Translate this page into:
Anticancer and microbial activities of gold nanoparticles: A mechanistic review
⁎Corresponding author. nhaljarba@pnu.edu.sa (Nada H. Aljarba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
According to WHO reports, the emergence of antibiotic resistance together with limited discovery of newer and effective anticancer and antimicrobial chemotherapeutics remained of utmost concern to human health. In addition, the poor solubility, stability, and side effects that lead to inefficiency of current anticancer and antimicrobial therapy encouraged research into new strategies to combat such resilient disease. Various metal nanoparticles (MNPs) such as gold (Au), silver (Ag), and others synthesized from natural or chemical sources have displayed potential biological properties against a wide range of microbial infections caused by multi-drug resistant bacteria. Remarkably, gold nanoparticles (Au-NPs) have gained particular concern, due to their biocompatibility, ease of surface functionalization, and their optical properties. Several studies have been carried out to investigate the antibacterial potentials of Au-NPs and findings showed that these NPs triggers microbial and cancer cell damage as a result of oxidative stress, membrane and DNA damage. In this review, we present a concise description of a broad update on Au-NPs isolated from natural and chemical sources that render it application in biomedical research as therapeutic agents. The review focuses mainly on the anticancer and antimicrobial activities of Au-NPs in conjunction with microorganism being prompted by different biocompatible origin and its future prospects these infectious systems. Finally, we summarize new possibilities for Au-NPs-based biochemical systems as an effective medical nanotechnology based therapeutic.
Keywords
Gold
Nanoparticles
Antibacterial activities
Resistance
Nanomedicine
1 Introduction
NPs are generally less than100 nm in size in one of their dimensions. Due to this size advantage, researchers have focused on development of various NPs as novel opportunities in diagnosis, designing nano-based devices with pharmaceutical applications such as stratification of disease, staging, and management of response to therapy (Rao et al., 2017). Encapsulation of conventional drugs within NPs increases half-life of the drug with improved uptake through the cell membrane and controlled release of therapeutic agents at the target site in addition, the small size allows the drug to evade the body's immune system. Due to small size and distinctive coating of NPs, they easily hold hydrophobic anticancer drugs to exact site in body reduced modification by immune system. The therapeutic roles of NPs also confer them with a better treatment for drug-resistant bacteria strains. Various NPs are synthesized from on metals such as Ag, Au or other materials such as, silica (SiO2), fullerene, quantum dots, carbon nanotubes (CNTs) and magnetic NPs have been formulated (Ahari et al., 2020).
Among these NPs, our study has been focused on Au-NPs due to their peculiar characteristics in various biomedical applications. The features like its chemical inertness and resistance to surface oxidation, makes Au-NP an ideal candidate for nanoformulation. Moreover, high stability, low cytotoxicity, biocompatibility, and multi-functional potential offered by Au-NPs makes it potential candidate for drug delivery system (Eleraky et al., 2020). It is well established that the Au-NPs with phytochemicals have been extensively used for their antiviral, antiallergic, anti-inflammatory, antioxidant and antitumor properties. Nevertheless, Au-NPs have shown potential applications for the delivery of antitumor agent’s, such as cisplatin, oxaliplatin and paclitaxel through the detection of DNA. Besides these, Au-NPs are also excellent drug carriers, which can tune the antibacterial effects of drugs and play a crucial role for effective antibacterial strategies against some resistant bacteria. The photothermal property of Au-NPs can be apply for photothermal treatments to induce anti-bacterial activity (Gubitosa et al, 2018). Au-NPs may also be functionalized by attaching cations, surface ligands, low-temperature plasma and other potential antibacterial agents to their surface to enhance the antibacterial characteristics. The antibacterial properties of functionalized Au-NPs have been shown to have considerable potential to overcome antibacterial resistance. The Au-NPs synthesize from a variety of plant sources, have been applied in the treatment of different kinds of cancers such as breast cancer cells MCF-7, Hep2 and A549 cells. As such, several researchers aimed to develop low-cost, effective, eco-friendly Au-NPs to cure cancer and microbial infections (Rajeshkumar, 2016; Rajeshkumar et al., 2021).
Despite its potential benefit for treating numerous cancers, the application of Au-NPs is limited because of its inability to specifically target cancer or infected cells with cytotoxic effect on healthy and infected cells once administered inside the body due to the facts that Au being a biologically inert metal causes low elimination and thereby leads to cumulative deposition by retention of Au in a specific part of body (Gao et al., 2011). About 90% of Au injected intravenously remains in circulation and other fraction i.e., 10% is accumulated in liver for more than one week (Huang, 2006). Such accumulation is not too much hazardous even after repeated administration, but have found to cause hemolysis in vitro cell.
Dykman and his co-workers have been studied applications of Au-NPs particularly immunological properties (Dykman and Khlebtsov, 2012). Yang and his co-workers reviewed the pharmacokinetics applications of Au-NPs specially focusing on optical properties (Yang et al., 2015). Umapathi and his co-workers have demonstrated a new approach to improve the anticancer potential of Au-NPs by developing a strong corona of curcumin and isonicotinic acid hydrazide (INH) around them. Their investigations on human lung squamous carcinoma (LK-2) and human lung fibroblast (TIG-120) cells indicated the selective toxicity of functionalized NPs, which act as an excellent carrier and stabilizer for curcumin and INH molecules are shown in Fig. 1 (Umapathi et al., 2020). Gupta and his group demonstrated Au-NPs is capable of inhibiting the growth of cancerous cells with the help of photothermal (Gupta and Malviya, 2021). Antimicrobial activity and creatinine adsorption capacity studies was carried out by Rehan and his groups using Au and Ag-NPs with silk fibers. Khan and his groups focused on plant-based Au-NPs and reported its more applications such as antimicrobial, antioxidants, hepatoprotective, anti-cancer therapeutic potential, in drug delivery etc. of Au-NPs (Khan et al., 2019). Besides these, Au-NPs have also been used in radiation therapy due to their unique radio-sensitizing characteristics. Su et al. which were characterized a theranostic iodine-125-labeled cRGD-Au-NPs, in tumor-targeting radio-sensitizer and CT/ SPECT imaging agent. Incorporation of 125I into Au-NPs helps in in vivo nuclear imaging for radiotracer. It has also been found that in their work, the use of Au was not forced to increase the intensity of CT, but to enhance the Au-NPs stability and high loading capabilities, as well as their bio-distribution and pharmacokinetics properties. Fig. 2 summarized a lot of applications of Au-NPs in various field on the basis of literature survey.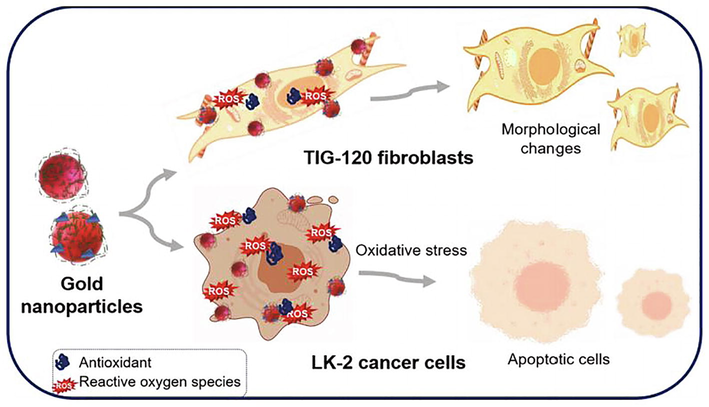
Schematic representation of the morphological changes and apoptosis in human lung fibroblast (TIG-120) and human lung squamous carcinoma (LK-2) cells treated with Au-NPs (Umapathi et al., 2020).
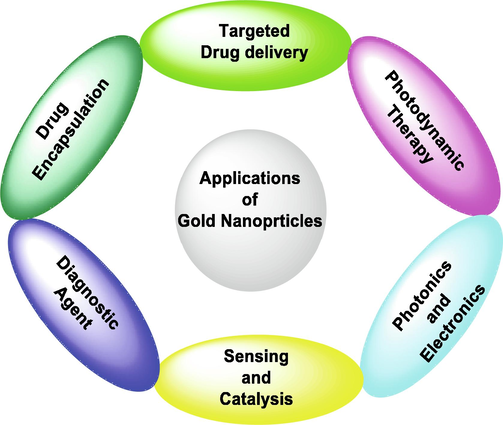
General applications of Au-NPs.
This review article aims to collect biological activities on Au-NPs with main focus on anticancer and antimicrobial activity. The classification has been done based on the raw materials from where they are extracted.
