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine. 2016;23:641-653.
- [Google Scholar]
- Clausenidin from Clausena excavata induces apoptosis in hepG2 cells via the mitochondrial pathway. J. Ethnopharmacol.. 2016;194:549-558.
- [Google Scholar]
- 8-Cetylberberine inhibits growth of lung cancer in vitro and in vivo. Life Sci.. 2018;192:259-269.
- [Google Scholar]
- Xu, M.R., Wei, P.F., Suo, M.Z., Hu, Y., Ding, W., Su, L., Zhu, Y.-D., Song, W.J., Tang, G.H., Zhang, M., 2019. Brucine suppresses vasculogenic mimicry in human triple-negative breast cancer cell line MDA-MB-231. Biomed Res. Int. 2019.
- Sanguinarine inhibits growth of human cervical cancer cells through the induction of apoptosis. Oncol. Rep.. 2012;28:2264-2270.
- [Google Scholar]
- Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells. Cancer Lett.. 2000;157(1):65-75.
- [Google Scholar]
- Inhibitory effects of O-methylated isoflavone glycitein on human breast cancer SKBR-3 cells. Int. J. Clin. Exp. Path.. 2015;8(7):7809.
- [Google Scholar]
- Zhao, Z., Liu, B., Sun, J., Lu, L., Liu, L., Qiu, J., Li, Q., Yan, C., Jiang, S., Mohammadtursun, N., 2019. Baicalein inhibits orthotopic human non-small cell lung cancer xenografts via Src/Id1 pathway. Evidence-Based Complement. Altern. Med. 2019.
- Daidzein induces choriocarcinoma cell apoptosis in a dose-dependent manner via the mitochondrial apoptotic pathway. Mol. Med. Rep.. 2018;17(4):6093-6099.
- [Google Scholar]
- Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer. 2017;17:1-10.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102919.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







