Anticancer activity of ethyl-acetate fraction of Sorbaria tomentosa and compounds identification through HPLC and LC-MS analysis
⁎Corresponding authors. shabnam.chem@pu.edu.pk (Shabnam Javed), amna.iags@pu.edu.pk (Amna Shoaib)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sorbaria tomentosa (Lindl.), is a graceful shrub bearing a science-based health benefits. This study aimed at assessing the anticancer potential of ethyl-acetate fractions (EtOAc) of S. tomentosa, and to identify bioactive natural compounds using HPLC and LC-MS techniques. Prior to the chromatographic analysis, four sub-fractions (SPE-1-SPE-4) were obtained from solid phase extraction (SPE) of EtOAc of the methanolic plant extract. SPE-2 fraction exhibited considerable levels of cytotoxicity (IC50: 50–75 µg/mL) than the rest of the sub-fractions with IC50 ranging in between 230 and 370 µg/mL against A-549, HepG2, and EI-138 human the cancer cell lines. SPE-2 was injected into a semi-preparative reverse HPLC-based-PDA revealing the occurrence of phenolic compounds. LC-MS analysis resulted in the identification of four bioactive phenolic compounds namely trans-p-sinapoyl-β-D-glucopyranoside (1), dunnianoside F (2), linoside A (3), and asplenetin (4) in SPE-2. In conclusion, the isolated compounds from the SPE-2 sub-fraction are the potential source of marked phenolic compounds that might be a promising option as anticancer agents in both cancer prevention and treatment.
Keywords
Solid phase extraction
Semi-preparative-HPLC
LC-MS
Phytochemical profiling
Phenolic
1 Introduction
Cancer is a malignant disease accounting for 10 million deaths in 2020 globally. Treating cancer is a highly complex process, and the most popular drugs (e.g. cytostatics) impart several detrimental effects, such as immunosuppression, thrombocytopenia, and neutropenia (Ferlay et al., 2021). Therefore, pharmacologically active ingredients (e.g. vitamins, polyphenols, and plant-derived bioactive compounds) are preferred as adjunctive therapies due to their excellent proliferative, proapoptotic, anti-inflammatory, antioxidant, and healing properties (Siddiqui et al., 2022). Plant-derived compounds especially phenolic have shown promising chemopreventive properties such as antioxidants (cellular defense by inducing antioxidants and scavengers of oxidants), antimutagenic, and anti-inflammatory (Sharifi-Rad et al., 2023). The plant phenolic are also known to arrest the cell cycle through the induction of apoptosis, regulation of carcinogen metabolism, and expression of ontogenesis. Besides, these compounds provide protection to DNA and cells, hence preventing the relapse and recurrence of cancer (Siddiqui et al., 2022; Sharifi-Rad et al., 2023).
Additionally, the utilization of botanical derivatives for phytopharmaceuticals is surpassing an efficient, safer, and cost-effective platform in the modern medical field. Only ∼ 5 % of investigated species of higher plants have been so far explored for phytopharmaceutical drugs, many species are yet to be explored as potential sources of biologically active compounds (Zheleznichenko et al., 2023). In this regard, natural products from the members of the family Rosaceae, or the rose family are regarded as the go-to source of drugs for human and animal ailments (Christenhusz et al., 2016). Sorbaria is a one of the genus of is a small Asiatic genus of rose family consisted of around 9 species. All of its species exhibited the occurrence of bioactive flavones, phenols, and polyphenols in excess, since, hold significant medicinal value as an antioxidant, anti-inflammatory, antitumor, analgesic, anti-anoxia, and anti-fatigue activity (Javed et al., 2021). Sorbaria is native to tropical Pakistan, mainly comprised of S. sorbifolia, S. kirilowii, S. grandiflora, and S. tomentosa.
Sorbaria tomentosa (Lindl.), locally famous as “Karhee or Berre” is a large, woody sprawling shrub (1.5–3 m tall), bears fern-like leaves (1–8 cm long, and 2–4 cm wide), brown obtuse buds (2 mm long), and small, creamy white flowers (25 cm long, 4 mm in diameter, and 7 cm wide), which blooms from July to September (Izhar et al., 2019). This large woody sprawling shrub is cultivated by local inhabitants of the Himalayas as barrier plantings to keep animals out of fields and gardens, and cities can use this plant to improve air quality by creating green walls with plants due to their potential to tolerate atmospheric pollution. Additionally, S. tomentosa is utilized as an ornamental plant and source of fuel (Hamayun et al., 2006). The whole plant has been recommended for the treatment of different diseases due to its antioxidant, anti-inflammatory, antitumor, analgesic, anticancer, antifungal, and hepatoprotective potential (Zheleznichenko et al., 2023). For an instant, its stem, leaves, and fruits utilized to prevent an allergic response and inflammation (Pankaj and Varma, 2013). The mixture of inflorescence with mustard oil used to minimize inflammation on the skin (Hamayun et al., 2006) and the paste of the flower with milk can comfort burn injuries (Kumar et al., 2009). The occurrence of a reasonable amount of carbohydrates, proteins, and fat provides a substitute for a high-calorie diet in anorexia nervosa incidence (Garber et al., 2013).
Over and above, the methanolic extract of S. tomentosa has shown antitumor effect and phytotoxicity activity, while ethanolic extract has marked antioxidant activity and stabilization potential for sunflower oil (Inayatullah et al., 2007). Javed and Shoaib (2021) found methanolic extract and its n-hexane and ethyl acetate fractions of S. tomentosa to be the most potent against lung A-549, hepatocellular HepG2, and urinary bladder EI-138 cell lines. In continuation of their study, Javed et al. (2021), explored many important compounds of medicinal value including odd chain of fatty acids (e.g. 3,13-dimethylpentadecanoic acid, 2,4-dimethyltetradecanoic acid, 2,4-heptadecadienoic acid; ethyl ester, 2-butyl cyclopropane dodecanoic acid, and heptadecanoic acid; ethyl ester) from the n-hexane fraction of S. tomentosa through GC–MS analysis. Likewise, Naz et al. (2023) linked the anti-diabetic activity of chloroform and methanolic extracts of S. tomentosa with the presence of an abundance of compounds like oleic acid, α-sitosterol, 8-octadecenoic acid, lupeol, etc. Moreover, different phytochemicals (e.g. noreugenin, wogonin, daucosterol, quercetin-3-glucuronide, kaempferol-3-xyloside, trifolin, tannins, phenol-carboxylic acids, catechins, etc.) have been documented in the extract of S. sorbifolia and S. pallasii through HPLC techniques (Zheleznichenko et al., 2022). So far, the literature concerning the occurrence of biologically active substances in S. tomentosa is still fragmentary.
