Translate this page into:
Anticancer and antioxidant efficacy of silver nanoparticles synthesized from fruit of Morinda citrifolia Linn on Ehrlich ascites carcinoma mice
⁎Corresponding authors. malsalhi@ksu.edu.sa (Mohamad S. AlSalhi), sdnesan1981@gmail.com (Sandhanasamy Devanesan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The M. citrifolia, commonly called starvation fruit, plays an essential role in daily dietary intakes, as it contains several vitamins, phytochemicals, and electrolytes. These constituents have several biological activities that are anti-tumor, antibacterial, antiviral, antifungal, and play a role in the immune system. The silver nanoparticles (AgNPs) were synthesized using a more uncomplicated and fast means using the M. citrifolia fruit samples. The Silver ions (Ag+) readily get reduced to (Ag0) to perform M. Citrifolia AgNPs hence the fruit extracts of M. Citrifolia can be considered potential lowering agents. The preliminary confirmation was done qualitatively by change of color into brownish-black, and the color change was observed due to bio-reduction and the Surface Plasmon Resonance (SPR) vibration. It was established by the absorbance peak at 430 nm using UV–Visible spectral analysis. Scanning Electron microscopy (SEM) showed the particle size ranging from 12 to 26 nm. Fourier-transform infrared spectroscopy (FTIR) confirmed the presence of the functional groups from synthesized M. citrifolia AgNPs. The biochemical studies were supported by the effect of antioxidant and anticancer properties of M. citrifolia AgNPs. The pathological changes were studied using hematoxylin, and Eosin staining in Albino mice, which was no tumor (Ehrlich ascites carcinoma) cell invasion exhibited in Fluorouracil treated group. The present investigation proves that the M. citrifolia fruit extract derived AgNPs is a promising antioxidant agent to treat liver cancer.
Keywords
Ehrlich carcinoma
AgNPs
Morinda citrifolia
Liver cancer
Antioxidant
1 Introduction
Nanomaterials are synthesized with a novel, eco-friendly, and sustainable physical, chemical, biological, and engineering processes. Nanotechnology is attained much significance in many sectors like health care, food and feed, cosmetics, environmental health, biomedical sciences, etc. (Pathkoti et al., 2017; Ahamed et al., 2010; Devanesan et al., 2020). Bio-based approaches for synthesizing nanoparticles are rapidly gained more attention due to their ease of synthesis, eco-friendliness, stable and biocompatible. Apart from the plant sources, bacteria, fungi, and seaweeds are potentially used for (NPs) synthesis (Algebaly et al., 2020; AlSalhi et al., 2016; Ameen et al., 2020; Valarmathi et al., 2020). The biogenic synthesis of NPs with plant extracts is more comfortable and safe to handle during the entire bio-synthetic process (Singh et al., 2020; AlSalhi et al., 2019).
In the biogenic synthesis method, the plant extracts were used to reduce and to cap agents, which have a vital role in the synthesis of NPs (Anu et al., 2020). The stabilizing and capping agents support making a perfect homogenous NPs and protecting surface aggregation (Alfuraydi et al., 2019). The reduced surface aggregation prevents the toxicity of synthesized NPs (Devanesan et al., 2020). In recent years, several researchers have reported the anti-tumor and antimicrobial activity of AgNPs (Mishra et al., 2017; Ameen et al., 2020; Valarmathi et al., 2020), and antioxidants properties with less cytotoxicity activities(Das et al., 2019).
The M. citrifolia (Noni) is extensively used in traditional medicine for over 2000 years (Wang and Su, 2001). Noni was reported with broad healing properties, comprising cancer activity (Wang et al., 2008). It has also been traditionally used to treat colds, flu, diabetes, blood pressure, depression, and anxiety (Ellethy, 2019). The noni fruit is rich in phenolic compounds like ellagic acid, gallic acid, quercetin, rutin, rosmarinic acid, caffeine, and chlorogenic acid, which are highly potent antioxidant quinol oxidase (NOX). NOX enzymes are responsible for reacting with oxygen and generating ROS, the free radical that damages the normal cell DNA (Divisi et al., 2006). The M. citrifolia fruit were used to treat menstrual cramps, liver diseases, and urinary tract infections (Mathivanan et al., 2005).
