Translate this page into:
Antibiotics production in optimized culture condition using low cost substrates from Streptomyces sp. AS4 isolated from mangrove soil sediment
⁎Corresponding author. dfarraj@ksu.edu.sa (Dunia A. Al Farraj),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The development of novel antibiotics with desirable properties from naturally available organisms is an on-going process in the field of microbial biotechnology. Mangroves are not extensively exploited for the isolation and screening of antimicrobial agents of the genus Streptomyces. The emergence of multidrug-resistant pathogens necessitates continuous search for novel antimicrobial agents. Also, searching of novel strains from pristine environments is an important approach for obtaining novel bioactive molecules. Streptomyces sp. AS4 was isolated from the mangroves. Antibiotic production was performed using substrates such as, apple pomace, pine apple peel, orange peel, rice bran, wheat bran, green gram husk, banana peel, pomegranate peel and black gram husk in solid state fermentation. Antibacterial activity was performed using Gram positive bacterial pathogen. The nutrient sources (carbon, nitrogen and ion) were optimized initially by one-variable-at-a-time approach and two level full factorial designs. In this study 129 actinomycetes were isolated from the mangroves. The isolates were assessed for their antibacterial activity. Among these isolates, Streptomyces sp. AS4 showed very strong antibacterial activity. Among these substrates, wheat bran showed enhanced production of antibiotics (209 U/g), whereas pomegranate peel supported very less quantity of antibiotics production (43 U/g) in a two level full factorial design. The process parameters were screened using traditional methods and statistical approach. Among the tested factors, moisture, yeast extract and CaCl2 significantly influenced the antibiotics production. Central composite design and response surface methodology enhanced antibiotics production by Streptomyces sp. AS4 by 10 fold than non-optimized medium. Streptomyces sp. AS4, a mangrove isolate showed hyper production of antibiotics. The strain AS4 utilized various agro-wastes including wheat bran for antibiotics production. Wheat bran proved to be a potent solid substrate for the production of antibiotics in an industrial scale. The actinomycetes strain, Streptomyces sp. AS4 and its antibiotics may have great application against various drug resistant organisms.
Keywords
Antibiotics
Drug resistant
Solid state fermentation
Streptomyces
1 Introduction
Streptomyces spp. have been the important source of almost all types of bioactive secondary metabolites that have significant uses like anti-cancer and anti-viral compounds and in agriculture as insecticide, antiparasitic and herbicidal compounds (Watve et al., 2001; Al-Dhabi et al., 2016, 2019a,b,c,d,e). Hence, isolation and screening of promising new strains of potent actinomycetes with novel antibiotics is an interesting and continuous process (Hacene et al., 1998; Forar et al., 2006; Al-Dhabi et al., 2018a,b, 2014; Arasu et al., 2017, 2013a,b, 2019). About 70% of potent antimicrobial agents are synthesized by actinomycetes groups and Streptomyces account to more than 80% of the all the antimicrobials in the world. Streptomyces species are potential sources of new secondary metabolites that have various potentialities like immunosuppressive, anticancer and antimicrobial activities (Rajeswari et al., 2015; Arasu et al., 2013a,b, 2015; Arokiyaraj et al., 2015; Balachandran et al., 2015). These Streptomyces spp. are frequently explored for various antibacterial and antifungal drug discoveries. Hence, novel antimicrobials may act through unique mechanisms that could treat various diseases and disorders (Wu et al., 2009; Yu et al., 2011; Arokiyaraj et al., 2015; Balachandran et al., 2015; Rajkumaria et al., 2019; Valsalam et al., 2019a,b). The production of novel secondary metabolites by Streptomyces spp. is mainly based on species, culture conditions and nutritional factors in the fermentation medium (Durante-Mangoni et al., 2009). The antibiotics such as, neomycin, streptomycin, tobramycin and paromomycin comes under Aminoglycosides group. These are produced by Micromonospora spp. or Streptomyces spp. (Takahashi and Igarashi, 2018). Aminoglycosides show potential antimicrobial activity against various pathogenic microorganisms, including Gram-negative and Gram-positive bacteria, protozoa and mycobacteria. These aminoglycosides are highly stable and have potential synergistic activity with other commercially available antibiotics (Thakur et al., 2009). However, the synthesis of secondary metabolites (antibiotics) by Streptomyces spp. varies widely based on culture conditions. The production of antibiotics can be lost completely or can be increased if the fermentation conditions or media compositions changed (Ibrahim and Elkhidir, 2011). In antibiotics production, both the environmental factors such as, temperature, pH, fermentation period and culture medium composition significantly influence the process. To enhance and achieve maximum production of antibiotic by the potent actinomycetes, it is very important to optimize the environmental conditions and nutritional factors. Optimization of culture medium by statistical approach using response surface methodology (RSM) is mainly used to minimize the process (Poulikakos and Falagas, 2013). Also, this helps to prepare biomolecules in commercial scale using low cost substrates and to use the suitable environmental conditions to improve the production of antibiotics. Aminoglycosides are important choice, however it is not in use for the past 30 years because of their toxicity. However, these drugs have the potential application against multiple drug resistant pathogens (Nicasio et al., 2008; Wang et al., 2017). In recent years, there is an increase in drug resistance against currently available various antimicrobial agents, including, cephalosporins, carbapenems, fluoroquinolones, penicillins, and aminoglycoside, hence synergistic property of these drugs may be useful to treat various diseases (Navarrete-Bolanos et al., 2017). Minor alterations in the composition of media significantly influence the quality and quantity of secondary metabolite production along with overall metabolic homeostasis of the organisms. The optimization of medium components is highly complicated because any substance that supports microbial growth can be considered as a novel substrate (Feng et al., 2011; Kong et al., 2014; Kavitha et al., 2016; Bruntner et al., 2005).