2 Biological activity of Au-NPs
Biological activity of Au-NPs has been studied on the basis of various important characteristics of Au (III) that help to synthesize Au-NPs from different materials. Au is extensively used as nontoxic nanomaterials but those materials which are used in the preparation and modification of Au-NPs may be toxic. Toxicity may be evidenced as the high concentration of Au-NPs, but these NPs produce apparent antibacterial effects. At specific concentrations, these NPs have been shown to have no toxic effects on normal cells (Rao et al., 2017). Modified Au-NPs not only show good antimicrobial activity against standard strains but also have unique anticancer activity against bacteria. Au (III) conjugated to different drugs systems has exhibited to intensify their efficacy against bacteria. Au-NPs-aminoglycosides coated membranes have also been shown to be efficient antibacterial agents against various types of bacteria like E. coli, M. luteus, P. aeruginosa and S. aureus (De et al., 2016). Au nanorods or nanospheres showed these interactions with teichoic acid (negative charge) on B. cereus (positive charge). In brief, we have now discussed the various biological activities of Au-NPs using a diagram which has been shown in Fig. 3.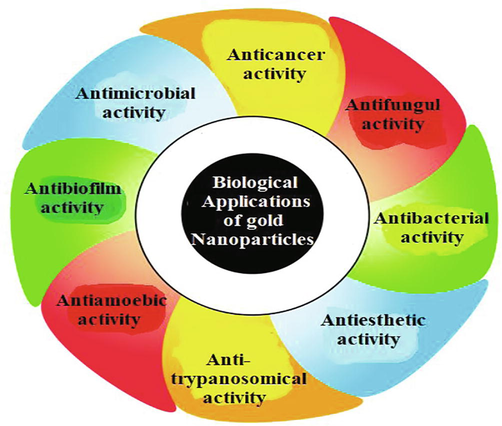
Different biological activities of Au-NPs.
2.1 Anti-cancer activity of Au-NPs
With nanomedicine, microorganisms such as E. coli, M. luteus, P. aeruginosa and S. aureus can be conjugated with Au-NPs with the surface modification of NPs. The modified surface of Au-NPs confers these agents as a specific function of nanobio blend which make molecules able to be used in biomedicine for target drug delivery. Since, Au-NPs are well known NPs for their significant capability of blending due to their large surface area, which allows them to be conjugated to different chemicals substances, like antibacterial agents and various biomolecules. Hence, biosynthesized Au-NPs can be functionalized or adsorbed by biological peptides to deliver drug to a targeted cell/tissue (Siegel et al., 2019). NPs target the tumor cells through accumulation and entrapment. The process is also defined by retention and permeation effect imposed in cancerous cells because of improper lymphatic flow and angiogenic vessels. Therefore, the entration of these NPs accumulates more or selectively inside cancerous cells as compared to the normal cells. Conjugation of these microbes at the Au surface via cell surface receptors, such as antibodies, peptides and antibiotics against cells infected by tumor can increase the residence of these nanoparticles, thus enabling their use in diagnosis and therapy (Fig. 4). Au-NPs with two functional domains showed biocompatibility for their use in drug delivery without entering the cell. For example, Au-NPs conjugated with anti-VEGF have been shown to enhance the induction of apoptosis in CLL cells as compared to these antibodies alone (Kim et al., 2009).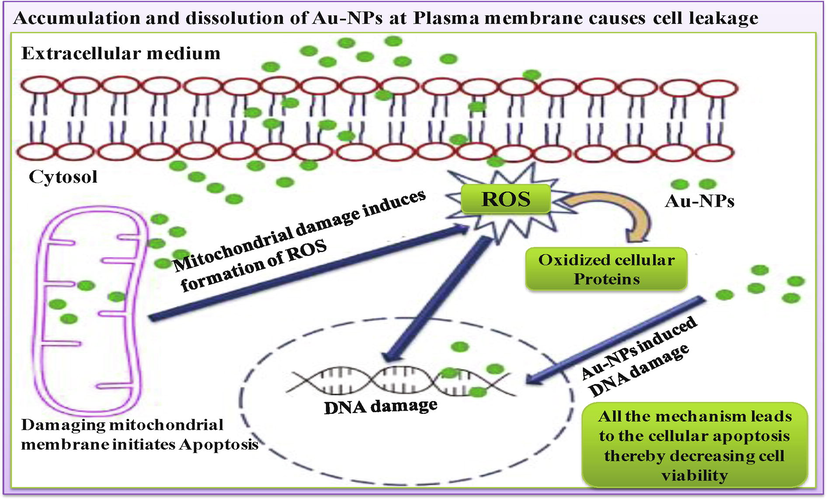
Possible mechanism of action of Au-NPs on cancer cell lines (Singh et al., 2019).
Currently, Au-NPs have been getting popular for their use in diagnosis and treatment of cancer. Conjugations of Au-NPs with adamantane and cyclodextrins seem to possess photothermal effects on cancer cells (Wang et al., 2010). Furthermore, mixtures of Au-NPs with few other magnetic NPs have been used to target specific types of cells during cancer cell imaging. A study revealed that iron Nano shells coated with Au have shown inhibitory effect on colorectal and oral cancer cells without affecting normal cells (Wu et al., 2011). The cytotoxicity in this experiment showed dependence on age of Au-NPs and were seen to be released at slow rate into human cell lines because of presence of iron. Another study proved that the apoptosis of metastatic cancer cells was observed by targeted detection of cancer cells with Au-NPs functionalized and labeled with fluorescent heparin. The detection of cancer cells here is totally based on quenching and regaining of fluorescence of heparin. Heparin on functionalization with heparin loses fluorescent behavior because of quenching which is regained on its cleavage by heparinase/heparinase. Therefore, heparin functionalized Au-NPs can be useful in both diagnosis as well as treatment of cancer (Lee et al., 2010). Fig. 5 describes the possible mechanism of action of Au-NPs on cancer cell lines.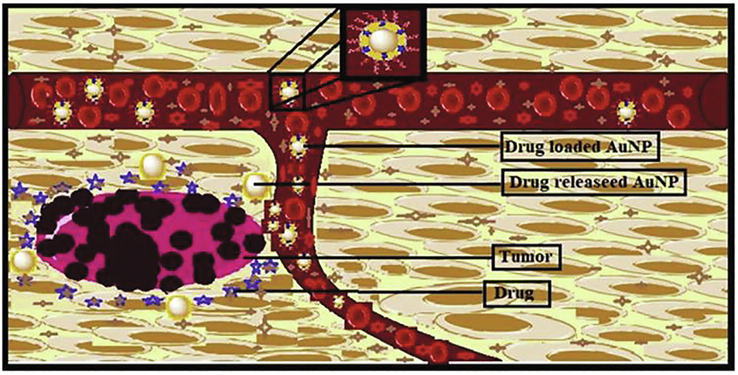
An imaginary demonstration of Au-NPs as a targeted drug delivery system for cancer treatment (Bergen et al., 2006).
The conjugation of polyamidoamine dendrimer-folic acid and fluorescein isothiocyanate has been successfully used in imaging and specific targeting of tumor cells. Dendrimers containing terminal amines, which can be acetylated leading to a possibility of synthesizing different functionalized Au-NPs. e.g., functionalizing these dendrimers containing Au-NPs with folic acid have been used to target tumors cells in vitro. These functionalized Au-NPs specifically shown the interaction with HeLa cells due to the presence of folic acid receptors which not affects normal cells (Shi et al., 2009). To demonstrate the anticancer activity, researchers have used PEGylated NPs conjugated with folic acid to target cancer cells and also the derivative of thiol-PEGylated tamoxifen was developed which was selectively used in targeted delivery of Au-NPs to breast cancer cells in vitro (Dreaden et al., 2011). Demonstration of Au-NPs as a targeted drug delivery system for cancer treatment has been shown in Fig. 6.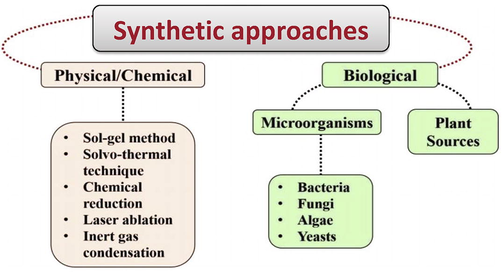
Various approaches for the synthesis and development of Au-NPs.
Studies reveal that Au-NPs functionalized with peptides, fluorophores, aptamers, cell adhesion molecules etc., have ability to target specific tissues, thereby they are useful in imaging of tumors, targeting drug delivery and detecting apoptosis. Similarly, the octreotide peptide functionalized Au-NPs have also been used in bio-imaging of neuro-endocrine carcinomas (Surujpaul et al., 2008). Au-NPs was biosynthesized from flower Couroupita guianensis which shows their anticancer potential on MTT assay, apoptosis in staining of DAPI, DNA fragmentation, and comet assay in DNA damage.