The antioxidant molecules maintain free radicals into balance, thus lowering the risk of oxidative stress. It is well documented that many herbal antioxidants reduce the toxicity due to oxidative stress and prevent the damage to normal cells. The whole mechanism were elicited by several reports (Palu et al., 2008; Devanesan et al., 2020). Besides, sensible evidence that antioxidants reversed nephrotoxicity caused by oxidative stress due to one or the other reasons. And antioxidants protected the kidney cells from the toxicity elicited by CIS (Gupta and Singh, 2013). Ehrlich ascites carcinoma [EAC] is one of the commonest, and it appeared firstly as spontaneous murine mammary adenocarcinoma (Saravana Kumar et al., 2010). EAC lacks tumor-specific transplantation antigen, which can see a rapid proliferation of nearly found all mouse hosts (Abdel-Gawad et al., 2016; Ali et al., 2018).
Generally, chemotherapy is a standard treatment method for various types of cancers; however, most of the chemotherapeutic drugs showed limited impact along with multiple side effects. Hence to find out a safe treatment protocol, the present research was carried out to evaluate the properties of M. citrifolia fruit extract derived AgNPs and their biological effects, against antioxidant and anticancer activity using mice models with EAC-induced carcinoma .
2 Materials and methods
2.1 Preparation of M. Citrifolia fruit extract
The M. citrifolia fresh fruit sample was collected and cleaned with distilled water to remove the unwanted dust particles over the fruits. Five grams of fruit sample was sliced into small pieces and submerged into 150 ml of distilled water and then blended for 10 min. The aqueous fruit extract was filtered with Whitman No. 1 filter paper, and it was collected. The fruit extract was stored at 4 °C for further evaluation studies.
2.2 Synthesis of M. Citrifolia mediated AgNPs
Forty-five ml of 1 mM aqueous (AgNO3) solution was added with 5 ml of fruit extract to the conical flask. The flask was then incubated at room temperature; after 4 h, the reaction mixture turned into brownish dark in colour. The AgNPs obtained were purified by centrifugation at 15,000 rpm for 15 min (AlSalhi et al., 2016). The semisolid AgNPs was air-dried and collected as powder. The synthesized AgNPs was subjected to different characterization followed by biological applications.
2.3 Characterization of M. citrifolia-AgNPs
2.3.1 UV–vis spectroscopy
The characteristic surface plasma resonance (SPR) peak of the AgNPs was documented with UV–Vis spectroscopy (Perkin Elmer Spectrophotometer), capable of scanning the wavelength ranging from 260 to 800 nm.
2.3.2 FTIR analysis
The functional group of synthesized AgNPs was analyzed using FTIR system (Shimadzu Corporation, Kyoto, Japan), which was used to detect the characteristic peaks ranging from 400 to 4000 cm−1 with the resolution of 2 cm−1.
2.3.3 Scanning Electron microscopy (SEM) analysis
The morphology of synthesized M. citrifolia-AgNPs was performed by using SEM (JSM-7600F).
2.4 Animals models
The male Swiss albino mice weighing 150–200 g were used in this study. The mice were maintained thermoneutral temperature conditions before to start the experiments. The mice were kept at 20 °C and 26 °C and fed with pelleted to avoid stress reasonably well. During handling the mice, the experimental protocols were followed by ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (CPCSEA),with a reference 1416/Po/a/11.
2.4.1 Transplantation of tumor cells
EAC cells were obtained from the department of biochemistry Raja Serfoji College, Tanjavur. The EAC cells were conserved in vivo in Swiss albino mice by intraperitoneal injection of 2 × 106 cells/mice. Before the intraperitoneal administration of EAC cells, they were washed with normal saline and allowed to develop the ascitic tumor for 7 to 10 days. Ascitic fluid was collected from EAC cell-borrowed each mouse (days 9 of tumor-bearing) and measure viable EAC cells under the microscope. Each test mice received 0.1 ml cancer cell suspension containing 2 ×106 cells administrated intraperitoneal route.