During the last 20 years, new spectroscopic techniques for structural identification have emerged, which facilitated the speed and accuracy of phytochemical analysis (Wolfender et al., 1998). HPLC-PDA (high-resolution mass spectrometry- photodiode array) methods have gained more interest as the reliable, cost-effective, rapid, and accurate choice for the analysis of phytochemicals (Chroho et al., 2022). Qualitative information on chemical constituents, purity of the chromatographic peaks, and multiple wavelengths of chromatograms can easily be obtained by PDA (Khan et al., 2018). HPLC–PDA profiling has been found effective in the identification of bioactive compounds mainly phenolic compounds in Bougainvillea glabra (Saleem et al., 2020). However, like the UV spectrum, the structural information provided by HPLC is generally limited, whereas the mass spectrometer (MS) detection system coupled with the interface is relatively efficient in structural characterization (Kumar, 2017). So far, LC-MS (liquid chromatography-mass spectrometry) is regarded as a rapid, essential, sensitive, and versatile analytical technique for the identification of unknown compounds to achieve noise reduction and sensitivity improvements (López-Fernández et al., 2020). Furthermore, the soft-ionization technique namely electrospray ionization (ESI) has gained momentum in the determination of the molecular mass of the biological samples and has solved the problem of high polarity and ionization of thermally unstable proteins. ESI is the most successful interface used in LC–MS configuration, where the positive ion mode (ESI + ) is generally preferred as more compounds are expected to ionize in this mode (Altemimi et al., 2017). Therefore, LC–ESI/MS offers a novel powerful method to spot the unidentified bioactive compounds present in the plant extracts through high separation capacity of HPLC and precise structural and functional characterization by MS.
This research aimed to appraise the anticancer potential of ethyl-acetate fractions of S. tomentosa against three cancer cell lines viz., A-549 (lung adenocarcinoma), HepG2 (hepatocellular carcinoma), and EI-138 (urinary bladder cancer cell), and to provide a comprehensive phytochemical profile of S. tomentosa through HPLC-PDA and LC-MS techniques.
2 Materials and methods
2.1 Fractionation and solid-phase extraction (SPE) of ethyl acetate (EtOAc) fraction
The methanolic extract of a freshly ground plant of S. tomentosa (No. GC. Bot. Herb. 816) was partitioned with different solvents, concentrated under vacuum to obtain fractions comprised of n-hexane (21 g), dichloromethane (28 g), and ethyl-acetate (35 g) (Javed et al., 2021).
The dried EtOAc fraction (2 g) was processed for HPLC fractionation by dissolving in 20 % methanol (10 mL). Four sub-fractions (SPE1-SPE4) were eluted using 200 mL of 80:20 (285 mg), 50:50 (380 mg), 20:80 (261 mg), and 00:100 (225 mg) H2O: methanol step gradient thorough reverse-phase C18 SPE column (Starta, 10 g C18 Silica capacity). The sub-fractions were dried at low temperatures not exceeding 45 °C under vacuum and reduced pressure and a freeze dryer (Deng et al., 2019).
2.2 Cytotoxic activity of SPE by MTT assay [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide]
All three cancer lines were acquired from the European Collection of Authenticated Cell Cultures, Salisbury, UK, and cultured on RPMI-1640 medium amended with fetal bovine serum (10 %). The cytotoxicity activity of SPE1-SPE4 was analyzed through an MTT assay. Briefly, the single cell suspension was seeded in 96-well plates (1 mL/well = 1 × 103 cells/mL), incubated at 37 °C for 24 h, followed by the addition of 1 mL of SPE concentration (0.005, 0.01, 0.05, 0.1, and 0.5 mg/mL). The MTT dye reagent (1 mL) was added after another incubation period of 24 h, and after that loaded plates were kept for 48 h at 37 °C. The reaction terminated with the addition of isopropanol and the samples were measured for absorbance at 570 nm (Javed and Shoaib, 2021).
The data acquired on the anticancer activity were analyzed by variance analysis followed by LSD Test using computer software Statistix 8.1. The graphs were plotted using Microsoft Excel version 2013.
2.3 Semi-preparative HPLC
As the SPE-2 fraction showed the maximum cytotoxic potential, therefore, this sub-fraction was utilized for solid-phase extraction and sample purification using a semi-preparative HPLC system (Khan et al., 2018). Briefly, the samples were prepared, separated, and analyzed on an Agilent 1260 Infinity Series semi-preparative HPLC. The obtained chromatograms were visualized at 220, 254, 280, and 320 nm. Later, sub-fractions of SPE-2 fraction 50 % (MeOH: H2O) at 254 nm were collected manually for further semi-preparative and analytical HPLC.
2.4 Analytical HPLC
Agilent 1260 Infinity series analytical HPLC system (diode array detector or DAD monitoring 200–500 nm) was used to assess the purity of each peak of SPE-2. The primary signal for DAD was noted with a 2 nm step at 254 nm. Both mobile solvents contained 0.05 % TFA (flow rate 1 mL/min at 40 °C). Each peak purity was checked by the sharpness of the peak and the UV profile (PDA) detected by Poly View 2000-Diode Array spectral processing software. An elution gradient was used with solvents A (method 1: the mobile phase was a 30:100 methanol/water gradient with 40 min run time and 10 uL injection sample dissolved in MeOH) and solvent B (method 2: the mobile phase was 10 % acetonitrile: 90 % water with 30 min run time, then ramped at a higher gradient to 100 % acetonitrile over 5 min, finally back to 10 % acetonitrile for next 5 min recorded with 10 uL injection sample).
2.5 LC-MS
An 1100 series HPLC (Aglient Technologies, Inc., USA) fixed with binary pumps, auto-sampler, degasser, and DAD measuring absorbance between 180 and 400 nm was utilized. The reverse phase analytical column [Aqua 5 µm C-18 (250 × 4.6 mm)] was used as the stationary phase and the temperature was maintained at 40 °C. A linear gradient of 10–90 % MeOH: H2O containing 0.05 % TFA was used in LC-MS analysis of each collected sub-fraction with 1 mL/min flow rate for 45 min.
The mass spectrometer, Bruker esquire 3000 series, (LR-MS) (Bruker Daltonik GmbH, Bremen, and Germany) equipped with online LC was used to find mass spectra of S. tomentosa collected sub-fractions. For LC-MS analysis, atomic pressure chemical ionization (APCI) interface having positive ionization voltages scanning between 100 and 1200 amu was applied. Nebulizer pressure was adjusted to 60 psi, capillary voltage at 4000 V, and drying gas flow rate 5 L/min with the vaporizing temperature of 395 °C (Khan et al., 2018).