2 Materials and methods
2.1 Isolation of actinomycetes for the production of secondary metabolites
In this study, soil sediments were collected from mangrove forests using a spatula in the labelled aseptic containers and aseptically closed. Further, the collected sample was transported to the laboratory covered with ice cubes to maintain the temperature about 2–8 °C. All samples were initially serially diluted by standard method and actinomycetes were isolated using spread plate method. Actinomycetes isolation agar has been used for the isolation of actinomycetes. The culture media was supplemented with gentamycin sulphate (25 µg/ml) and nystatin (50 µg/ml) to minimize bacterial and fungal contamination, respectively. All plates were incubated from 14 to 28 days. Further, the culture was purified by continuous streaking method on starch agar plates.
2.2 Screening of actinomycetes for secondary metabolites production
The selected 129 actinomycetes isolates were inoculated into Erlenmeyer flask (250 mL) containing (g/L) peptone-4, yeast extract-8 and starch-20. Inoculum was prepared separately for all 129 isolates and incubated for 14 days at 28 ± 2 °C using an orbital shaker. After two weeks, secondary metabolites were analysed by disc diffusion method. For initial screening, B. subtilis was used as an indicator organism. From the 129 actinomycetes strain one actinomycete was selected for secondary screening. The selected actinomycete, AS4 showed potent activity against B. subtilis. It was cultured in liquid medium for 14 days as described earlier and after incubation, the antimicrobial agents were extracted with solvents such as, ethyl acetate, methanol, ethanol, acetone and hexane. Further, the applied solvent was completely evaporated and antimicrobial activity was carried out using Kirby-Bauer method (Rao et al., 2012). All fractions were loaded on a sterile disc (6 mm), air dried and placed on bacterial culture plates. To the control plate, only solvent was loaded on a sterile disc (6 mm) and dried. Then the diameter of zone of inhibition was indirectly considered as antibiotic titre as suggested (Maxwell et al., 1994). One antibiotic unit was defined as the amount of solvent extract required to give 1.0 mm annular clearing around a sterile disc under standard experimental condition.
2.3 Identification of actinomycetes
The potent antimicrobials producing actinomycetes strain was grown on starch casein agar medium and the isolate was identified based on pigment production, morphological characters, aerial hyphae and morphology of spores. The selected strain was grown on starch casein agar medium for 14 days. Then, genomic DNA was isolated according to the manufactures instructions (Merck, Bangalore, India). Finally, the amplified partial 16S rRNA gene was sequenced and has been submitted to Genbank.
2.4 Substrates
The substrates such as, apple pomace, pine apple peel, orange peel, rice bran, wheat bran, green gram husk, banana peel, pomegranate peel and black gram husk were collected. All substrates were either air dried for two weeks or dried in an oven at 60 °C for eight hours. The residue was passed through standard mesh size (30 mesh sieve).
2.5 Production of antimicrobial agents by solid state fermentation (SSF)
Streptomyces sp. AS4 was cultured in SSF with various substrates. 5% (v/w) inoculum was introduced throughout the experiments and the culture was incubated for eight days at 28 °C. The strain AS4 was grown in an Erlenmeyer flasks containing 10 g solid substrate (apple pomace, pine apple peel, orange peel, rice bran, wheat bran, green gram husk, banana peel, pomegranate peel and black gram husk). Sodium phosphate buffer (0.1 M, pH 7.2) has been used to adjust the moisture content of the solid substrate at 70% level. The solid substrate was sterilized for 30 min at 121 °C to eliminate spores, if any with the solid biomass. After 8 days of incubation, the fermented medium was extracted with 100 mL phosphate buffer. The Erlenmeyer flask was kept on a rotary shaker for 30 min at 150 rpm. Finally, the crude antibiotic was separated by centrifugation (5000g, 10 min) and ethyl acetate was added (double volume) with the sample and used as crude extract after complete evaporation.