2.4.2 Grouping of experimental animals
Group-I (Normal Control) was served as normal control treated with saline control (5 ml/kg i.p.)
Group-II – (Cancer Induced) was served as EAC tumor control group.
Group-III (Cancer Standard drug) was served as EAC tumour control administered with 5 – Flouro uracil (5-FU) (20 mg/kg, i.p.) for 14 days.
Group-IV- (Cancer + Plant sample) was served as EAC treated tumor control treated with M. citrifolia mediated AgNPs (2 mg/kg/bwt) for 14 days.
The standard drug, Flouro uracil 20 mg/kg and the plant extract were administered after 72 h of tumor cells inoculation. All the drugs were administered for 14 days continuously, and the biochemical parameters were evaluated.
2.4.3 Sample collection
The blood samples from each mouse were collected by sino-orbital puncture using micro-capillary tubes after the start of the experiment (48 h). Blood samples were withdrawn in clean and dry test tubes containing ethylene diamine tetraacetic acid (EDTA) and then centrifuged at 3000 rpm for 15 min. The red blood cell (RBC) count and white blood cell (WBC) count were measured using standard procedures (Ochiai et al., 2018).
The supernatant was used for superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and Lipid hydroperoxide (LPO) assays.
2.4.3.1 Assay of antioxidant enzymes
The antioxidants enzymes such as SOD, CAT, and GPx activity were as assayed according to the methodology described by Kakkar et al. (1984), Sinha (1972) Rotruck et al. (1973) respectively.
2.4.3.2 Statistical analysis
The obtained result was expressed as the mean value ± SD. The group comparisons were performed using one-way analysis of variance (ANOVA) test with significance P < 0.05.
3 Results and discussion
The appearance of brownish-black color evidenced the bio-reduction, and the colour change confirms the formation of AgNPs (Fig. 1). M. citrifolia fruit aqueous extract showed a good reducing activity in converting Ag+ ions to Ag0, which was visually observed. During this synthesis process, the AgNPs were formed and gave a brownish-black color due to the excitation of Surface Plasmon Resonance. In the course of reduction time, the solutions were becoming darker and stable within a few hours (Devanesan et al., 2018; Sathishkumar et al., 2012). The UV–Vis absorption spectra of the synthesized AgNPs is shown in Fig. 2. The absorption peak observed at 430 nm, indicated the formation of AgNPs from M. citrifolia. The obtained UV–Vis spectrum result was by the earlier findings of (Devanesan et al., 2020; Alfuraydi et al., 2019)
a-b: Visual observation of color changes- colorless solution to brownish-black color formation of AgNPs.
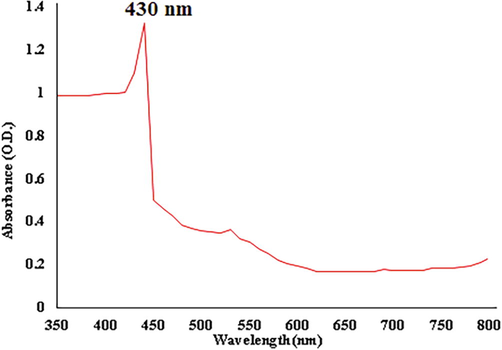
UV–Vis spectroscopic analysis of AgNPs.