3 Results
The methanolic extract of the S. tomentosa plant was fractionated with n-hexane, dichloromethane, and ethyl-acetate, yielding 21, 28, and 35 g, respectively. Purification of the major organic ethyl-acetate fraction by repeated HPLC led to the separation of four different sub-fractions (SPE1-SPE4). Cytotoxicity assays were conducted with the ethyl-acetate sub-fractions (SPE1-SPE4) and doxorubicin was taken as standard control. Doxorubicin (standard control) exhibited high IC50 values ≤ 11 µg/mL against all three cell lines, while the variable cytotoxicity was depicted by different sub-fractions. The IC50 of SPE-2 (EtOH) was 50, 60, and 75 µg/mL against HepG2, EI-138, and A-549, respectively. The IC50 of SPE-1, 3 & 4 (EtOH) were in the range of 230–370 µg/mL against three cancer cell lines (Table 1; Fig. 1).
| Ethyl-acetate sub-fractions | IC50 values (µg/mL) | ||
|---|---|---|---|
| A-549 | HepG2 | EI-138 | |
| SPE-1 | 280 ± 1.2 | 320 ± 0.7 | 230 ± 1.8 |
| SPE-2 | 75 ± 2.4 | 50 ± 1.0 | 60 ± 1.4 |
| SPE-3 | 220 ± 1.7 | 250 ± 2.0 | 370 ± 2.0 |
| SPE-4 | 250 ± 1.3 | 370 ± 1.4 | 280 ± 2.3 |
| Control (Without Drug) | > 1000 | > 1000 | > 1000 |
| Doxorubicin | 9.70 ± 1.82 | 8.50 ± 3.21 | 9.50 ± 3.21 |
± indicates standard errors of the mean of three replicates.
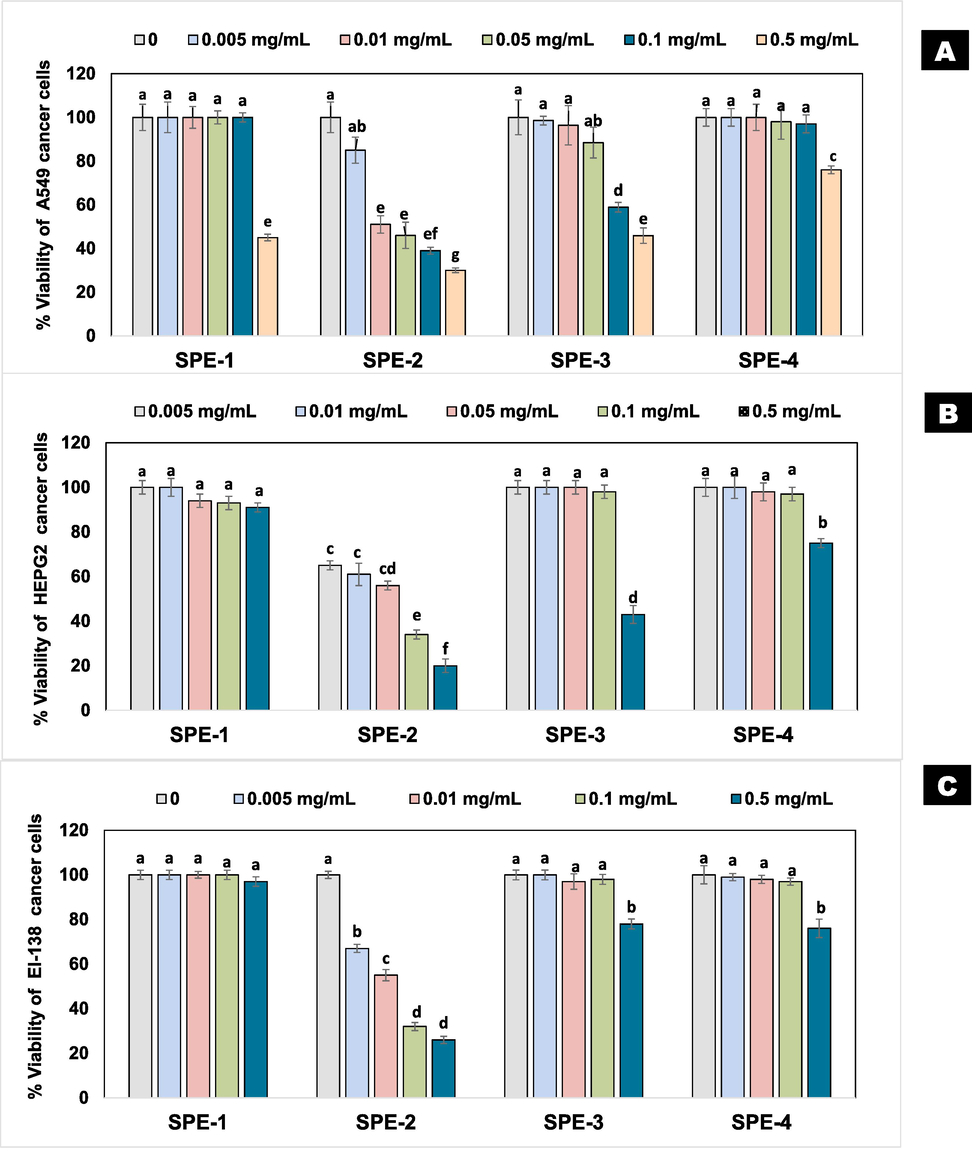
- Cytotoxic activity of Sorbaria tomentosa EtOAc (SPE) fractions against human cancer cell lines lung A-549 (A), hepatocellular HepG2 (B), and urinary bladder EI-138 (C). Error bars indicate standard errors of the mean of three replicates. Values with different letters show significant difference (P ≤ 0.05) as determined by LSD-test.
The SPE-2 fraction was selected for further fractionation in order to explore the possible chemical composition responsible for the potential cytotoxic effect by comparing the retention time and UV spectra of the reference compounds. The semi-preparative and analytical HPLC chromatograms of the SPE-2 fraction at 220, 254, 280, and 330 nm generated similar chromatograms (Figs. 2 & 3). Only peaks with UV response at 254 nm were collected in both semi-preparative and analytical HPLC due to the characteristics of peaks (tall, sharp, and relatively narrow).