To simplify anticancer properties, we have further classified these NPs with anti-cancer activity based on the extract from which they are obtained. The anticancer activity of Au-NPs using a marine bacteria Entercoccus sp. against lungs and liver cancer cells (Kumar et al., 2016). Hamed and his coworkers synthesized Au-NPs from a soil bacteria Streptomyces griseus which were found in sediment of the Suez Gulf, Egypt., out of total nine actinomycetes in which one was succeeded to produce Au-NPs. These NPs when tested for biomedical application showed anticancer activity against cancer cell lines Colon carcinoma cells (HCT-116) and breast carcinoma cells (MCF-7) (Hamed et al., 2019). Munawer and co-workers used a bio fabricated Au-NPs conjugated with Commiphora wightii, with a fungus named endophytic Cladosporium sp. and were studied for their anticancer properties on breast cancer cell line MCF-7. These NPs were observed to have anticancer activity. In addition, nature contains abundant of wellspring plants. Au-NPs have been obtained from plant extracts via green routes as reducing agents and stabilizers have accumulated intense interest in past decades because of their peculiar properties. The simple characteristics for Au-NPs obtained from plants include the collection of various parts of plants which describe as the plant extracts play a vital role both as stabilizers and reducing agents to obtain plant-Au-NPs. biomolecules from plant extracts, including poly-phenols, alkaloids, flavonoids, polysaccharides, reducing sugars, vitamins, amino acids and proteins have been used in the reduction of gold as AuIII to Au0. Then, they stabilize the Au-NPs by covering the outer surface of the Au-NPs to prevent agglomeration (Munawer et al., 2020). Lokina and co-workers produced Au-NPs using grapes fruit extract having crystalline nature and spherical shape and shown anticancer activity against HeLa cell lines (Lokina and Narayanan, 2013). Barai and groups also synthesized Au-NPs using the extract from the stem bark of Nerium oleander that was very effective in apoptosis of cancer cells such as breast cancer (MCF-7) (Barai et al., 2018). Patel and his coworkers synthesized Au-NPs by eco-friendly approach using leaf extract of Sasa borealis plant and show their anticancer activity on HEK293 cells with AGS cells due to their toxic effects (Patil et al., 2018). Various approaches which have been discussed for the synthesis and development of Au-NPs were shown by Fig. 7.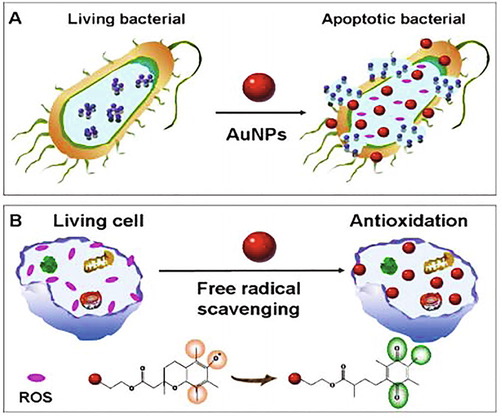
Schematic illustration of the plant-Au-NPs used in antibacterial (A) and antioxidant (B) applications (Qiao et al., 2021).
To show the anticancer activity of Au-NPs, the plant extract Marsdenia tenacissima were used to show their application as in vitro anticancer drug in lung cancer cells i.e., A549 cells (Sun et al., 2019). Patel and his coworkers synthesized Au-NPs using flower extract namely, Lonicera japonica. The activity was found on normal HEK293 cells i.e., kidney cells of human embryo which showed its non-toxic effect and dose dependent anticancer activity on cervix cancer (HeLa) cells (Patil et al., 2019). Another extract of algae Sargassum incisifolium was used to synthesize Au-NPs and showing their toxicity on HT-29 and MCF-7 cancer cells which were nontoxic in nature (Mmola et al., 2016). A green approach was applied in the synthesis of Au-NPs from Abies spectabilis plant extract to show their anticancer activities on cancerous cells of bladder (T24) (Wu et al., 2019). Modified Au-NPs were biofunctionalized using aqueous Gymnema sylvestre extract which show anti-cancer activity against HT29 cell line (Arunachalam et al., 2014). Synthesis of Au-NPs using halotolerant Microalga dunaliella salina by green methodology to show the anti-cancer activity against MCF7 and MCF 10A cancer cells. (Singh et al., 2019). Au-NPs have been also synthesized from brown seaweed Sargassum glaucescens and their anticancer activity was tested against liver (HepG2), cervical (HeLa), leukemia (CEM-ss) and breast (MDA-MB-231) cell lines (Ajdari et al., 2016). The Au-NPs from eight green extracts of various plant parts have been synthesized and their anticancer activities have been studied on MCF 7 breast cancer cell lines (Priya et al., 2015). Au-NPs have also been obtained from leaf extract of anax notoginseng to show the anticancer activity on PANIC-1 cells in an environment friendly manner (Wang et al., 2019). Similarly, the Au-NPs have also been synthesized from extract of Citrus macroptera fruit and their study have been performed for anticancer activity on three different cancerous HepG2 cells (liver cancer cell line), tA 549 (alveolar basal epithial cells), MDA-MB 468 (breast cancer cell) (Majumdar et al., 2019). Green methodology was also used to synthesize Au-NPs from aqueous Alternanthera Sessilis extract and shown their anticancer activity against cervical cancer cells (HeLa) (Qian et al., 2019). Au-NPs were also synthesised from extract of Gloriosa superba tuber, Pongammia pinnata leaf extract and shown their anti-cancer activity against human breast adenocarcinoma (MCF-7) cancer cells (Govindaraju et al., 2020). Kajani and co-workers have been synthesized Au-NPs from ethanolic extract of Taxus baccata, which show anticancer activity against different cancerous cells like breast (MCF-7), cervical (HeLa) and ovarian (Caov-4) (Kajani et al., 2016). The Au-NPs have been found to show high potential in cancer therapies of few plant extracts like Catharanthus roseus (CR) and Carica papaya (CP) plant extracts used to synthesize Au-NPs with different shape and sizes. NPs are found to show potential against different cancer cells: Lymphomas, breast cancer, Hodgkin’s disease, Leukemia, acute Lymphocytic, soft tissue sarcomas, Neuroblastoma, and multiple Myeloma (Muthukumar et al., 2016). Fig. 8 describes the illustration of the plant-Au-NPs used in antibacterial and antioxidant applications.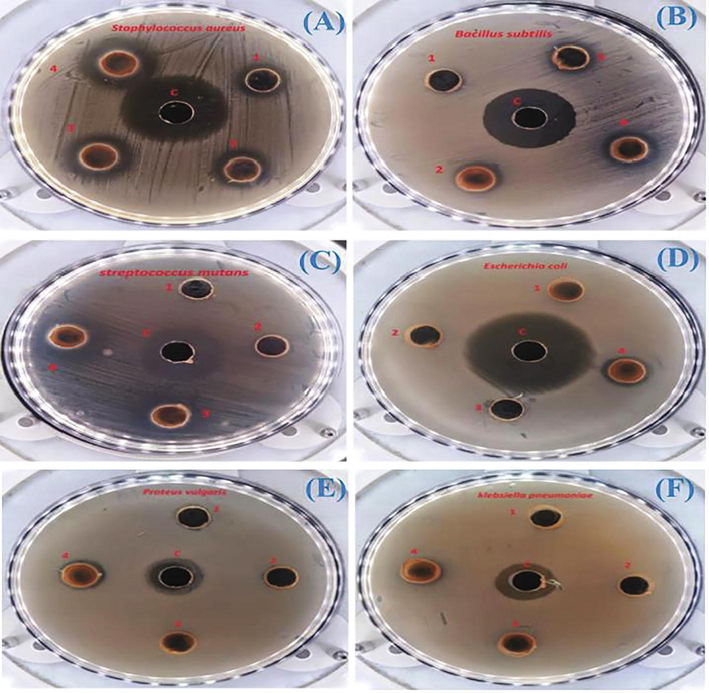
Antimicrobial activity of synthesized Au-NPs against gram positive and negative organisms: (A) Bacillus substillis, (B) Micrococcus luetus, (C) Staphylococcus aureus, (D) Streptococcus mutants, (E) E. coli, and (F) Proteus vulgarius (Thangamani and Bhuvaneshwari, 2019).
Au-NPs also, derived from different chemicals. The modified Au-NPs with 11-mercaptoundecanoic acid, on conjugation with chloroquine were found to show anticancer activity against breast cancer cells (MCF-7). The different concentrations of GNP-Chl conjugates were used against MCF-7 cancer cells. The assayed viability was seen via trypan blue that give IC50 value of 30 ± 5 μg·mL−1 (Joshi et al., 2012). Studies reveals that the Au-NPs were obtained from chemicals using green synthesis methods and stabilized by Resveratrol whose formation was confirmed by plasmon resonance band at 537 nm. These NPs were used as carrier for anticancer drug against glioma carcinoma cell line (LN 229) (Mohanty et al., 2014).
Daduang and groups have been developed the Au-NPs by conjugation with gallic acid and used it as a drug delivery for anticancer effect. The study was performed on cervical cancer cells, infected by HPV type 16 (CaSki), 18 (HeLa) (Daduang et al., 2015). Hanora and co-workers successfully synthesized Au-NPs dispersed in aqueous medium and studied their anticancer activity. They used both green chemical method as well as biosynthesis process to synthesize these nano particles. They conjugated NPs with citrus pectin, sodium alginate, chitosan and fermented fenugreek powder (aqueous extract) by gamma radiation. It was observed that green biosynthesis provides superior results as compared to green chemical synthesis. The NPs synthesized showed anti-cancer activity against different EAC cells (Hanora et al., 2016).