2.6 Initial screening of variables by one-variable-at-a-time approach
Wheat bran was used as the substrate until otherwise stated. 10 g wheat bran was weighed and introduced into all 100 mL Erlenmeyer flasks. To evaluate the effect of carbon sources, various carbon sources such as sucrose, glucose, starch, maltose, trehalose and xylose were added at 1% (w/w) level. The nitrogen sources such as, ammonium sulphate, beef extract, peptone, yeast extract and casein were added at 1% (w/w) to screen nitrogen sources. To analyze the mineral sources, the divalent ions such as, Mg2+, Ca2+, Fe2+, Mn2+, Co2+, Cu2+ were added with the substrate separately at 0.1% level (w/w). The moisture content of the solid substrate (wheat bran) was adjusted to 65% level and sterilized for 30 min as described previously. About 5% inoculum was introduced and incubated for 8 days at 28 ± 2 °C. After 8 days antibiotics extraction and assay was performed as described earlier.
2.7 Screening of variables by statistical approach
Statistical method has been used frequently to screen the optimum factors influencing antibiotics production. In this study, the factors such as, initial moisture content (50%–90%) and pH of the culture medium (6–9), carbon source (sucrose, glucose, starch, maltose, trehalose and xylose), nitrogen source (ammonium sulphate, beef extract, peptone, yeast extract and casein) and ionic sources (Mg2+, Ca2+, Mn2+, Co2+, Fe2+, Cu2+) were screened. The selected factors were analyzed at two different levels (low (−) and high (+). A statistical approach was deployed (two level full factorial experimental design) to identify the important factors influencing antibiotics production. Three experimental runs were performed and an average value was taken as response Y.
3 Central composite design (CCD) and response surface methodology
The CCD has been frequently used to optimize the concentrations of the variables in order to enhance the production of antibiotics. The selected factors were analyzed at five different levels (−α, −1, 0, +1 and +α). Design expert 10.0.6.2 was used to analyze the variables at five different levels, for the selected three variables (moisture, yeast extract and glucose) 20 experimental trials has been performed. These 20 trials include, six centre points, six axial points and eight factorial points. 10 g of wheat bran was weighed and transferred in 100-mL Erlenmeyer flask, and the designed amount of yeast extract and CaCl2 was added. The pH of the culture medium was fixed as optimum level. The culture medium was mixed carefully and sterilized for 30 min. All flasks were inoculated and SSF was performed as described earlier. After which, antibiotics production was assayed in triplicate analysis. The mean value of the response Y (antibiotics production) was obtained.
4 Results and discussion
4.1 Screening of Streptomyces sp. AS4 for antibiotics production
In this study, 129 Actinomycetes strains were isolated from three stations in mangroves in South West Coast of India. A total of 99, 20 and 10 actinomycetes were obtained in station I, II and III, respectively. The isolated actinomycetes showed various shape and colour. These isolates showed diverse in response to texture. To screen antimicrobial activity, B. subtilis was selected as an indicator organism. The cell free extract was used for antimicrobial activity analysis. Among 129 Actinomycetes, 84% of the strains showed antibacterial properties. Among these, Streptomyces sp. AS4 showed better antibacterial activity; hence this strain was selected for maximum production of antibacterial substances. Actinomycetes were isolated based on their morphological characters such as, different shape, varying size and variation in colouration. The colour of aerial mycelium was white, melanoid pigment – negative, reverse side pigment – negative, soluble pigment – negative and spore chain – rectiflexibiles. Based on morphological characters, this actinomycete was tentatively identified as Streptomyces sp. Further, it was characterized by various experiments. Results revealed that the selected isolate was able to grow between pH 6.0 and 8.5, between 20 and 40 °C. However, optimum pH was found to be 7.0 and 30° was optimum for the growth of the actinomycete. The isolated Streptomyces sp. was oxidase-positive, catalase-positive and chitinase-positive, and was able to hydrolyze starch. Based on 16S rRNA gene sequencing, the lead molecule producing Streptomyces was identified as Streptomyces sp. AS4. Actinomycetes, mainly from the genus Streptomyces has been isolated from various aquatic environments showed antimicrobial activity against varieties of drug resistant bacteria (Sengupta et al., 2015). Due to limited availability of antibacterial drugs in clinical applications and development of multiple drug resistance, there is an urgent need for development of novel lead molecules with limited side effects. The potential antibacterial activity of actinomycetes from the genus Streptomyces strains is well known. However, very limited studies are performed on actinomycetes from mangrove ecosystem which are very rich in actinomycetes biodiversity. Hence, searching of novel antibiotics from the mangrove environment is an important objective of this study. Limited information is available on isolation of Streptomyces sp. from the mangrove soils. In a study, recently isolated nine actinomycetes from mangroves are proven to be excellent candidates for the production of antibiotics (Malek et al., 2014). It was also reported that mangrove environments are becoming very important sources for Streptomyces sp. for the production of lead molecules due to their adaption towards varying tidal gradients and varying salinity (Mahalaxmi et al., 2010).