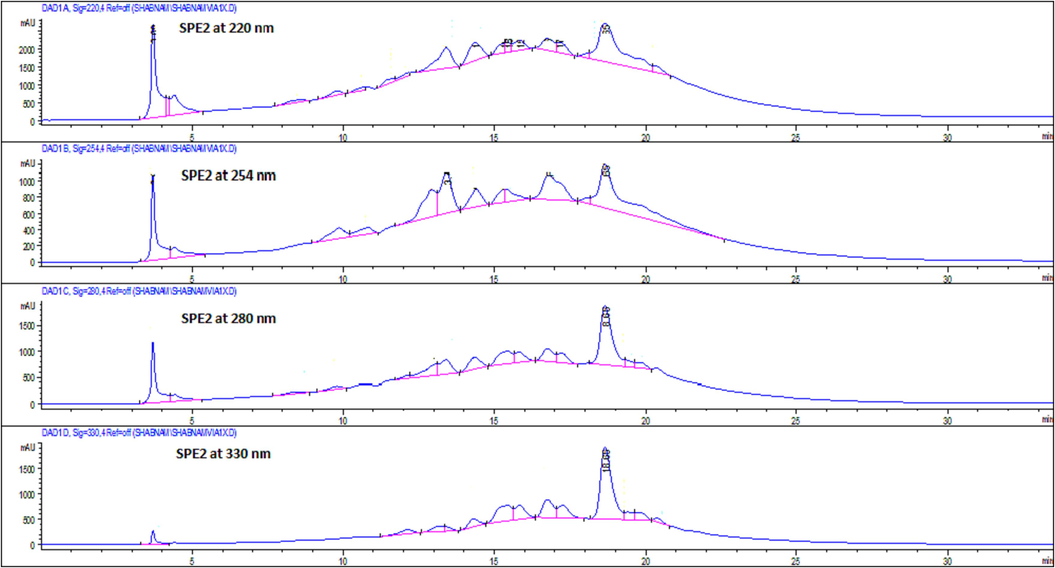
- Semi-preparative HPLC chromatogram of SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa at 220, 254, 280 and 330 nm.
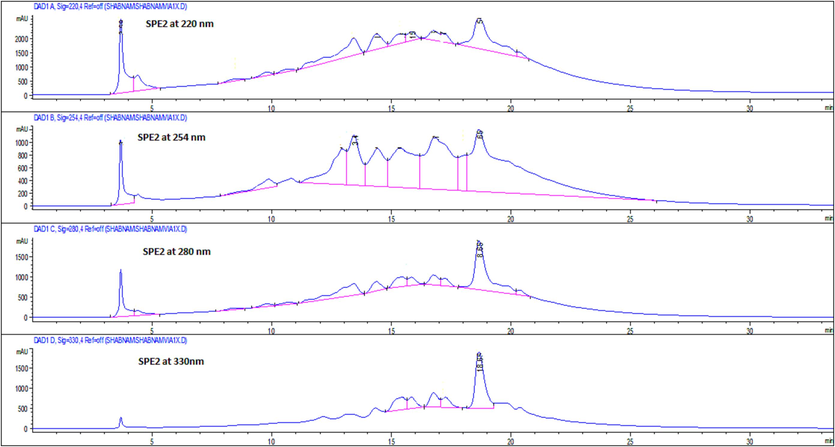
- Analytical HPLC chromatogram of SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa at 220, 254, 280, and 330 nm.
Semi-preparative HPLC separated five distinct peaks at a retention time of 13.11, 14.40, 15.30, 16.80, and 18.70 at 254 nm from SPE-2 (Table 2; Fig. 4). The analytical HPLC chromatogram of each of the five peaks of SPE-2 further identified different peak areas by method 1 (Fig. 5) and method 2 (Fig. 6), and UV spectra at different retention times are presented in Fig. 7. Acetonitrile as the organic solvent in method 2, widely separated peaks than method 1.
| Peak Number | Retenion time (min) |
|---|---|
| 1 | 13.11 |
| 2 | 14.40 |
| 3 | 15.30 |
| 4 | 16.80 |
| 5 | 18.50 |
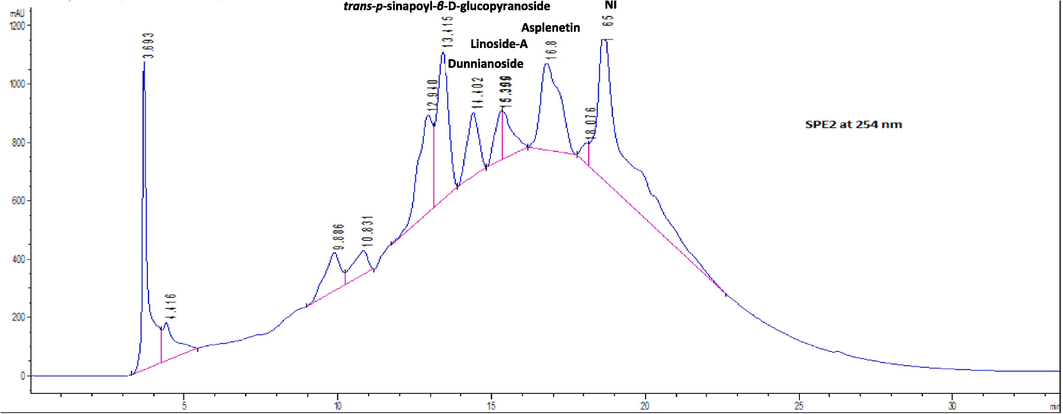
- Semi-preparative HPLC chromatogram of SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa at 254 nm indicating sub-fractions (1–5) represented by peaks.
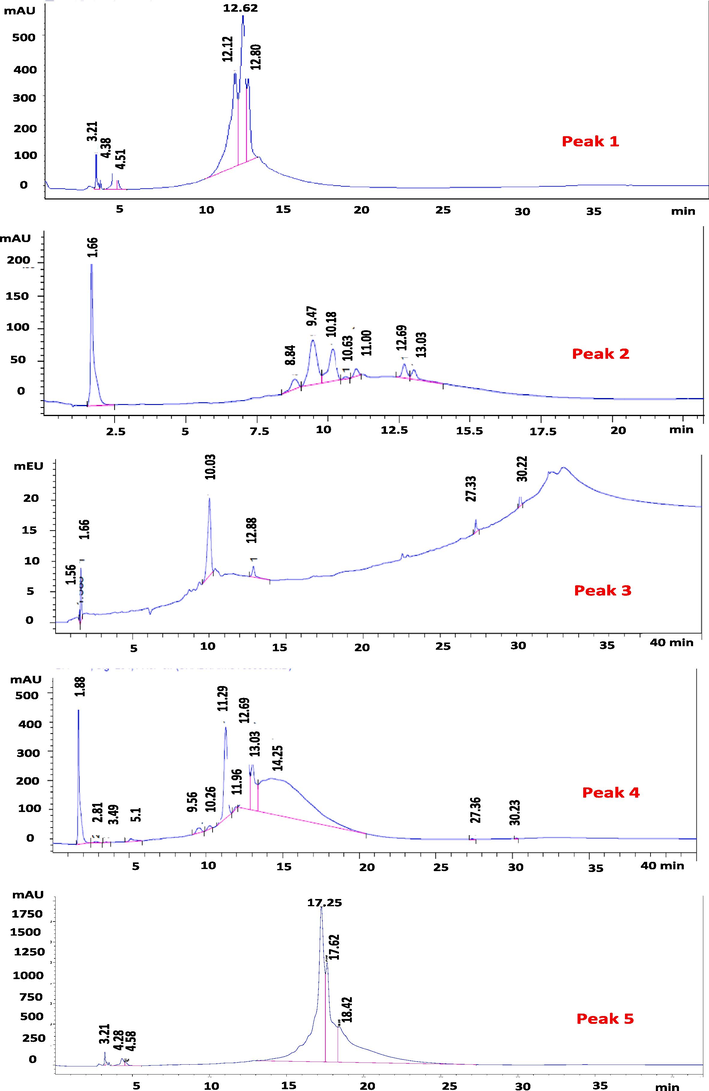
- Analytical HPLC chromatogram of the compounds from SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa at 254 nm indicating different peaks in the five peak areas by method 1.