Recently, we synthesized a new type of Au-NPs, which were prepared by functionalization with peptide and a thioctic acid–DMPGTVLP peptide (TA-peptide) conjugate. These were found to show anti-cancer activity against breast cancer cell lines MCF-7 and T47D (Akrami et al., 2021). The synthetic purpose of Au-NPs which were obtained from 3-butoxy-2-hydroxypropyl 2-(2,4-dihydroxyphenyl) acetate using green synthesis approach. The medicinal value for these capped NPs was investigated by checking their anticancer activity on liver cancer (HepG2) cells and found better approach towards best anticancer agents (Ashokkumar et al., 2014). Recently, the Au-NPs were also conjugated to chitosan under gamma radiation to provide these NPs with enhanced anticancer activity towards HepG-2 and CaCo-2 cell lines (Sokary et al., 2020). Hoshyar and co-workers synthesized controlled size Au-NPs using antioxidant crocin as a reducing agent. These crocin functionalized Au-NPs was employed as anticancer agents to treat breast cancer cells and others such as MCF-7, MDA-MB-231, PC-3, HepG2 and HL-60 (Hoshyar et al., 2016). Au-NPs have also been synthesized using a protein, i.e., apo-α-LA (alpha helical protein), which have been used in to selectively target breast cancer cells like MF7. It can also be seen that coating of this apo-protein over Au-NPs enhances their anti-cancer activity by several folds (Yarramala et al., 2015). Recently, eight different proteins were used to synthesize the Au-NPs using green methodology. Two proteins failed to produce the Au-NPs, however, six succeeded in synthesis of Au-NPs. The Au-NPs, thus obtained from protein assisted synthesis were studied for their anti-cancer activity against three different cancer cells i.e., Human colorectal cancer cells (HCT116), human cervical cancer cells (HeLa) and squamous carcinoma cells (SCC-7) (Joseph et al., 2014).
Rao and his group synthesiszed Au-NPs from silk fibre as reducing agent on HAuCl4·xH2O Then they have been investigated it on Jurkat cell cell line and were found to show anti-cancer activity (Rao et al., 2017). Tomoaia and co-workers have also been synthesized the Au-NPs by capping them with Doxorubicin and Resveratrol. These were found to show anti-cancer activity against two human cervical cancer cells i.e., HeLa and CaSki cells (Tomoaia et al., 2015). The anti-cancer applications of Au-NPs from above studied sources have been listed in Table 1.
S. No.
Reducing agent used
Type of Reducing agent used
NPs characteristics (nm)
Cell lines
Outcome
Ref.
1.
Enterococcus sp
marine bacteria
Size: 6 – 13
Shape: sphericalHepG2 and A549
Atable gold nanoparticles show more significant anticancer activity against HepG2 and A549 cells at 100 μg concentration of nanoparticles.
Kumar et al., 2016
2.
Streptomyces griseus
Soil Bacteria
Size: 19 – 28
Shape: sphericalHCT-116); MCF-7
Anticancer activity against two different cancer cell lines Colon carcinoma cells (HCT-116) using 61.9 ug/well and breast carcinoma cells (MCF-7) using 46.6 ug/well
Hamed et al., 2019
3.
Endophytic Cladosporium sp.
Fungi
Size: 5 – 10
Shape: HexagonalMCF-7
Showed anti-cancer activity in breast cancer cell line MCF-7 (IC5038.23 µg/mL) through the induction of apoptosis
Munawer et al., 2020
4.
licorice root extract
Plant (root)
Size: 26.47–63.25
Shape: CircularThe human breast cancer (MCF-7) and liver cell-lines (HePG-2) by MTT assay.
The inhibitory concentration (IC50) value of licorice root extract-AuNPs was 23 µg/ml towards HepG-2 cellline while that of 50 µg /ml towards MCF-7 cell line.
Al-Radadi et al., 2021
5.
Pituranthos tortuosus aqueous extract
Plant (aerial parts)
Size: 5 to 15 nm
Shape: sphericalHepatocellular carcinoma (HepG-2) and human colon carcinoma (HCT-116) cell lines using the MTT assay.
AuNPs cytotoxic activity (IC50) was 23.60 µg/ml for (HCT-116) and IC50 = 6.27 µg/ml for HepG-2)
Abd El-Moaty et al., 2021
6.
Marine bacterium Vibrio alginolyticus
Microorganisms
Size: 50–100 nm
Shape: monodispersed, irregular shapedThe human colon carcinoma cell line (HCA-7) using the (MTT) assay
The IC50 was 15 µg / mL, and the maximum inhibition of the cell death was (>75%) obtained when treating 25 µg /mL
Shunmugam et al., 2021
7.
Aqueous extract Crassocephalum rubens (AECR)
Plant (leaves)
Size: 20 ± 5 nm
Shape: sphericalThe human breast cancer (MCF-7) and colorectal cancer (Caco-2) cells using the (MTT) assay
The in vitro cytotoxicity of the AECR-AuNPs was (125 and 250 μg /mL) during 24 and at all concentrations tested during 48 h
Adewale et al., 2020
8.
Jasminum auriculatum extract
Plant (leaves)
Size: 8–37 nm
Shape: sphericalThe human cervical cancer cell lines (Hela) using the (MTT) assay
The inhibitory effect in the proliferation of the human cervical cancer cell line with the IC50 value of 104 μg/mL
Balasubramanian et al., 2020
9.
Pongammia pinnata extract
Plant (leaves)
Size: 16nm
Shape: sphericalThe human breast cancer cell line (MCF-7) using the (MTT) assay
The inhibitory effect in the proliferation of the human breast cancer cell line with the IC50 of 1.85 μg/mL
Govindaraju et al., 2020
10.
Petroselinum crispum extract
Plant (leaves)
The particle shape and size is diverse because difference in the amount of the plant extract added during the synthesis
The human cancerous colorectal cell line using MTT assay
The 50% minimum inhibitory concentration (IC50) was determined as 89.1, 56.83, 71.51, 71.16 and 84.39 for the pure plant extract and AuNPs(A), AuNPs(B), AuNPs(C), and AuNPs(D), respectively
El-Borady et al., 2020
11.
Anacardium occidentale extract
Plant (leaves)
Size: 10–30 nm
Shape: sphericalThe human breast cancer cell line (MCF-7)
The inhibitory effect in the proliferation of the human breast cancer cell line with the IC50 of 6 μg/mL
Sunderam et al., 2019
12.
Grapes fruit extract
Aqueous extract
Size: Nano range
Shape: sphericalHeLa cell lines
A promising and effective antibacterial agent against the multidrug resistant strains of various bacterias
Lokina and Narayanan, 2013
13.
Algae Sargassum incisifolium
Aqueous extract
Size: 12.38
Shape: spherical(HT-29, MCF-7)
gold nanoparticles displayed negligible toxicity against cancerous (HT-29, MCF-7) and non-cancerous (MCF-12a) cell lines
Mmola et al., 2016
14.
Abies spectabilis
Plant extract
Size: 108.6
Shape: sphericalBladder cancer T24 cells
cytotoxicity effects on anticancer activity against T24 cells by MTT assay
Wu et al., 2019
15.
Brown seaweed Sargassum glaucescens
Size: 3.65 ± 1.69
Shape: sphericalcervical (HeLa), liver (HepG2), breast (MDA-MB-231) and leukemia (CEM-ss)
anticancer effect of SG-stabilized AuNPs is via the intrinsic apoptotic pathway on cervical (HeLa), liver (HepG2), breast (MDA-MB-231) and leukemia (CEM-ss) cell lines
Ajdari et al., 2016
16.
Panax notoginseng
Leaf
Size: 12- 80
Shape: sphericalPANC-1 cells
anticancer activity in pancreatic cancer PANC-1 cell lines and induced cytotoxicity, ROS and apoptosis by intonating intrinsic apoptotic gene expressions in PANC-1 cells
Wang et al., 2019
17.
Aegle marmelos
fruit extracts
Size: 18
Shape: sphericalhuman breast cancer cell line (MCF-7)
in vitro anticancer activity was confirmed by MTT assay on the human breast cancer cell line MCF-7 at different concentrations
Vijayakumar et al., 2018
Eugenia jambolana
fruit extracts
Size: 16
Shape: spherical
soursop
fruit extracts
Size: 28
Shape: spherical
18.
Backhousia citriodora (B. citriodora)
leaf extract
Size: 8.40 ± 0.084
Shape: sphericalMCF-7 breast cancer cell line and the HepG2 liver cancer cell line
Au-NPs showed a significant dose-dependent reduction in the viability of the MCF-7 breast cancer cell line and the HepG2 liver cancer cell line with IC50 values of 116.65 and 108.21 µg, respectively
Khandanlou et al., 2018
19.
Crassocephalum rubens leaf
Aqueous extract
Size: 20 ± 5
Shape: sphericalMCF-7 and Caco-2 cell lines
Significant anticancer activity of the AECR-AuNPs on MCF-7 and Caco-2 cells was noted at (125 and 250 μg/mL).
Adewale et al., 2020
20.
Marsdenia tenacissima (MT),
Herbal extracts
Size: 30-50
Shape: sphericalliver cancer HepG2 cells
MT-AuNPs were analyzed for cytotoxicity property against HepG2 cells by MTT analysis and found anticancer activity of biogenic AuNPs through in-vivo studies
Li et al., 2019
21.