4.2 Production of antibiotics by solid state fermentation
In the present study antibiotics were produced using Streptomyces sp. AS4 in solid state fermentation using apple pomace, pine apple peel, orange peel, rice bran, wheat bran, green gram husk, banana peel, pomegranate peel and black gram husk. Among these substrates, using wheat bran showed to have enhanced production of antibiotics (209 U/g), whereas pomegranate peel showed very less quantity of antibiotics production (43 U/g). The other substrates showed antibiotics production between 63 and 187 U/g substrate. SSF has various advantages than submerged fermentation, and SSF provides natural environment to actinomycetes than submerged fermentation. Solid substrates play significant role in the production of various antibiotics (Ellaiah et al., 2004; Liu et al., 2009). In this study antibiotics yield produced by Streptomyces sp. AS4 was higher than previous reports about Streptomyces marinensis for the production of antibiotics in solid state fermentation. Substrates such as concentrated mineral and raspberry seed powder have been used to enhance the production of neomycin. In an industrial point of view, to reduce the production of lead molecules, various agro-residues were screened for antibiotics production (Darakchieva et al., 1987; Hanrahan and Lu, 2006).
4.3 Influence of nutrients on antibiotics production-preliminary analysis
To optimize the nutritional factors, various nutrient sources were added to wheat bran separately (Figs. 1a–c). Among the carbon sources, glucose enhanced the production of antibiotics in SSF (267 U/g). The effect of nitrogen sources on antibiotics production was analyzed using ammonium sulphate, beef extract, peptone, yeast extract and casein that were added at 1% (w/w). The addition of yeast extract (249 U/g), ammonium sulphate (240 U/g) and beef extract (220 U/g) significantly influenced the antibiotics production. The effects of supplementation with various minerals were evaluated at 0.1% level and were found to have potentil impact on antibiotics production. Supplementation of copper sulphate (278 U/g dry wheat bran) and ferric chloride (269 U/g substrate) showed the maximum production of antibiotics after 8 days of incubation. Our findings confirmed the influence of various nutrient factors on the production of antibiotics by Streptomyces sp. AS4. Glucose emerged as an efficient carbon source on antibiotics production. This result was similar with the results of antibiotics production by S. hygroscopicus and S. griseus in solid state fermentation (Zhou et al., 2015; Gesheva et al., 2005; Berdy, 1989). It was previously reported that various metal ions, including, iron, zinc, manganese, critically affect the growth of bacteria and stimulate or suppress the production of antibiotics (Bevan et al., 1995; Hanrahan and Lu, 2006). Additionally, iron also positively influenced the production of antibiotics based on the concentration in the culture medium (Zhou et al., 2015).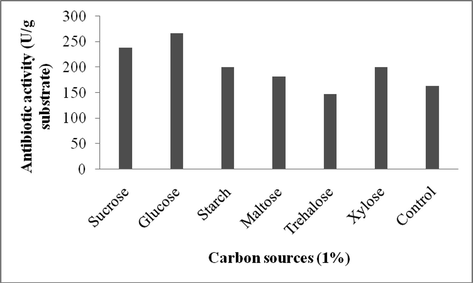
Effect of carbon source (1%) for the production of antibiotics using Streptomyces sp. AS4 in SSF.
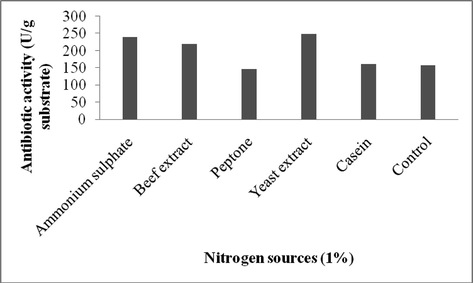
Effect of nitrogen source (1%) for the production of antibiotics using Streptomyces sp. AS4 in SSF.