FTIR spectral analysis of AgNPs from M. citrifolia showed phenolic compounds, amines, alcohol, ether, and esters with respective peaks at 3430.14, 2912.07, 2852.67, 1642.63, 1469.63, 1249.75, 1215.65, 1113.16, 1070.96, and 752.82 cm−1 (Fig. 3). The FTIR spectra of the synthesized AgNPs showed strong alcohols and phenolic group stretching at 3430.14 cm−1, another stretching of alkane group at 2912.07, 2852.67 and 1469.84 cm−1, N–H bending vibrations of primary amines at 1642.63, C-O stretching corresponding to 1249.75, 1215.65, 1113.16, 1070.96 cm−1, while C–H oop bends of aromatics at 752.82 cm−1. The current result proved the presence of active compounds as supportive evidence of previously reported work of Andean blackberry fruit-inspired AgNPs (Kumar et al., 2017), M. domestica fruit AgNPs (Umoren et al., 2014), and also L. ovalifolium fruits mediated AgNPs (Moldovan et al., 2018).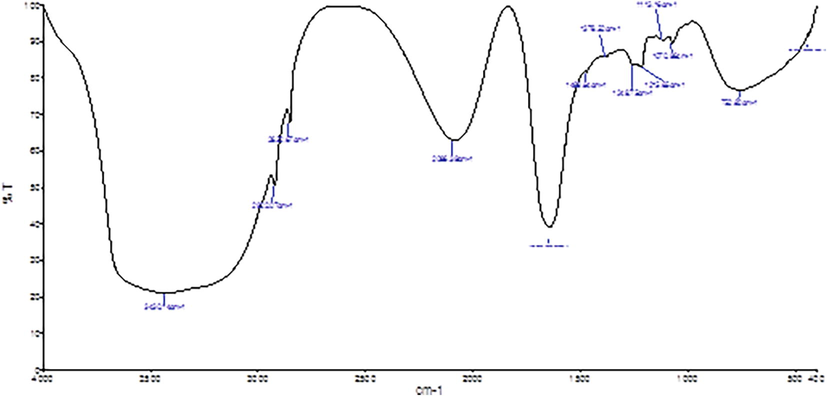
FTIR spectra of AgNPs synthesized by fruit extracts of M. citrifolia.
The SEM images of the synthesized AgNPs from fruit extract of M. citrifolia revealed that morphology of AgNPs not aggregated each other’s. The particle size distribution of AgNPs in diameter was ranging from 12 to 26 nm (Fig. 4 a & b). Several studies were reported smaller, and non-aggregated NPs reduced the cytotoxicity and improved the penetration volume in particular diseases (Devanesan et al., 2020; Kumar et al., 2017).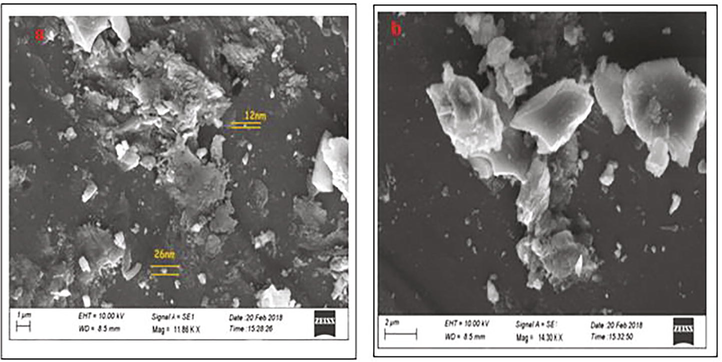
(a) SEM analysis of AgNPs (b). High resolution of individual AgNPs focused by SEM.
The effect of M. citrifolia on antioxidant enzymes (SOD, CAT, GPx, and LPO) of four groups are shown in Table 1 and Fig. 5(a, b). The level of these enzymes was significantly decreased in EAC-induced mice (Group II) compared to all the other three groups (Group I, III, and IV). In contrast, the LPO level was extremely high in EAC-induced mice (Group II) compared to all the other groups (I, III, and IV). The effect of synthesized AgNPs from M. citrifolia plays a vital role in maintaining EAC-induced Swiss albino mice's antioxidant capacity. The synthesized AgNPs proved the treatment effects are closely related to the standard drugs. Numerous studies have been reported that SOD and CAT levels were decreased the cancers it may due to increased LPO levels (Ellethy, 2019; Weydert and Cullen, 2010; Tsai et al., 2011). ROS induce procarcinogens, stimulate LPO, prevent the enzymatic system, and stimulate the cellular redox modified in the antioxidant defensive systems. The M. citrifolia AgNPs blocked oxidative dress, and LPO levels treated EAC bearing mice.