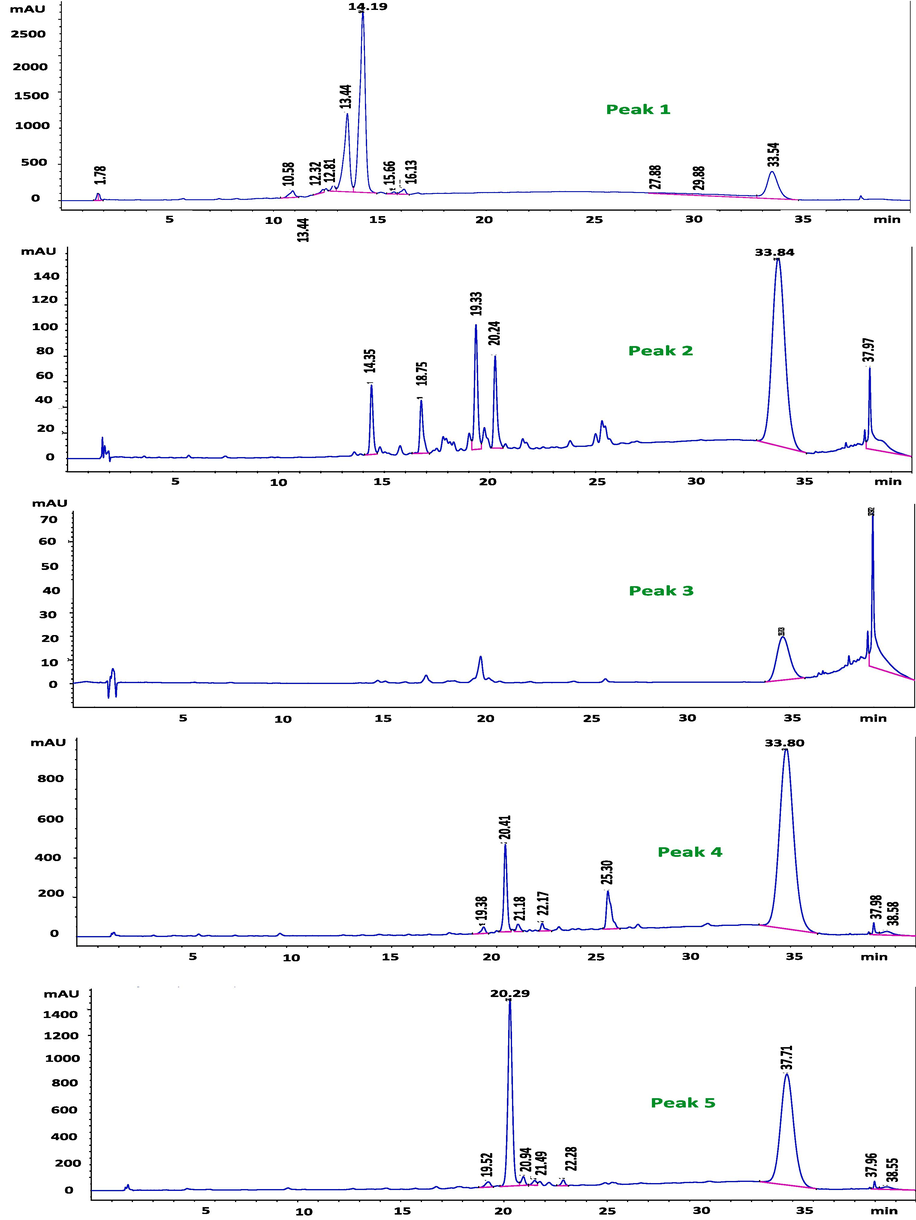
- Analytical HPLC chromatogram of the compounds from SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa at 254 nm indicating different peaks in the five peak areas by method 2.
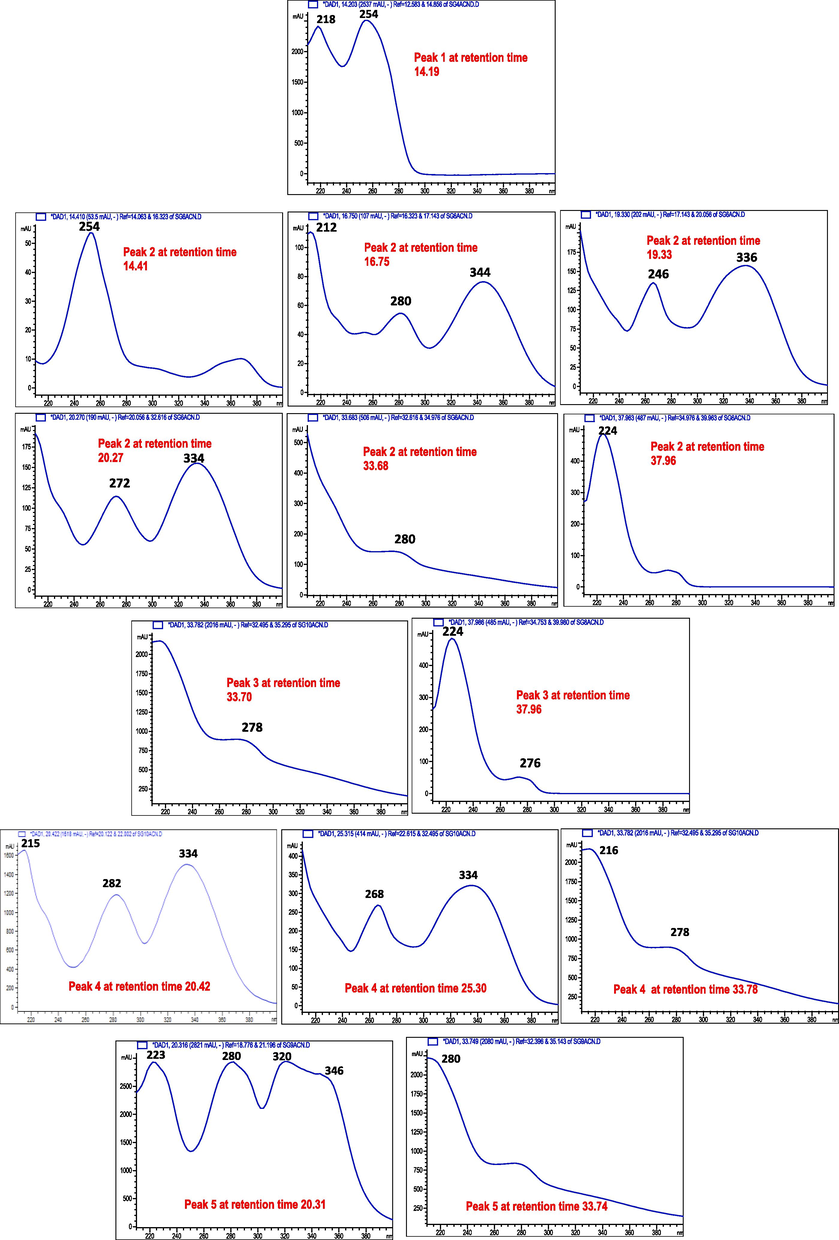
- UV spectra of the compounds of from peak 1–5 of the SPE-2 sub-fraction of the ethyl-acetate fraction of Sorbaria tomentosa.