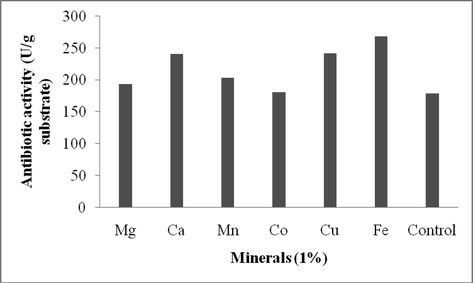
Effect of ionic source (1%) for the production of antibiotics using Streptomyces sp. AS4 in SSF.
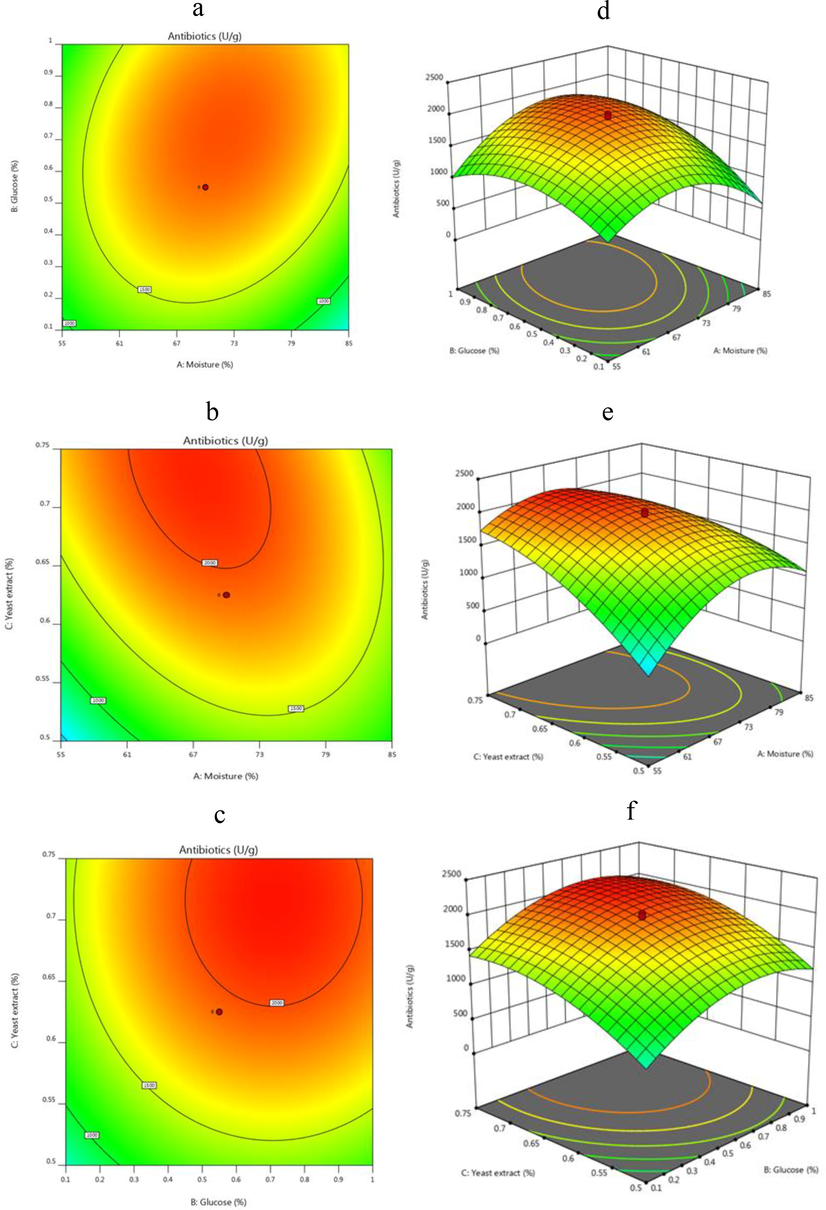
2D contour plot and 3D surface plots explaining the individual and interactive effects of factors on the antimicrobial activity of Streptomyces sp. AS4.
4.4 Screening of variables by statistical approach
A two-level full factorial design was employed for the designing and analysis of experimental data. Five variables were selected based on preliminary experiments comprising 32 experimental runs analysing five variables at two different levels (low and high or + and −). The selected independent factors examined and their levels, interactions were described in Table 1. The results in Table 1 show variations of antibiotics yield from 81 to 1452 U/g dry wheat bran of antibiotics production. This broad change in the yield reflects the account of culture medium optimization in SSF to accomplish enhanced production. The analysis of the results from the two-level full factorial design experiments reflects the interaction between tested variables. The F value of the designed model was 32.03 and the p value was <0.0001. The factors such as pH, glucose, yeast extract and CaCl2 positively influenced on the production of antibiotics. Among all these tested factors, glucose influenced more antibiotics production. Screening of variables using factorial experimental design has various advantages than traditional method and has been previously analysed (Wang et al., 2017). Factorial design helps to identify the variables by studying the interactive effects between variables simultaneously. Recently, Statistical approach to screen the independent variables to enhance the production of secondary metabolites from Micromonospora Y15was done in an effective manner (Wang et al., 2016) (Table 2).