Groups
SOD (IU/min/mg protein)
Catalase (IU/min/mg protein)
GPx (IU/min/mg protein)
LPO (μM/ g tissue)
I
07.60 ± 0.01
39.10 ± 0.00
27.20 ± 0.02
162.60 ± 0.04
II
04.80 ± 0.04*a
10.20 ± 0.10*a
09.60 ± 0.04*a
403.10 ± 0.08*a
III
06.90 ± 0.03*b
23.60 ± 0.02*b
22.30 ± 0.01*b
190.40 ± 0.06*b
IV
6.50 ± 0.01*b
26.50 ± 0.01*b
19.40 ± 0.02*b
205.10 ± 0.03*b
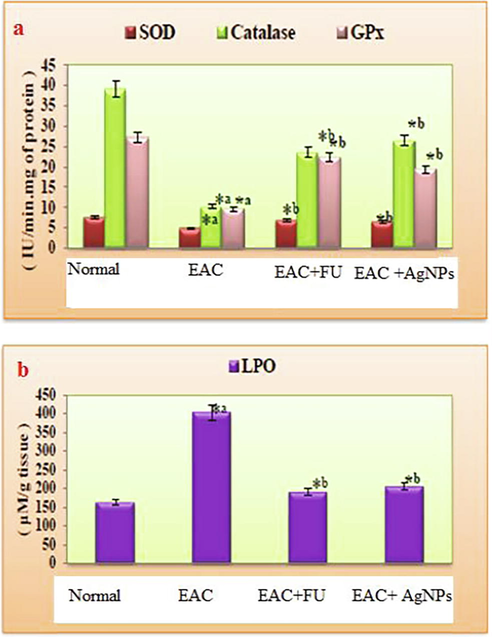
Effect of Nanoparticles synthesized from M. citrifolia on (a) SOD, Catalase, GPx and (b) LPO in different experimental groups of Swiss albino mice.
The effect of M. citrifolia on body weight, tumour volume, RBC and WBC in different experimental groups were given in Table 2. There was a significant (P < 0.05) increase in body weight, tumor volume in cancer-induced mice (Group I) when compared to normal control (Group II) and treated groups (Group III and IV). The WBC volume increased significantly in EAC bearing mice compared to normal control and treated groups. At the same time, RBC value was dramatically decreased in EAC mice as compared to normal control and treated groups. The M. citrifolia fruit-inspired AgNPs to interact with EAC mice and prevent the invade of the cancer cells and evidenced the effect of AgNPs closely related to standard chemotherapeutic drug activity. All values were expressed as mean ± SD.Statistically significant of *ap < 0.05 compared to Normal control group (I), *bp < 0.05 compared to EAC treated group (II).
Groups
Body Weight (g)
Tumor Volume (ml)
RBC (cells/ml × 106)
WBC (cells/ml × 103)
I
20.00 ± 0.01
00.00 ± 0.00
05.12 ± 0.02
07.40 ± 0.04
II
27.5 ± 0.04*a
07.50 ± 0.10*a
03.65 ± 0.04*a
16.10 ± 0.08*a
III
20.5 ± 0.03*b
01.20 ± 0.02*b
04.95 ± 0.01*b
09.50 ± 0.06*b
IV
22.5 ± 0.01*b
02.00 ± 0.01*b
04.20 ± 0.02*b
10.40 ± 0.03*b
The RBC level was decreased and increased WBC significantly (P < 0.05) in group II compared to group I normal control. The present studies' results were supported with previously existed of M. citrifolia fruit extract in EAC bearing mice (Ali et al., 2018; Rahman et al., 2017). The M. citrifolia fruit extract and methotrexate combination vaccination changed animal models' hematological parameters (Mhatre and Marar, 2016).