After the separation of peaks through HPLC, the identification of the compounds was performed through LC-MS. The chromatographic conditions for the LS-MS were optimized by method development, and the best resolution was acquired through a linear gradient elution using water and MeOH containing 0.1 % formic acid as the mobile phase. LS-MS on the SPE-2 fractions of an ethyl-acetate fraction of S. tomentosa unveiled the mass spectrometric detection in positive ion mode exhibiting peak distribution in the range of m/z 300–900. Four phenolic compounds viz., trans-p-sinapoyl-β-D-glucopyranoside (compound 1), dunnianoside F (compound 2), linoside A (compound 3) and asplenetin (compound 4) were identified from the SPE-2 of EtOAc fraction of S. tomentosa (Table 3; Fig. 8).
| No. | Name | Positive ion (m/z): | MW |
|---|---|---|---|
| 1 | trans-p-sinapoyl-β-D-glucopyranoside | [M + H] + 387 [M + Na] + 409 |
386 |
| 2 | Dunnianoside-F | [M + H] + 453 [M + Na] + 475 |
452 |
| 3 | Linoside-A | [M + H] + 679 [M + Na] + 701 |
678 |
| 4 | Asplenetin | [M + H] + 681 [M + Na] + 703 |
680 |
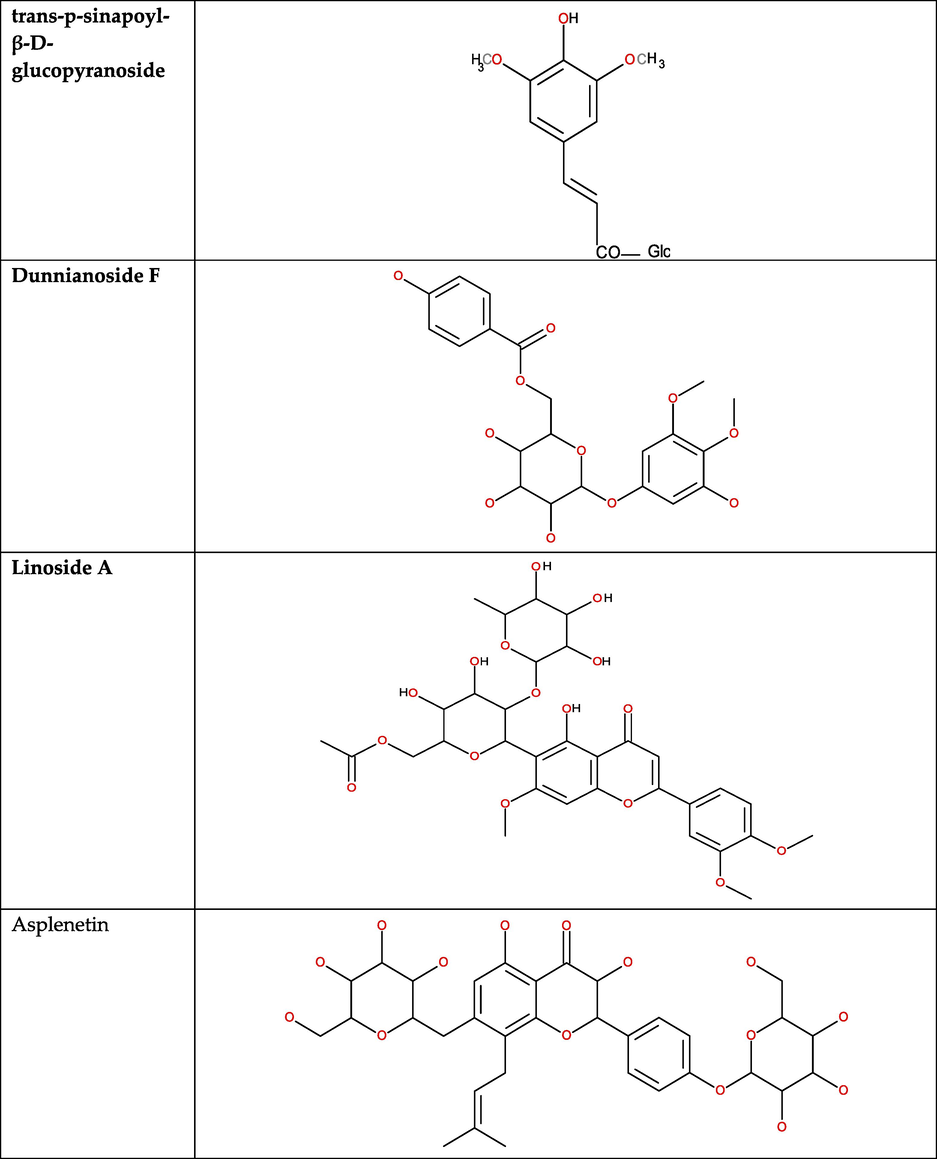
- Components identified in the SPE-2 of the ethyl-acetate fraction of Sorbaria tomentosa.