Dragon fruit extract
Fruit extracts
Size: 10–20
Shape: sphericalMCF-7 breast cancer cells,MDA-MB-231 cells
The DF extract and DF-AuNPs induced significant growth inhibition of MCF-7 breast cancer cells.
Divakaran et al., 2018
22.
Dracocephalum kotschyi
Leaf extract
Size: 7.9–22.63
Shape: sphericalHeLa and K562 cell lines
Biological results exhibited that Au-NPs displayed a dose-dependent cytotoxicity with IC50: 196.32 and 152.16 μg/ml against K562 and HeLa cell lines as well.
Dorosti et al., 2016
23.
M. acuminata colla.
flower extract
Size: 10.1–15.6
Shape: sphericalMCF-7 and VERO cells
In vitro anticancer efficacy (MCF-7) and toxicity (VERO) of AuNPs, flowers extracts were performed by MTT assay. IC50 value for DPPH analysis was at 390 μg and 460 μg for extracts respectively.
Valsalam et al., 2019
24.
Actinidia deliciosa
Fruit extract
Size: 20
Shape: sphericalHCT116 cells
AuNPs showed 71% viability at highest concentration (350 μg/mL) using MTT assay, which provides promising approach for alternative nano-drug development
Naraginti et al., 2017
25.
Jasminum auriculatum
Leaf extract
Size: 8–37
Shape: sphericalHela cancer cells
Au NPs revealed that the nanoparticles manifested a significant dose-dependent inhibitory effect in the proliferation of the human cervical cancer cell line with the IC50 value of 104 μg/mL.
Balasubramanian et al., 2020
26.
Chloroquinine
trypan blue
Size: ∼7
Shape: sphericalMCF-7 (Breast cancer cells)
The anticancer activity of chloroquine-gold nanoparticle conjugates (GNP-Chl) was well diagnosed on MCF-7 breast cancer cells.
Joshi et al., 2012
27.
Resveratrol
Doxorubicin
Size: ∼35
Shape: sphericalLN 229
The MTT assay using fibroblast cells from explants tissue revealed the biocompatibility of R-GNPs. Cytotoxic activity of doxorubicin loaded R-GNPs against glioma carcinoma cell line (LN 229), showed the suitability of R-GNPs as a carrier for anticancer drugs
Mohanty et al., 2014
28.
citrus pectin
Green chemivals
Size: 21-31
Shape: sphericalEAC cells, T-cell lymphoma
(TCP), B-cell lymphomas, Thyroid Papillary carcinoma (FRO) and breast cancer (MCF7), human lymphocytes and meningiomaProduction of smallest size particles and more effective as anticancer as follow: AuNPs green chemical synthesis (Citrus Pectin 1%, metal concentration 1 mM, pH 7 and radiation dose 5 kGy, anticancer IC50 EAC = 21.5 μg/ml and CACO = 24.4 μg/ml)}, anticancer IC50 EAC = 4.8 μg/ml and CACO = 5.45 μg/ml)}.
Hanora et al., 2016
sodium alginate
Chitosan
Size: 13-24
Shape: spherical
Fermented fenugreek powder
29.
thioctic acid–DMPGTVLP, TAP@AuNPs
Peptide
Size: 3.52–26.2
Shape: sphericalBreast cancer cell lines MCF-7 and T47D
Treatment of the cells with TAP@AuNPS resulted in greater release of cytochrome c following caspase-3/7 activation compared with free TA-peptide. The cytosolic level of adenosine triphosphate for TAP@AuNPs was higher than in controls.
Akrami et al., 2021
30.
3-butoxy-2-hydroxypropyl 2-(2,4-dihydroxyphenyl) acetate
Cajanus cajan
Size: 9-41
Shape: sphericalliver cancer (HepG2) cells
Anticancer activity has been studied using liver cancer cells and cytotoxic mechanism has been evaluated using MTT, Annexin-V/PI Double-Staining Assay, Cell cycle, Comet assay and Flow cytometric analysis for apoptosis.
Ashokkumar et al., 2014
31.
Chitosan
Gamma irradiation
Size: Nano range
Shape: sphericalHepG-2 and CACO-2 cell lines
The Cs/Au nanocomposites inhibited the proliferation of cancer cells more than chitosan
Sokary et al., 2020
32.
Croin
Surface Plasm
Size: 4-10
Shape: sphericalMCF-7, MDA-MB-231, PC-3, HepG2 and HL-60
The anti-cancer effect of AuNPs was determined using MTT and LDH tests.
Hoshyar et al., 2016
33.
apo-α-lactalbumin
Protein
Size: 10–16
Shape: sphericalMouse fibroblast cells (L929), Breast cancer MCF-7 cells
Au-NPs kill 75% of MCF-7 cells, at the same concentration these are capable of killing only 30% of HeLa cells
Yarramala et al., 2015
34.
Doxorubicin
Phosphate buffer saline
Size: 53
Shape: sphericalHeLa and CaSki cells
Cytotoxic effects of Resv-Dox mixtures and Dox-GNPs complexes have been found for the first time in HeLa and CaSki cells.
Tomoaia et al., 2015
Resveratrol
Size: 50
Shape: spherical
35.
Kaempferol 3-O-β-D-apiofuranosyl-7-O-α-L-rhamnopyranoside
p-nitrophenol
Size: 37
Shape: sphericalMCF-7 cancer cells
AuNPs also displayed strong DPPH radical scavenging compared to the flavonoid extract, with an IC50 of 30.56 μg/mL.
Oueslati et al., 2018
2.2 Anti-microbial activity
Antimicrobial agents are used to minimize population of molds, bacteria, and fungi. These substances inhibit the growth of microorganisms on lot of surfaces to prevent infection. The antimicrobial agents with broad-spectrum are considered as perfect for use in hygienic environments such as hospitals, schools, and commercial kitchens. Some of active metal ingredients used as antimicrobial agents include Au and Zn. Various studies have shown the antibacterial effect of Au-NPs with good potential an elaborate that antibacterial activities of Au-NPs (Nagaraj et al., 2012).
2.2.1 Anti-bacterial activity
Since the antibacterial activity of NPs strongly depends on the size, the smaller dimensions of Au-NPs impart high activity against the various studied microbes such as S. aureus and K. pneumonia, S. mutans, B. subtilis, E. coli, P. vulgaris etc. These activities of newly synthesized Au-NPs have been examined on the above-mentioned gram-positive and negative organisms by Bhuvaneshwari et al. (Fig. 5). The antibacterial activities of NPs have been investigated on various microbes like E. faecalis, E. coli, P. aeruginosa, S. typhimurium, V. fluvialis, and V. damsel, and yeast Candidaal-bicans (Sunderam et al., 2019). Manjunath and co-workers synthesized Au-NPs using biogenic synthesis from seaweed named Sargassumwightii derived endophytic fungi Cladosporium cladosporioides. These Au-NPs were assessed for their biological activity against E. coli, S. aureus, B. subtilis, P. aeruginosa and findings showed the AuNP showed highest activity against S. aureus, and least activity against B. subtilis (MTCC 441) amongst reported microbes. It was observed that roughly 50% of E. coli bacteria were killed after 3 min and 80% after 6 min. No bacterial stains were present after 10 min of photoactivation (Manjunath et al., 2017).
The natural extracts bearing bioactive pharmacophore has been widely utilized for the formulation of NPs via green synthesis methods as results of metal ion reduction in a one-step. Plant extracts have an effective analyzer in testing free radicals and well-defined antioxidant property. NPs are of great scientific and technical importance as they form a bond between large materials and tiny particles like atoms at molecular level. Au-NPs are flexible in nature, resourceful and can be modified into different forms based on the need from one to other types of tasks and it becomes a good rival for Au. Au-NPs can attack effectively various forms of bacteria and viruses when compared to Ag and other NPs. On comparing with other chemically synthesized NPs, Au-NPs are less hazardous and less toxic to the environment.
Rajathi and his group synthesized Au-NPs from Stoechospermum marginatum, to show their anti-bacterial activity against Klebsiella oxytoca, Pseudomonas aeruginosa, Enterobacter faecalis, Salmonella typhimurium, Klebsiella pneumonia and Proteus vulgaris and found that the AuNPs to be more effective on Klebsiella pneumonia (Rajathi et al., 2012). Bimetallic NPs have been synthesized by the combination of Ag-Au in different ration using a marine red alga named Gracilaria sp and were investigated for bioactivity against different gram positive and gram negative bacteria such as Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhii and Escherichia coli of which first two were found to be killed (Ramakritinan et al., 2013). Adavallan and co-workers also synthesized Au-NPs from leaf extract of Morus alba (mulberry) and used as anti-bacterial agent against human pathogen. These biosynthesized Au-NPs showed inhibition of a gram-negative bacterium (Vibrio cholera) and a gram-positive bacterium (Staphylococcus aureus) (Adavallan and Krishnakumar, 2014). Green synthetic technique was used to synthesise Au-NPs from HAuCl4 utilizing leaf extract of Papaya. Investigating into biomedical applications of these NPs showed they act as good antibacterial agents against both gram positive as well as gram negative bacteria’s like Pseudomonas putida and Staphylococcus aureus (Sunkari et al., 2017). Recently, Vinay et al., (2020) have synthesized Au-NPs from seed extract of Elaeocarpus ganitrus using hydrothermal path and observed the anti-bacterial activity against P. desmolyticum and S.aureus (Vinay et al., 2021). Cuurently, Rauf and gropus have been synthesized Au-NPs from leaf extract of Mentha longifolia that found their use in killing stains of K. pneumonia, S. aureus and B. subtilis (Rauf et al., 2021).