The pathological changes were studied using hematoxylin and Eosin staining and photographed using a camera fixed light microscope at 45X. Liver section of the control mice (A) showing the normal histological appearance of liver, including hepatic cells (HC), Kupffer cell (KC), and centrally located nuclei (N) in Fig. 6. The Liver section micrograph showed the sinusoidal infiltration of carcinoma cells along with lymphocytes (arrows) and the development of cytoplasmic vacuolation in the hepatocytes. The liver section of Fluorouracil (FU) treated group showed no tumor cell invasion, few lymphocytic infiltration, and nearly normal arrangement of hepatocytes. Liver section of the plant extract-treated group showed mild vacuolization, apoptosis with very few mononuclear cells infiltration (arrowheads).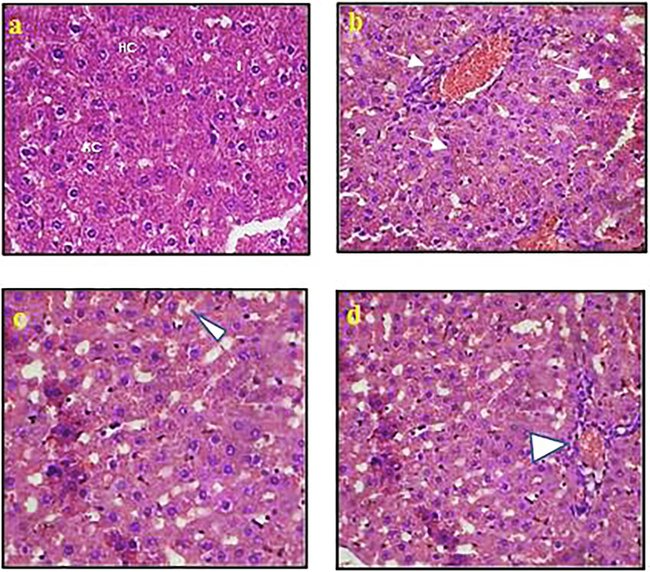
Pathological changes using haematoxylin and Eosin staining (a) Normal control; (b) EAC control; (c) EAC + FU; (d) EAC + AgNPs.
The M. citrifolia juice seems to preserve the liver from chronic exogenous calcium tetra chloride vulnerability (Wang et al., 2008). Previous reports have exhibited that tumor cell causes liver damage and prevents the normal metabolic process in hepatic cells, leading to alter the serum enzymes activity (Ali et al., 2017; Ohkawa et al., 1979). The current EAC + FU and EAC + AgNPs were observed to reduce the tumor volume compared to EAC bearing mice, respectively. At the same time, tumor cell viability was significantly reduced in FU treated and AgNPs treated EAC bearing mice; the result was supported for previously reported by M. citrifolia fruit (Ali et al., 2017). The plant-based AgNPs enhanced cytotoxicity effects on different cancer cell lines (Murugesan et al., 2019). Earlier studies also proved the efficiency of plant-inspired AgNPs activities against rat splenocytes (Sengottaiyan et al., 2016; Aravinthan et al., 2015). Based on the finding of our results, recommend that the synthesized M. citrifolia fruit AgNPs have the potentiality of antioxidant and anti-tumorigenic against EAC tumors.
4 Conclusion
M. citrifolia (Noni) has been widely used in folk medicine in several countries. M. citrifolia contains more than 200 phytochemicals, which use to treat numerous diseases, especially anticancer activity. The current study exhibits, the nanoparticles synthesis from aqueous extract of M. citrifolia Linn's and to evaluated the effective in preventive drug to the tumor growth in ascitic and solid tumour EAC mice. The biochemical parameters were growing up its antioxidant and anticancer properties of AgNPs. M. citrifolia components and Ag0 enhanced the interaction of each cell and prevented the tumor cell proliferation in EAC bearing mice. Overall, the M. citrifolia fruit AgNPs have acted as an antioxidant and anticancer agent in EAC mice.
Acknowledgment
The authors are grateful to the researchers supporting project number (RSP-2020/68), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdel-Gawad, E.I., Hassan, A.I., Awwad, S.A., 2016. Efficiency of calcium phosphate composite nanoparticles in targeting Ehrlich carcinoma cells transplanted in mice. J. Adv. Res. 7, 143–154. doi:10.1016/j.jare.2015.04.001
- Ahamed, M., AlSalhi, M. S., Siddiqui, M.K.J., 2010. Silver nanoparticle applications and human health. Clin. Chim. Acta 411, 1841–1848. doi.org/10.1016/j.cca.2010.08.016.