Run
A:Moisture
B:pH
C:Glucose
D:Yeast extract
E:CaCl2
Antibiotic activity
%
%
%
%
U/g
1
90.00
9.00
1.00
1.00
0.10
455
2
60.00
9.00
1.00
1.00
0.10
349
3
90.00
6.00
1.00
1.00
0.10
1001
4
60.00
6.00
1.00
1.00
0.10
698
5
90.00
9.00
0.10
1.00
0.10
351
6
60.00
9.00
0.10
1.00
0.10
501
7
90.00
6.00
0.10
1.00
0.10
276
8
60.00
6.00
0.10
1.00
0.10
399
9
90.00
9.00
1.00
0.10
0.10
801
10
60.00
9.00
1.00
0.10
0.10
359
11
90.00
6.00
1.00
0.10
0.10
258
12
60.00
6.00
1.00
0.10
0.10
259
13
90.00
9.00
0.10
0.10
0.10
432
14
60.00
9.00
0.10
0.10
0.10
980
15
90.00
6.00
0.10
0.10
0.10
399
16
60.00
6.00
0.10
0.10
0.10
81
17
90.00
9.00
1.00
1.00
0.01
784
18
60.00
9.00
1.00
1.00
0.01
1452
19
90.00
6.00
1.00
1.00
0.01
439
20
60.00
6.00
1.00
1.00
0.01
369
21
90.00
9.00
0.10
1.00
0.01
412
22
60.00
9.00
0.10
1.00
0.01
241
23
90.00
6.00
0.10
1.00
0.01
288
24
60.00
6.00
0.10
1.00
0.01
251
25
90.00
9.00
1.00
0.10
0.01
231
26
60.00
9.00
1.00
0.10
0.01
102
27
90.00
6.00
1.00
0.10
0.01
640
28
60.00
6.00
1.00
0.10
0.01
399
29
90.00
9.00
0.10
0.10
0.01
442
30
60.00
9.00
0.10
0.10
0.01
301
31
90.00
6.00
0.10
0.10
0.01
271
32
60.00
6.00
0.10
0.10
0.01
269
Source
Sum of Squares
df
Mean Square
F-value
p-value
Model
2.546E + 06
23
1.107E + 05
32.03
<0.0001
B-pH
1.123E + 05
1
1.123E + 05
32.51
0.0005
C-Glucose
2.282E + 05
1
2.282E + 05
66.02
<0.0001
D-Yeast extract
1.303E + 05
1
1.303E + 05
37.71
0.0003
E-CaCl2
15664.50
1
15664.50
4.53
0.0659
AB
46818.00
1
46818.00
13.55
0.0062
AC
18721.13
1
18721.13
5.42
0.0483
AD
29890.13
1
29890.13
8.65
0.0187
BC
28560.50
1
28560.50
8.26
0.0207
CD
2.727E + 05
1
2.727E + 05
78.91
<0.0001
CE
43512.50
1
43512.50
12.59
0.0075
DE
39200.00
1
39200.00
11.34
0.0098
ACD
32640.13
1
32640.13
9.45
0.0153
ACE
1.166E + 05
1
1.166E + 05
33.75
0.0004
ADE
21424.50
1
21424.50
6.20
0.0375
BCD
64800.00
1
64800.00
18.75
0.0025
BCE
97461.13
1
97461.13
28.20
0.0007
BDE
5.881E + 05
1
5.881E + 05
170.17
<0.0001
ABCD
1.378E + 05
1
1.378E + 05
39.88
0.0002
ABCE
1.599E + 05
1
1.599E + 05
46.27
0.0001
ABDE
21528.13
1
21528.13
6.23
0.0372
ACDE
34060.50
1
34060.50
9.86
0.0138
BCDE
2.824E + 05
1
2.824E + 05
81.71
<0.0001
ABCDE
23005.13
1
23005.13
6.66
0.0326
Residual
27645.88
8
3455.73
Cor Total
2.573E + 06
31
Antibiotic activity = +452.81 + 59.25B + 84.44C + 63.81D + 22.13E − 38.25AB + 24.19AC − 30.56AD − 29.87BC + 92.31CD − 36.87CE − 35DE − 31.94ACD + 60.37ACE + 25.87ADE + 45BCD − 55.19BCE- 135.56BDE − 65.63ABCD + 70.69ABCE + 25.94ABDE + 32.62ACDE − 93.94BCDE − 26.81ABCDE
4.5 Optimization of antibiotics by central composite design and response surface methodology
The CCD experiment was not only employed to analyse the interactions among the important three selected variables (yeast extract, glucose and pH) but also to find their optimum levels. The experimental results listed in Table 3 were analysed using multiple regressions. Antibiotic activity was found to be maximum at run 9 (2159 U/g substrate). In order to evaluate the maximum antibacterial activity corresponding to the optimum levels of 0.835% yeast extract, 70% moisture and 0.55% glucose. The second-order polynomial model was proposed to evaluate the optimum levels of these selected variables. The second-order polynomial model for antibacterial activity was shown in the following equation:
Run
A:Moisture
B:Glucose
C:Yeast extract
Antibiotics
%
%
%
U/g
1
95.2269
0.55
0.625
426
2
70
1.30681
0.625
1823
3
70
0.55
0.625
1980
4
70
0.55