The biogenic Au-NPs have been synthesized from leaf extract of Jasminum auriculatum when investigated for their biomedical applications showed good anti-bacterial activity against human pathogenic bacteria (Streptococcus pyogenes, E. coli, S. aureus and Klebsiella pneumonia) (Balasubramanian et al., 2020). The Au-NPs have also been synthesized from a mixture of leaf extracts of Carica papaya and Catharanthus roseus which were used to evaluate the anti- anti-bacterial activity against S. aureus, Bacillus subtilis, Escherichia coli and Proteus vulgaris (Muthukumar et al., 2016). Au-NPs may have bactericidal effects because of the presence of chemicals (Au-ions, surface coating agents, and chemicals involved in their synthesis) coexisting in are not completely removed. Bacterial drug resistance globally has become a serious threat, minimizing the effective antibiotic options; therefore, novel methodologies that improve antimicrobial action are urgently needed (Wang et al., 2010). Emam and his coworkers showed the synthesis of Au-NPs from starch as reducing agent and H2O2 as reduction enhancer in their study. The anti-bacterial activity of these NPs was tested against four microbes; two of gram positive and two gram negative. The gram-positive bacteria were S. aureus and B. subtilis whereas gram negative bacterial species were E. coli and P. aeruginosa. These NPs showed significant bactericidal effect on samples of S. aureus at minimum inhibitory concentration about 960 μg·mL−1 (Emam et al., 2017). Recently, Kurtjak and his groups have been synthesized Au-NPs by functionalizing them with arginine and hydroxyapatite having presence of Au (III) ions. The anti-bacterial investigation of these particles was performed on three bacteria’s P. aeruginosa, E. coli and S. aureus and found that effect of Au-NPs, however, better inhibition against P. aeruginosa MW1 strain due to presence of Au (III) ions (Kurtjak et al., 2017).
P. aeruginosa is considered as most tolerant bacteria showing high resistance against anti-biotics because of its capacity of mutation. Nazari and groups have been synthesized Au-NPs by functionalization with various antibiotics such as methicillin, erythromycin, vancomycin, penicillin G, clindamycin and nalidixic acid used to investigate against P. aeruginosa, Staphylococcus aureus and Escherichia coli and found most effective against P. aeruginosa (Nazari et al., 2012). Recently, Au-NPs have been synthesized by capping with curcumin isolated from Curcuma pseudomontana with Hematite (α-Fe2O3) and found the antibacterial activity against Pseudomonas aeruginosa, S. aureus, Bacillus subtilis and E. coli. These NPs were tested for their anti-bacterial effects and showed the lethal effect against E. Coli (Muniyappan et al., 2021). The antibacterial activity based on surfactants which were used to synthesize star shaped Au-NPs with anisotropic nature and showed great anti-bacterial activities against Propio nibacterium acres (Huynh et al., 2021). Fig. 8 describes the antimicrobial activity of synthesized Au-NPs against gram positive and negative organisms of various kinds, respectively. The applications of Au-NPs towards showing antibacterial activity have been listed in Table 2.
S.No.
Reducing agent used
Type of Reducing agent used
NPs characteristics (nm)
Type of microbial
Outcome
Ref.
1.
Licorice root extract
Plant (root)
Size: 26.47–63.25
Shape: CircularFive bacterial strains Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhii
Au-NPs synthesized using Licorice root extract exhibit good antibacterial activity to Gram negative bacteria
Al-Radadi et al., 2021
Five fungal cultures Aspergillus niger, Candida albicans, Fusarium oxysporum, Aspergillus flavus and Penicillium citrinum
AuNps is on the whole as good as the standard for nystatin Antifungal agent
2.
Pituranthos tortuosus aqueous extract
Plant (aerial parts)
Size: 5-15
Shape: spherical
Helicobacter pylori strains
The AuNPS showed anti-H. pylori activity, particularly against the multi-drug resistant strains with an MIC value of 15, 63 (mg/ml).
Abd El-Moaty et al., 2021
0.3
3-Aminopropyltrimethoxysilane (APTMS)- chitosan
Biopolymers
Size: 4
Shape: spherical
Salmonella enterica Serovar typhimurium L12031 bacterium
APTMS and gold nanoparticles, which possessed effective antibacterial and interact with the cell membrane of the bacterium S. Typhimurium, causing its death.
Virgili et al., 2021
0.4
Curcumin (CUR-AuNPs)
Isolation from rhizomes of Curcuma pseudomontana(plant)
Size: 20
Shape: sphericalTwo Gram positive bacteria Bacillus subtilis and Staphylococcus aureus and two Gram negative bacteria Pseudomonas aeruginosa and Escherichia coli
The CUR-AuNPs have effective antibacterial activity
Muniyappan et al., 2021
0.5
Jasminum auriculatum extract
Plant (leaves)
Size: 8–37
Shape: sphericalHuman pathogenic bacteria (Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli and Klebsiella pneumonia)
The biogenic gold nanoparticles using Jasminum auriculatum leaf extract were showed massive antimicrobial commotion against human fungal and bacterial pathogens
Balasubramanian et al., 2020
The human fungal pathogenic (Candida albicans, Aspergillus fumigatus, Lecanicillium lecanii and Trichoderma viride)
0.6
Pongammia pinnata extract
Plant (leaves)
Size: 16
Shape: sphericalMycobacterium tuberculosis
The gold nanoparticle treatment was effective against the drug sensitive M. tuberculosis with the MIC of 10 μg/mL
Govindaraju et al., 2020
0.7
Chitosan derived from squilla shell wastes
Biopolymers
Size: 80-82
Shape: sphericalBacterial cultures (gram positive) Staphylococcus sp., Bacillus sp and Bacterial cultures (gram negative) Escherichia coli, Proteus sp., Pseudomonas sp., Serratia sp. and Klebsiella sp
Au NPs showed significant antimicrobial action against several pathogenic bacteria and fungi.
Kalaivani et al., 2020
0.3
fungal cultures: Aspergillus niger, Aspergillus flavus
0.8
Anacardium occidentale extract
Plant (leaves)
Size: 10–30Shape: spherical
The microbial strains Bacillus subtilis and Escherichia coli
AuNPs elicited an increased activity against the pathogens
Sunderam et al., 2019
9.
Stoechospermum marginatum
leaf extract
Size: 18.7–93.7
Shape: Hexagonal and triangle
P. aeruginosa, K. oxytoca, E. faecalis, K. pneumonia, S. typhimurium, and P. vulgaris
Biosynthesized nanoparticles exhibited excellent antibacterial activity
Rajathi et al., 2012
10.
Gracilaria sp
Marine red alga
Size: 20–30,
Size: 30-40
Shape: Round or spherical or poly-disperse
S. aureus, Klebsiella pneumoniae, Salmonella typhii and Escherichia coli
Bimetallic NPs of 1:3 concentration showed zones of inhibition against the pathogenic bacteria such as Staphylococcus aureus and Klebsiella pneumoniae rather than Ag NPs and Au NPs
Ramakritinan et al., 2013
11.
Mentha longifolia
leaves extracts
Size: 13.45 ± 2
Shape: sphericalK. pneumoniae, S. aureus, B. subtilis
AuNP exhibited good in vitro antibacterial and anti-oxidant activities
Rauf et al., 2021
12.
Nerium oleander
Stem bark extract
Size: 20–40
Shape: Mostly Spherical with hexagonal, triangular and rod
M. tuberculosis by Luciferase Reporter Phage (LRP) assay and rifampicin resistant M. tuberculosis
in vitro anticancer activity of the stabilized AuNPs on MCF-7 cell lines significantly killing the cancer cells at 74 μg/mL.
Barai et al., 2018
13.
Jasminum auriculatum
Leaf extract
Size: 8–37
Shape: spherical
S. pyogenes, S. aureus, E. coli and K. pneumonia
The inhibitory effect in the proliferation of the human cervical cancer cell line with the IC50 value of 104 μg/mL
Balasubramanian et al., 2020
14.
Dracocephalum kotschyi
Leaf extract
Size: 7.9–22.63
Shape: spherical
E.coli, K.pneumonia, P.aeruginosa, Enterobacter sp. and S.aureus
Biological results exhibited that Au-NPs displayed a dose-dependent cytotoxicity with IC50: 196.32 and 152.16 μg/ml against K562 and HeLa cell lines as well.
Dorosti et al., 2016
15.