- Alfuraydi, A.A., Devanesan, S., Al-Ansari, M., AlSalhi, M.S., Ranjitsingh, A.J., 2019. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B 192, 83–89, doi.10.1016/j.jphotobiol.2019.01.011
- Algebaly, A.S., Mohammed, A.E., Abutaha, N.M., Elobeid, M.M., 2020. Biogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential. Saudi J. Biol. Sci. 27, 1340–1351. doi:10.1016/j.sjbs.2019.12.014.
- Ali, H., Yesmin, R., Satter, M.A., Habib, R., Yeasmin, T., 2018. Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells. J. King Saud Univ. Sci. 30, 386–392. doi.org/10.1016/j.jksus.2017.05.009.
- Evaluation of beneficial effects of morinda citrifolia L. in presence of cisplatin on ehrlich’s ascites carcinoma bearing mice. IJPSR. 2017;40:305-312.
- [CrossRef] [Google Scholar]
- AlSalhi, M.S., Devanesan, S., Alfuraydi, A.A., Vishnubalaji, R., Munusamy, M.A., Murugan, K., Nicoletti, M., Benelli, G., 2016. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 11, 4439–4449. doi.org/10.2147/IJN.S113193.
- AlSalhi, M.S., Elangovan, K., Ranjitsingh, A.J.A. Murali, P., Devanesan, S., 2019. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 26, 970–978. doi.org/10.1016/j.sjbs.2019.04.001.
- Ameen, F., AlYahya, S., Govarthanan, M., ALjahdali, N., Al-Enazi, N., Alsamhary, K., Alshehri, W.A., Alwakeel, S.S., Alharbi, S.A., 2020. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 1202, 127233. doi.org/10.1016/j.molstruc.2019.127233.
- Anu, K., Devanesan, S., Prasanth, R., AlSalhi, M.S., Ajithkumar, S., Singaravelu, G., 2020. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud Univ. Sci. 32, 2520–2526. doi.org/10.1016/j.jksus.2020.04.018.
- Aravinthan, A., Govarthanan, M., Selvam, K., Praburaman, L., Selvankumar, T., Balamurugan, R., Kamala-Kannan, S., Kim, J.H., Koildhasan, M., 2015. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 10, 977–1983 doi.org/10.2147/IJN.S79106.
- Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.) PLoS ONE. 2019;14:e0220950
- [Google Scholar]
- Devanesan, S., Ponmurugan, K., AlSalhi, M.S., Al-Dhabi, N.A., 2020. Cytotoxic and antimicrobial efficacy of silver nanoparticles synthesized using a traditional phytoproduct, asafoetida gum. Int. J. Nanomed. 15, 4351–4362. doi.org/10.2147/IJN.S258319.
- Devanesan, S., AlSalhi, M.S., Balaji R.V., Ranjitsingh, A. J. A., Ahamed, A., Alfuraydi, A.A., AlQahtani, F.Y., Aleanizy, F.S., Othman, A.H., 2018. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett. 13, 315. doi.org 10.1186/s11671-018-2731-y.
- Potential antitumor activity of nonsteroidal anti-inflammatory drugs against Ehrlich ascites carcinoma in experimental animals. Int. J. Health Sci. (Qassim).. 2019;13:11-17.
- [Google Scholar]
- Gupta, R.K., Singh, N., 2013. Morinda citrifolia (Noni) alters oxidative stress marker and antioxidant activity in cervical cancer cell lines. Asian Pac. J. Cancer P. 14, 4603–4606. doi:10.7314/apjcp.2013.14.8.4603.
- A modified spectrophotometric assay of SOD. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Kumar, B., Smita, K., Cumbal, L., Debut, A., 2017. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 24, 45–50. doi.org/10.1016/j.sjbs.2015.09.006.
- Review on the current scenario of Noni research: taxonomy, distribution, chemistry, medicinal and therapeutic values of Morinda citrifolia. Int. J. Noni Res.. 2005;1:1-16.