4 Discussion
Any extract/compound can be regarded as very active (IC50 ≤ 20 μg/mL), moderately active (IC50 > 20–100 μg/mL), weakly active (IC50 > 100–1000 μg/mL), and inactive (IC50 > 1000 μg/mL) based on at least 50 % of the cancer cell survivability (IC50) (Baharum et al., 2014). Accordingly, SPE-1, SPE-3, and SPE-4 sub-fractions were regarded as weakly active having IC50 in the range of 230–370 µg/mL against cancer cell survivability. However, SPE-2 fractions can be kept under moderately active groups, and may also specify the occurrence of compounds having cytotoxic potential (Javed et al., 2021). The presence of the different bioactive compounds e.g. polyphenols, and flavonoids were suggested for the cytotoxic response by an ethyl-acetate fraction of S. tomentosa against HepG2, EI-138, and A-549 cancer cell lines (Jang et al., 2020). Phenolic contents in the ethyl-acetate extract of Vernonia amygdalina have also shown cytotoxic effects and morphological alterations in the human glioblastoma cell line (U-87) (Cock, 2011). Phenolic compounds have been found as antioxidants, apoptosis, and target specificity against cancer cell lines in the study of BinRohin et al. (2017). Moreover, the concentration of the sub-fractions also determined the degree of lethality, and it was found to be directly proportional to the increasing concentration of the fraction from 0.005, 0.01, 0.05, 0.1, and 0.5 mg/mL (Khan et al., 2018). Nevertheless, distinct concentrations of the biocompounds may alter the anticancer activities of the sub-fractions.
Greater sensitivity and accuracy of HPLC-PDA/ESI-MS method make it as a potential diagnostic tool for the characterization and quantification of specific phenolic compounds (Chroho et al., 2022). Chromatograms obtained either through semi-preparative or analytical HPLC were similar, and the concentration of those compounds might be related to the polarity and capacity of solvation (Lee et al., 2021). Miliauskas et al. (2006) reported better results for non-polar compounds (e.g. flavonoids) through the semi-preparative column and did not find any advantage for polar compounds over an analytical column. However, only peaks with UV response at 254 nm were collected in both semi-preparative and analytical HPLC as the peaks were tall, sharp, and relatively narrow, which may indicate the separation method efficiently removed a component from a mixture. Additionally, peaks with UV response at 254 nm are ascribed to all compounds having one or more benzene rings. More specifically, the literature showed that prominent peaks of the phenolic compounds are obvious in the UV–Vis spectrum (260, 280, 325, and/or 350 nm), and a more generic wavelength for phenolic is found at 260 nm (Sanches et al., 2022). Therefore, the occurrence of phenolic compounds in the ethyl-acetate sub-fraction of S. tomentosa might be speculated through spectroscopic analysis.
Further analysis of peaks at 254 nm through a semi-preparative HPLC chromatogram of SPE-2 separated five distinct peaks at a retention time of 13.11, 14.40, 15.30, 16.80, and 18.70. To select the appropriate mobile phase to elute the subfractions preferably to baseline, two different organic solvents were checked in two methods (method 1: methanol and method 2: acetonitrile) (Khan et al., 2018). The analytical HPLC chromatogram of SPE-2 with Acetonitrile (organic solvent) in method 2, widely separated peaks, which may reveal maximum purification of the compounds. Shorter retention can be expected for equal proportions of organic to water with Acetonitrile as it exhibit more elution strength than methanol for reversed-phase chromatography (Hopkins, 2019).
Followed by HPLC, the chromatographic conditions for the LS-MS were optimized, and the best resolution was acquired (MeOH containing 0.1 % formic acid) (Khan et al., 2018). LS-MS on the SPE-2 fractions showing peak distribution in the range of m/z 300–900. The peaks were existing mainly at odd m/z and consisted of clusters of peaks at each nominal mass, which is consistent with earlier findings showing analogous m/z distributions (Santos et al., 2018). Trans-p-sinapoyl-β-D-glucopyranoside, dunnianoside F, linoside A, and asplenetin were identified from the SPE-2 of EtOAc fraction of S. tomentosa.
Trans-p-sinapoyl-β-D-glucopyranoside (compound 1) protonated molecular ion peak at m/z 386 [M + H] + determining the molecular formula of C17H22O10 Compound 1 has been reported in many plants (Xiao et al., 2017). It holds anti-inflammatory, anticancer, anti-diabetic, and antihypertensive properties on human health and also provides protection to the nervous, respiratory, and digestive systems beyond their well-known antioxidant and antibacterial activities (Xiao et al., 2017). Moreover, the antioxidant potential of sinapoyl (malate and glucose) is comparable to conventional antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), or Trolox (Nguyen et al., 2021).
Dunnianoside F (compound 2) is an antioxidant phenolic glycoside, exhibiting a protonated molecular ion peak at m/z 453 [M + H] + and [M + Na] + 475 determining the molecular formula of C21H24O11, previously obtained from the roots of Trichoderma hypoxylon (Zhang and Yin, 2022). Linoside A (compound 3), showed anti-inflammatory and antibacterial activity, displayed a protonated molecular ion peak at m/z 679 [M + H] + and [M + Na] + 701 determining the molecular formula of C32H38O16. Linezolid is approved oxazolidinone, regarded as safe and effective in treating resistant Gram-positive infections in neutropenic cancer patients (Smith et al., 2003). Asplenetin (compound 4) is an isoprenylated flavonoid, displayed a protonated molecular ion peak at m/z 681 [M + H] + and [M + Na] + 703 determining the molecular formula of C20H20O7. It was reported in Launaea asplenifolia and Prunus armeniaca and, being flavonoids, the anticancer, antioxidant, anti-inflammatory, anti-Infective, wound healing, and antiviral properties of asplenetin might have been predicted (Das et al., 2023).
Therefore, it was found that the anticancer activity of sub-fractions of the EtOAc fraction of S. tomentosa was due to the abundance of phenolic compounds. Due to the occurrence of polyhydroxy groups in aromatic ring of phenolic compounds, these have proven for strong antioxidant properties through oxidant scavengers, metal chelators, oxidase inhibitors, and antioxidant enzyme cofactors (Rashid et al., 2007).
Solid phase extraction (SPE) of EtOAc fraction led to the isolation of four fractions (SPE 1–4). Cytotoxicity assays identified SPE-2 as the most potent SPE fraction against cancer cell lines by MTT assay. Semi-preparative and analytical HPLC of SPE-2 fraction followed by LC-MS analysis identified trans-p-sinapoyl-β-D-glucopyranoside, dunnianoside F, linoside A, and asplenetin as bioactive phenolic compounds present in the S. tomentosa.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication
Consent for publication
All authors approved the manuscript to be published.
CRediT authorship contribution statement
Shabnam Javed: Conceptualization, Data curation, Methodology, Visualization. Aneela Anwar: Writing – review & editing. Iqra Javiad: Writing – review & editing. Amna Shoaib: Visualization, Writing – original draft. Hossam M. Aljawdah: Funding acquisition. Prashant Kaushik: Funding acquisition.