Starch
(H2O2)
Size: 48.0 ± 28.6Shape: spherical
S. aureus, B. subtilis, E. coli and P. aeruginosa
AuNPs (11.74 nm) was produced by using 2 g/L starch, 1% H2O2 and 10 g/L NaOH, where, reducing sugars detected to be 0.46 g/L. The produced nanogold showed good catalytic activity in reduction of p-nitroaniline and accelerate the reduction percentage from 12.1% to 39.2% in 2 h
Emam et al., 2017
16.
Hematite (α-Fe2O3)
Rhodamine B
Size: 50 −150
Shape: spherical Escherichia coli
Hematite products, with and without gold decoration, exhibited an impressive antibacterial effect and showed the lethal effect in E.coli.
Alp et al., 2020
17.
Surfactants
ascorbic acid
Size: 137-189
Shape: sphericalPropionibacterium acnes
Au-NPs have significant effect that gold nanostars could be the prospective agent for replacing antibiotics in acne treatment
Huynh et al., 2021
18.
sodium selenite salt
bark, leaf, and flower extracts
_
E. coli, M. luteus, B. subtilis and K. pneumoniae
antintifungal activity of Au nanoparticles against Aspergillus sp. at higher concentration (200 mg/L) was exhibited with the inhibition zone 0.502 and 0.125 cm2
Mondal et al., 2021
19.
Luteolin tetraphosphate
–
Size: 9
Shape: spherical
Aeromonas hydrophila and Escherichia coli
Minimum Inhibitory Concentration (MIC) of nanoparticles of all strains was in the concentration range of 0.125 to 0.5 μg/mL. The synthesized Ag-NPs showed superior antifungal activity against (C. glabrata) compared to Se-NPs and Au-NPs.
Lotfali et al., 2020
20.
Bovine serum albumin
Choistan derived from squilla shell wastes
Size: 5
Shape: sphericalStaphylococcus aureus
The cytotoxic effect of the synthesized Au NPs against MCF-7 cell lines was assessed by MTT assay with IC50 value of 250 μg mL−1.
Kalaivani et al., 2020
21.
Candida albicans
Vancomycin (antibiotic)
_
Enterococci
Treatment with the nanocomplex significantly reduced the expression levels of the ERG11 gene in fluconazole-resistant C. albicans isolates and the iNOS gene in macrophages
Rahimi et al., 2019
22.
Colistin (antibiotic)
seeds extract
Size: 5
Shape: sphericalE. coli
Au-NPs showing anti-bacterial activities also showed high potential as a fungicidal
Zayed et al., 2019
2.2.2 Anti-fungal activity
Fungi have been problematic for causing some of the major diseases such as Mycoses, Candidiasis, Mycotoxicoses, Actinomycosis, Otomycosis, Aspergillosis, Peniciliinosis etc. Au-NPs have been investigated as great anti-fungal. The spherical Au-NPs from different parts i.e., bark, leaf, and flower of Moringa oleifera and showed the antifungal activity against Aspergillus sp at high concentration of about 200 mg·L−1 (Mondal et al., 2021). No adverse effect of these NPs was seen on species Chironomus sp. A group of researchers investigated Au-NPs against fungal strains of Candida glabrata present in vaginal tract of human beings. These strains were shown to be resistant to drug amphotericin B but not Au-NPs at concentration range 0.125 to 0.5 μg·mL−1 (Lotfali et al., 2020). Recently, the Au-NPs with spherical morphology (size 80–82 nm) were synthesized from squilla shell wastes (chitosan) and found their anticancer and anti-bacterial properties which inhibits the growth of fungi Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus and Candida albicans (Kalaivani et al., 2020).
Au-NPs synthesized by mixture of extracts isolated from Adiantum capillus veneris and Pteris quadriureta were shown to have antibacterial activity showed good potential against fungi such as Trichophytonrubrum, Aspergillus niger, Scedosporium apiospermum, Aspergillus fumigates and Aspergillus flavus (Rautray and Rajananthini, 2019). The minimum inhibitory concentration of the AuNP on these strains was 50 mg·mL−1. Au-NPs synthesized from the leaf extract of Croton Caudatus Geisel using one pot green approach and showing their antibacterial and antifungal activities with high potential as fungicidal agent (Kumar et al., 2016). The biogenic Au-NPs have been synthesized from Fruit extract Actinidia deliciosa showing both bactericidal and antifungal effects on fungi such as Candida albicans, Aspergillus fumigatus, Lecanicillium lecanii and Trichoderma viride (Balasubramanian et al., 2020). Using biogenic synthesis Au-NPs were synthesized from the endophytic fungus that was isolated from a sea weed Sargassumwightii and showing antibacterial as well as antifungal effects (Virgili et al., 2021).
Candida albicans is fungus and is one of the major causes of mortality and morbidity in the burned patients. These Candida albicans fungal strains have now been found resistant to fluconazole. A group of researchers have designed gold nano-particles by their conjugation with indolicidin that were found to show fungicidal effect against this fluconazole resistant Candida albicans (Rahimi et al., 2019). Zayed and his coworkers have been synthesized Au-NPs from seed extract of Pimpinella anisum utilizing green synthesis. These NPs besides showing anti-bacterial activities also showed high potential as a fungicidal against Candida albicans (Zayed et al., 2020). Another group of researchers synthesized Ag-Au alloy type NPs by micro-wave assisted synthesis. These NPs besides showing anticancer activity also showed anti-fungal activity over standard strains of Candida albicans (Jia et al., 2020).
3 Conclusion and future perspectives
As bacterial resistance is becoming one of the biggest threats to human health in the 21st century, the progress of antibacterial nanomaterials is an effective mode to overcome this issue. Antibacterial resistance can be caused due to the reduced intake or overexposure to antibiotics by patients. MNPs afford a widespread platform for therapeutic applications based on their unique physical and chemical properties and provide treatment for drug-resistant microbial infection. Au-NPs are an excellent biocompatible agent and are easily tuned, and their antibacterial properties can be enhanced by varying their structure and size. Furthermore, gold nanoparticles can also play a better antibacterial role for effective antibacterial strategies against some resistant bacteria.
This review has provided from past up to the most recent ones information on the advancement in the wide range of biological activities of Au-based nanoparticles, which have shown potential for biomedical applications against anticancer and microbial infections. For the purpose, we have explored both chemical and natural sources of Au-NPs, providing synergistic action with antibiotic, to prevent or solve problem of antibiotic resistance. Several examples of applications of Au-NPs of either chemical or natural origin have been reported which illustrate the different advantages that can be obtained by such a dual strategy, resulting in better biopharmaceutical properties of nanomedicine with improved therapeutic efficacy. We hope in the near future to have safe and operative nano Au-based therapeutics and further extending its applications and useful results.
CRediT authorship contribution statement
Nada H. Aljarba: Writing – original draft, Funding acquisition. Shah Imtiaz: Writing – original draft, Visualization. Naushad Anwar: Writing – review & editing. Ibtesam S. Alanazi: Writing – original draft. Saad Alkahtani: Writing – review & editing, Supervision.
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program to support publication in the top journal (Grant no. 42-FTTJ-44).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mulberry leaf extract mediated synthesis of gold nanoparticles and its anti-bacterial activity against human pathogens. Adv. Nat. Sci: Nanosci. Nanotechnol.. 2014;5(2):025018.
- [CrossRef] [Google Scholar]
- Method for producing antimicrobial nanofilms packaging cover based on titanium nano-dioxide through extrusion for extension of food shelf-life. Google Patents. 2020
- [Google Scholar]
- Novel gold nanoparticles reduced by sargassum glaucescens: preparation, characterization and anticancer activity. Molecules. 2016;21(3):123.
- [Google Scholar]
- Potential anticancer activity of a new pro-apoptotic peptide–thioctic acid gold nanoparticle platform. Nanotechn.. 2021;32(14):145101.
- [CrossRef] [Google Scholar]
- Biofunctionalized gold nanoparticles synthesis from gymnema sylvestre and its preliminary anticancer activity. Int. J. Pharm. Pharm. Sci.. 2014;6(4):423-430.
- [Google Scholar]
- Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloid. Surfaces B: Biointer.. 2014;123:549-556.
- [Google Scholar]
- Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J. Drug Deliv. Sci. Techn.. 2020;57:101620.
- [CrossRef] [Google Scholar]
- Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg.. 2018;5:10.
- [Google Scholar]
- Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev.. 2015;16(1):169-174.
- [Google Scholar]
- Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev.. 2012;41(6):2256-2282.
- [Google Scholar]
- Nanomedicine Fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics.. 2020;12(2):142.
- [CrossRef] [Google Scholar]
- Generation of biocompatible nanogold using H2O2–starch and their catalytic/antimicrobial activities. Eur. Polym. J.. 2017;90:354-367.
- [Google Scholar]
- Effect of gold nanoparticles on glutathione depletion-induced hydrogen peroxide generation and apoptosis in HL7702 cells. Toxicol. Lett.. 2011;205(1):86-95.
- [Google Scholar]
- Unveiling the anticancer and antimycobacterial potentials of bioengineered gold nanoparticles. Process Biochem.. 2020;96:213-219.
- [Google Scholar]
- One pot environ-mental friendly synthesis of gold nanoparticles using Punica Granatum Juice: a novel antioxidant agent for future dermatological and cosmetic applications. J. Colloid Interface Sci.. 2018;521:50-61.