0.625
1976
5
70
0.55
0.625
1897
6
44.7731
0.55
0.625
391
7
70
0.55
0.625
1879
8
55
1
0.5
7
9
70
0.55
0.835224
2159
10
70
0.55
0.625
2019
11
85
1
0.5
1120
12
70
0.55
0.625
1885
13
55
1
0.75
1049
14
70
0.55
0.414776
360
15
55
0.1
0.75
1359
16
55
0.1
0.5
291
17
85
0.1
0.75
501
18
85
0.1
0.5
574
19
70
−0.206807
0.625
48
20
85
1
0.75
1091
Antibiotics = +1941.07 + 46.78A + 258.27B + 368.57C + 216.25AB − 276.50AC + 2.25BC − 552.59A2 − 366.27B2 − 251.72C2
The R2 of this designed CCD model was 0.9085 and the adjusted R2 was 0.8261(Table 4). In this study, the adjusted determination coefficient (R2) confirmed the importance of statistical design, exhibiting minor experimental error and a fit regression equation (Wang et al., 2016; Wang and Liu, 2008). The saddle or elliptical nature of contour plot showed the significance of the good interactions between the respective variables. Fig. 2a–c shows the contour plot and 3D response surface plot for the antibacterial activity generated by the predicted CCD model. It could be evident from Fig. 2 (a-f), the high antibacterial activity of 2159 U/g could be observed at 70% moisture, 0.835%yeast extract and 0.55% glucose. According to the model equation, we predicted that a maximum antibacterial activity of 2199 U/g could be achieved at 70% moisture, 0.82% glucose and 0.725% yeast extract. The predicted response (2199 U/g substrate) was verified, 2214 U/g substrate of antibacterial activity, which was very close to the predicted response. RSM has been used for the production of streptomycin (Sharma et al., 2009) and neomycin (Messaoudi et al., 2012). The newly optimized medium enhanced antibacterial activity upto 10 times higher than the un optimized medium.
Source
Sum of Squares
df
Mean Square
F-value
p-value
Model
1.001E + 07
9
1.112E + 06
11.03
0.0004
A-Moisture
29885.77
1
29885.77
0.2964
0.5981
B-Glucose
9.110E + 05
1
9.110E + 05
9.03
0.0132
C-Yeast extract
1.855E + 06
1
1.855E + 06
18.40
0.0016
AB
3.741E + 05
1
3.741E + 05
3.71
0.0830
AC
6.116E + 05
1
6.116E + 05
6.07
0.0335
BC
40.50
1
40.50
0.0004
0.9844
A2
4.401E + 06
1
4.401E + 06
43.64
<0.0001
B2
1.933E + 06
1
1.933E + 06
19.17
0.0014
C2
9.131E + 05
1
9.131E + 05
9.06
0.0131
Residual
1.008E + 06
10
1.008E + 05
Lack of Fit
40.3
5
8.06
0.0001
2.492
Pure Error
17729.33
5
3545.87
Cor Total
1.102E + 07
19
5 Conclusion
A total of 129 Actinomycetes strains were isolated from the mangrove soil sediments. Among them, strain AS4 displayed high levels of potent bactericidal activities. Morphological characters and 16S rRNA analysis revealed that strain AS4 belongs to Streptomyces spp., Hence, it was named as Streptomyces sp. AS4. To enhance maximum production of antibiotics, solid state fermentation was the method deployed. Wheat bran proved to be an excellent choice as a solid substrate for enhanced production of antibiotics at the minimum production cost.
Acknowledgment
The authors extend their appreciation to The Researchers Supporting Project number (RSP-2019/108) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci. 2019
- [CrossRef] [Google Scholar]
- Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications. J. King Saud Univ. – Sci. 2019
- [CrossRef] [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol., B: Biol.. 2019;197:111529
- [Google Scholar]
- Arasu MV, Duraipandiyan V. Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B: Biol.. 2018;189:176-184.