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
Authors are thankful to HEJ, Research Institute of Chemistry, ICCBS for useful NMR and MS analysis and guidance at ICCBS, Pakistan, Medicinal Chemistry and Natural Products Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool, UK. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2023R1083), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants.. 2017;6(4):42.
- [Google Scholar]
- In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules.. 2014;19:18317-18331.
- [Google Scholar]
- Cytotoxicity study and morphological changes of different extraction for Bismillah leaf (Vernonia amygdalina) in human glioblastoma multiforme cell line (U-87) Biomed Research.. 2017;28:1472-1478.
- [Google Scholar]
- The number of known plant species in the world and its annual increase. Phytotaxa.. 2016;261:201-217.
- [Google Scholar]
- HPLC-PDA/ESI-MS analysis of phenolic compounds and bioactivities of the ethanolic extract from flowers of Moroccan Anacyclus clavatus. Plants.. 2022;11(24):3423.
- [Google Scholar]
- Problems of reproducibility and efficacy of bioassays using crude extracts, with reference to Aloe vera. Pharmacognosy Communications.. 2011;1:52-62.
- [Google Scholar]
- Evaluation of Mollugo oppositifolia Linn. as cholinesterase and β-secretase enzymes inhibitor. Frontier. Pharmacology.. 2023;13:990926
- [Google Scholar]
- Synthesis, spectroscopic study and radical scavenging activity of kaempferol derivatives: Enhanced water solubility and antioxidant activity. International Journal of Molecular Sciences.. 2019;20(4):975.
- [Google Scholar]
- Cancer statistics for the year 2020: An overview. International Journal of Cancer.. 2021;149(4):778-789.
- [Google Scholar]
- Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. Journal of Adolescent Health.. 2013;53(5):579-584.
- [Google Scholar]
- Thnopharmacology, Indigenous collection and preservation techniques of some frequently used medicinal plants of utror and gabral, district Swat, Pakistan. African Journal of Traditional, Complementary and Alternative Medicines.. 2006;3(2):57-73.
- [Google Scholar]
- The role of methanol and acetonitrile as organic modifiers in reversed-phase liquid chromatography. Chromatogrphy Today.. 2019;26:24-26.
- [Google Scholar]
- Biological evaluation of some selected plant species of Pakistan. Pharmaceutical Biology.. 2007;45:397-403.
- [Google Scholar]
- Antioxidant potential and stabilization studies of sunflower oil using Sorbaria tomentosa extract and its Cu (II)/Zn (II) Chelates. Revista De Chimie.. 2019;70:4193-4420.
- [Google Scholar]
- Sorbaria kirilowii ethanol extract exerts anti-inflammatory effects in vitro and in vivo by targeting Src/nuclear factor (NF)-kappaB. Biomolecules.. 2020;10:741.
- [Google Scholar]
- Javed, S., Shoaib, A., Mehmood, Z., 2021. Proximate, macro Elemental and GC-MS analysis of Sorbaria tomentosa. Pakistan Journal of Weed Science Research. 27 (1), 109–118.
- In Vitro Cytotoxic Evaluation of Sorbaria tomentosa. Pakistan Journal of Weed Science Research.. 2021;27(1):119-126.
- [Google Scholar]
- Cytotoxicity, in vitro anti-leishmanial and fingerprint HPLC-photodiode array analysis of the roots of Trillium govanianum. Natural Product Research.. 2018;32(18):2193-2201.
- [Google Scholar]
- Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs) Journal of Pharmaceutical Analysis.. 2017;7(6):349-364.
- [Google Scholar]
- An ethnobotanical study of medicinal plants used by the locals in Kishtwar, Jammu and Kashmir. India. Ethnobotanical Leaflets.. 2009;10:1240-1256.
- [Google Scholar]
- Mango rejects and mango waste; Characterization and quantification of phenolic compounds and their antioxidant potential. Journal of Food Processing and Preservation.. 2021;45(7):e15618.
- [Google Scholar]
- Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants.. 2020;9(6):479.
- [Google Scholar]
- Comparison of analytical and semi-preparative columns for high-performance liquid chromatography–solid-phase extraction–nuclear magnetic resonance. Journal of Chromatography a.. 2006;112(1–2):276-284.
- [Google Scholar]
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis. Open. Chemistry.. 2023;21(1):20220339.
- [Google Scholar]
- Sinapic Acid and Sinapate Esters in Brassica: Innate accumulation, biosynthesis, accessibility via chemical synthesis or recovery from biomass, and biological activities. Frontier in Chemistry.. 2021;9:664602
- [Google Scholar]
- Potential role of Spirulina platensis in maintaining blood parameters in alloxan induced diabetic mice. nternational Journal of Pharmacy and Pharmaceutical. Science.. 2013;5:450-456.
- [Google Scholar]
- Flavonoid glycosides from Prunus armeniaca and the antibacterial activity of a crude extract. Archives of Pharmacal Research.. 2007;30:932-937.
- [Google Scholar]
- HPLC–PDA polyphenolic quantification, UHPLC–MS secondary metabolite composition, and In vitro enzyme inhibition potential of Bougainvillea glabra. Plants.. 2020;9:388.
- [Google Scholar]
- Comprehensive analysis of phenolics compounds in citrus fruit peels by UPLC-PDA and UPLC-Q/TOF MS using a fused-core column. Food Chemistry.. 2022;14:100262
- [Google Scholar]
- Antiproliferative activity of neem leaf extracts obtained by a sequential pressurized liquid extraction. Pharmaceuticals.. 2018;11(3):76.
- [Google Scholar]
- Phenolic compounds as Nrf2 inhibitors: potential applications in cancer therapy. Cell Communication and Signaling.. 2023;21:89.
- [Google Scholar]
- Plants in anticancer drug discovery: From molecular mechanism to chemoprevention. BioMed Research International.. 2022;2022:5425485.
- [Google Scholar]
- Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant Gram-positive infections in cancer patients with neutropenia. Annal of Oncology.. 2003;14(5):795-801.
- [Google Scholar]
- Liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectroscopy for the screening of plant constituents. Journal of Chromatography.. 1998;794:299-316.
- [Google Scholar]
- Chemistry and bioactivity of Gardenia jasminoides. Journal of Food and Drug Analysis.. 2017;25(1):43-61.
- [Google Scholar]
- Characterisation of two unique sesquiterpenoids from Trichoderma hypoxylon. Mycology.. 2022;13(1):32-38.
- [Google Scholar]
- Zheleznichenko, T.V., Muraseva D,S., Erst, A.S.; Kuznetsov, A.A., Kulikovskiy, M.S., Kostikova, V.A., 2023. The influence of solid and liquid systems in vitro on the growth and biosynthetic characteristics of microshoot culture of Spiraea betulifolia ssp. aemiliana. International Journal of Molecular Sciences. 24(3), 2362.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103037.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