- [Google Scholar]
- Mhatre, B.A., Marar, T., 2016. Protective effect of Morinda citrifolia L. (fruit extract) on methotrexate-induced toxicities—hematological and biochemical studies. Cogent Biol. 2, 1. doi:10.1080/23312025.2016.1207879.
- Mishra, M.P., Sibanarayan, R., Shasank, S.S., Goutam, G., Debajyoti, D., Rabindra, N.P., 2017. In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. J. King Saud Univ. Sci. 29, 84–95. doi.org/10.1016/j.jksus.2015.05.007.
- Biosynthesis of silver nanoparticles using Ligustrum Ovalifolium fruits and their cytotoxic effects. Nanomaterials (Basel). 2018;8:627.
- [CrossRef] [Google Scholar]
- Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. Int. J. Nanomed.. 2019;14:5257-5270.
- [CrossRef] [Google Scholar]
- Blood biochemistry and hematological changes in rats after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci.. 2018;80:387-394.
- [CrossRef] [Google Scholar]
- Ohkawa, H., Onishi, N., Yagi, K., 1979. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. doi.org/10.1016/0003-2697(79)90738-3.
- Pathkoti, K., Manubolu, M., Hwang, H.M., 2017. Nanostructures: current uses and future applications in food science. J. Food Drug Anal. 25, 245–253. doi.org/10.1016/j.jfda.2017.02.004.
- Palu, A.K., Anne, H.K., Brett, J.W., Shixin, D., Jarakae, J., Leland, W., 2008. The effects of Morinda citrifolia L. on the immune system: Its molecular mechanisms of action. J. Ethnopharmacol. 115, 502–526. doi.org/10.1016/j.jep.2007.10.023.
- Anticancer activity and antioxidant potential of Aponogeton undulatus against Ehrlich ascites carcinoma cells in Swiss albino mice. Oncol. Lett.. 2017;14:3169-3176.
- [CrossRef] [Google Scholar]
- Selenium: biochemical roles as component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Saravana Kumar, J., Dilip, M., Wani, Z. A., Pal, H.C., Mahitosh, M., 2010. Effect of honey and eugenol on ehrlich ascites and solid carcinoma. J. Biomed. Biotechnol. 2010, 2314–6133. doi.org/10.1155/2010/989163.
- Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B Biointerfaces.. 2012;95:235-240.
- [CrossRef] [Google Scholar]
- Sengottaiyan, A., Mythili, R., Selvankumar, T., Aravinthan, A., Kamala-Kannan, S., Manoharan, K., Thiyagarajan, P., Govarthanan, M., Kim, J.H., 2016. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res. Chem. Intermed. 42, 3095–3103. doi.org/10.1007/s11164-015-2199-7.
- Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol. Rep. (Amst). 2020;31:e00427
- [CrossRef] [Google Scholar]
- Tsai, S.M., Hou, M.F., Wu ,S.H., Hu B.W., Yang, S.F., Chen, W.T, Chai, C.Y., Ma, H., Tsai, L.Y., 2011. Expression of manganese superoxide dismutase in patients with breast cancer. Kaohsiung J. Med. Sci. 27, 167–72. doi: 10.1016/j.kjms.2010.11.003.
- Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J. Mater. Environ. Sci.. 2014;5:907-914.
- [Google Scholar]
- Valarmathi, N., Ameen, F., Almansob, A., Kumar, P., Arunprakash, S., Govarthanan, M., 2020. Utilization of marine seaweed Spyridia filamentosa for silver nanoparticles synthesis and its clinical applications. Mater Lett. 263, 27244. doi.org/10.1016/j.matlet.2019.127244244.
- Cancer preventive effect of Morinda citrifolia (noni) Ann. NY Acad. Sci.. 2001;952:161-162.
- [Google Scholar]
- Hepatic protection by noni fruit juice against CCl(4)-induced chronic liver damage in female SD rats. Plant Foods Hum. Nutr.. 2008;63:141-145.
- [CrossRef] [Google Scholar]
- Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc.. 2010;5:51-66.
- [CrossRef] [Google Scholar]