- [Google Scholar]
- Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. BBA – Rev. Cancer. 2021;1875(2):188532.
- [CrossRef] [Google Scholar]
- Biosynthesis of gold nanoparticles using marine Streptomyces griseus isolate (M8) and evaluating its antimicrobial and anticancer activity. Egyp. J. Aqua. Biol. Fisheries. 2019;23(1):173-184.
- [Google Scholar]
- Synthesis and characterization of gold nanoparticles and their anticancer activity using gamma radiation. J. Chem. Pharm. Res.. 2016;8(3):405-423.
- [Google Scholar]
- Hoshyar, R., Khayati, G.R., Poorgholami, M., Kaykhaii, M., 2016. A novel green one-step synthesis of gold nanoparticles using crocin and their anti-cancer activities. doi: 10.1016/j.jphotobiol.2016.03.
- Gold nanoparticle-based immunochromatographic test for identification of Staphylococcus aureus from clinical specimens. Clin. Chim. Acta. 2006;373(1-2):139-143.
- [Google Scholar]
- One-pot, surfactant-free synthesis of gold nanostars and evaluation of their antibacterial effects against propionibacterium acnes. J. Nanomater.. 2021;2021:1-10.
- [Google Scholar]
- Synthesis of gold-silver nanoalloys under microwave-assisted irradiation by deposition of silver on gold nanoclusters/triple helix glucan and antifungal activity. Carbohyd. Polym.. 2020;238:116169.
- [CrossRef] [Google Scholar]
- Protein-coated pH-responsive gold nanoparticles: microwave-assisted synthesis and surface charge-dependent anticancer activity. Beilstein J. Nanotechnol.. 2014;5:1452-1462.
- [Google Scholar]
- The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloids Surf., B. 2012;95:195-200.
- [Google Scholar]
- Gold nanoparticles as potent anticancer agent: green synthesis, characterization, and in vitro study. RSC Adv.. 2016;6(68):63973-63983.
- [Google Scholar]
- Chitosan mediated gold nanoparticles against pathogenic bacteria, fungal strains and MCF-7 cancer cells. Intern. J. Biolog. Macromol.. 2020;146:560-568.
- [Google Scholar]
- Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv. Colloid Interface Sci.. 2019;272:102017.
- [CrossRef] [Google Scholar]
- Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc.. 2009;131:1360-1361.
- [Google Scholar]
- Antibacterial nanocomposite of functionalized nanogold and gallium-doped hydroxyapatite. Mater. Lett.. 2017;193:126-129.
- [Google Scholar]
- Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genetic Eng. Biotechn.. 2016;14(1):195-202.
- [Google Scholar]
- Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomater.. 2010;31(25):6530-6536.
- [Google Scholar]
- Antimicrobial and anticancer activity of gold nanoparticles synthesized from grapes fruit extract. Chem. Sci. Trans.. 2013;2(S1):S105-S110.
- [Google Scholar]
- Comparison of antifungal properties of gold, silver, and selenium nanoparticles against amphotericin B-resistant candida glabrata clinical isolates. Avicenna. J. Med. Biotechnol.. 2020;13(1):47-50.
- [Google Scholar]
- Synthesis of gold nanoparticles using citrus macroptera fruit extract: anti-biofilm and anticancer activity. Chem. Select.. 2019;4(19):5714-5723.
- [Google Scholar]
- Biogenic synthesis of gold nanoparticles by marine endophytic fungusCladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem.. 2017;63:137-144.
- [Google Scholar]
- Enhanced Antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using sargassum incisifolium aqueous extracts. Molecul.. 2016;21(12):1633.
- [Google Scholar]
- Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surf., B. 2014;114:138-143.
- [Google Scholar]
- Mondal, A., Chowdhury, S., Mondal, N.K., 2021. Insecticidal and fungicidal performance of bio-fabricated silver and gold nanoparticles. Int. J. Environ. Sci. Technol. doi: 10.1007/s13762-021-03181-w.
- Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Intern. J. Pharmaceu.. 2020;588:119729.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol.. 2021;3:117-124.
- [Google Scholar]
- Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem.. 2016;51(3):384-391.
- [Google Scholar]
- Phytosynthesis of gold nanoparticles using Caesalpinia pulcherrima (peacock flower) flower extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct.. 2012;7(3):899-905.
- [Google Scholar]
- The combination effects of trivalent gold ions and gold nanoparticles with different antibiotics against resistant Pseudomonas aeruginosa. Gold Bull.. 2012;45:53-59.
- [Google Scholar]
- Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles. Artificial Cells. Nanomed. Biotechn.. 2018;46(1):82-88.
- [Google Scholar]
- Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug Delivery Sci. Techn.. 2019;51:83-90.
- [Google Scholar]
- Anticancer studies of the synthesized gold nanoparticles against MCF 7 breast cancer cell lines. Appl Nanosci.. 2015;5(4):443-448.
- [Google Scholar]
- Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa) Artif. Cells. Nanomed. Biotechn.. 2019;47(1):1173-1180.
- [Google Scholar]
- Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of Candida albicans isolated from patients with burn infection. Inter. J. Nanomed.. 2019;14:5323-5338.
- [Google Scholar]
- Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (Kützing) Spectrochim. Acta A: Mol. Biomol. Spectrosc.. 2012;99:166-173.
- [Google Scholar]
- Employing sulphated polysaccharide (fucoidan) as medium for gold nanoparticles preparation and its anticancer study against HepG2 cell lines. Mater. Today Commun.. 2021;26:101975.
- [Google Scholar]
- Ramakritinan, C., Kaarunya, E., Shankar, S., Kumaraguru, A., 2013. Antibacterial effects of Ag, Au and bimetallic (Ag-Au) nanoparticles synthesized from red algae. In: Solid State Phenomena. Trans. Tech. Publ. R 201, 211–230.
- Green synthesis and biomedicinal applications of silver and gold nanoparticles functionalized with methanolic extract of Menthalongifolia. Artificial Cells. Nanomed. Biotechnol.. 2021;49(1):194-203.
- [Google Scholar]
- Therapeutic potential of green, synthesized gold nanoparticles. Bio. Pharm. Intern.. 2019;9:30-38.
- [Google Scholar]
- Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst.. 2009;134(7):1373.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Techn.. 2019;51:164-176.
- [Google Scholar]
- Sokary, R., Abu el-naga, M.N., Bakhit, M., Atta, S., 2020. A potential antibiofilm, antimicrobial and anticancer activities of chitosan capped gold nanoparticles prepared by γ–irradiation. https://doi.org/10.1080/10667857.2020.1863555.
- Anticancer activity of green synthesised gold nanoparticles from Marsdeniatenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artificial Cells, Nanomed. Biotechn.. 2019;47(1):4012-4019.
- [Google Scholar]
- In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J. Boil. Sci.. 2019;26(3):455-459.
- [Google Scholar]
- Microwave-irradiated green synthesis of gold nanoparticles for catalytic and anti-bacterial activity. J. Anal. Sci. Technol.. 2017;8(1)
- [Google Scholar]
- Gold nanoparticles conjugated to [Tyr3] Octreotide peptide. Biophys. Chem.. 2008;138(3):83-90.
- [Google Scholar]
- Green synthesis of gold nanoparticles usingSimarouba glaucaleaf extract and their biological activity of microorganism. Chem. Phys. Lett.. 2019;732:136587.
- [Google Scholar]
- Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf., B. 2015;135:726-734.
- [Google Scholar]
- Curcumin and isonicotinic acid hydrazide functionalized gold nanoparticles for selective anticancer action. Colloids Surf. A. 2020;607:125484.
- [CrossRef] [Google Scholar]
- In-vitro antibacterial, antioxidant and cytotoxic potential of gold nanoparticles synthesized using novel elaeocarpus ganitrus seeds extract. J. Sci.: Adv. Mater. Devices. 2021;6(1):127-133.
- [Google Scholar]
- Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surface Coatings Technol.. 2021;415:127086.
- [CrossRef] [Google Scholar]
- Photothermal effects of supramolecularly assembled gold nanoparticles for the targeted treatment of cancer cells. Angew. Chem. Int. Ed.. 2010;122(22):3865-3869.
- [Google Scholar]
- Synthesis of gold nanoparticles from leaf Panaxnotoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artificial Cells. Nanomed. Biotechn.. 2019;47(1):1216-1223.
- [Google Scholar]
- Cancer-cell-specific cytotoxicity of non-oxidized iron elements in iron core-gold shell NPs. Nanomedicine. 2011;7(4):420-427.
- [Google Scholar]
- Synthesis and characterization of gold nanoparticles from Abiesspectabilis extract and its anticancer activity on bladder cancer T24 cells. Artificial Cells. Nanomed. Biotechn.. 2019;47(1):512-523.
- [Google Scholar]
- Green synthesis, characterization and anticancer activity of luminescent gold nanoparticles capped with apo-α-lactalbumin. RSC Adv.. 2015;5(41):32761-32767.
- [Google Scholar]
- In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloid. Surfaces A: Physicochem. Eng. Asp.. 2020;585:124167.
- [Google Scholar]