- [Google Scholar]
- Characterization of silver nanomaterials derived from marine streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 2018::8(5).
- [Google Scholar]
- Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health 2019:549-556.
- [Google Scholar]
- Antifungal metabolites from sponge associated marine Streptomyces sp. strain (ERIMA-01) J. Pure Appl. Microbiol.. 2014;8(2):115-128.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol.. 2013;13(1):105.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Identification of novel quinine metabolite from marine actinomycetes with antifungal and anticancer bio-prospective. Fresenius Environ. Bull.. 2015;24(10a):3281-3287.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from Southern Western Ghats. South Indian J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- The discovery of new bioactive microbial metabolites: screening and identification. Amsterdam: Elsevier Science Publishers; 1989. p. :3-25.
- Identifying small-molecule lead compounds: the screening approach to drug discovery. Trend. Biotechnol.. 1995;13(3):115-121.
- [Google Scholar]
- Frigocyclinone, a novel angucyclinone antibiotic produced by a Streptomyces griseus strain from Antarctica. J. Antibiot.. 2005;58(5):346.
- [Google Scholar]
- Polyether antibiotic complex produced by Streptomyces hygroscopicus IM—110–81. Biotech. Bioind.. 1987;2(2):15-17.
- [Google Scholar]
- Do we still need the aminoglycosides? Int. J. Antimicrob. Agent.. 2009;33(3):201-205.
- [Google Scholar]
- Optimisation studies on neomycin production by a mutant strain of Streptomyces marinensis in solid state fermentation. Process Biochem.. 2004;39(5):529-534.
- [Google Scholar]
- Statistical optimization of medium components to improve the antibiotic activity of Streptomyces sp. 19G–317. Afr. J. Agri. Res.. 2011;6(19):4424-4431.
- [Google Scholar]
- Screening, isolation and characterization of antifungal producing actinomycete, Streptomyces strain RN+ 8. Afr. J. Biol. Sci.. 2006;2:73-82.
- [Google Scholar]
- Effects of nutrients on the production of AK-111-81 macrolide antibiotic by Streptomyces hygroscopicus. Microbiol. Res.. 2005;160(3):243-248.
- [Google Scholar]
- AH7, a non-polyenic antifungal antibiotic produced by a new strain of Streptosporangium roseum. Microbios. 1998;96(384):103-109.
- [Google Scholar]
- Application of factorial and response surface methodology in modern experimental design and optimization. Crit. Rev. Anal. Chem.. 2006;36(3–4):141-151.
- [Google Scholar]
- Response surface method as an efficient tool for medium optimisation. Trend. Appl. Sci. Res.. 2011;6(2):121-129.
- [Google Scholar]
- Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int. J. Biol. Macromol.. 2016;93:534-542.
- [Google Scholar]
- Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ. Sci. Poll. Res.. 2014;21(9):5983-5990.
- [Google Scholar]
- Optimisation of enzyme assisted extraction of silybin from the seeds of Silybum marianum by Box-Behnken experimental design. Phytochemical analysis. Int. J. Plant Chem. Biochem. Tech.. 2009;20(6):475-483.
- [Google Scholar]
- Corn husk as a novel substrate for the production of rifamycin B by isolated Amycolatopsis sp. RSP 3 under SSF. Process. Biochem.. 2010;45(1):47-53.
- [Google Scholar]
- Selective isolation of actinomycetes from mangrove forest of Pahang, Malaysia. In: International Conference on Agriculture, Biology and Environmental Sciences (ICABES'14): 2014, Indonesia. 2014.
- [Google Scholar]
- Stability and activities of antibiotics produced during infection of the insect Galleria mellonella by two isolates of Xenorhabdus nematophilus. Appl. Environ. Microbiol.. 1994;60(2):715-721.
- [Google Scholar]
- Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol.. 2012;32(1):129-134.
- [Google Scholar]
- An experimental strategy validated to design cost-effective culture media based on response surface methodology. Prep. Biochem. Biotech.. 2017;47(6):578-588.
- [Google Scholar]
- The current state of multidrug-resistant gram-negative bacilli in North America: insights from the Society of Infectious Diseases Pharmacists Pharmacotherapy. J. Human Pharma. Drug Ther.. 2008;28(2):235-249.
- [Google Scholar]
- Aminoglycoside therapy in infectious diseases. Expert Opin. Pharmaco.. 2013;14(12):1585-1597.
- [Google Scholar]
- Characterization of saltern based Streptomyces sp. and statistical media optimization for its improved antibacterial activity. Front Microbial.. 2015;21(5):753.
- [Google Scholar]
- Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B: Biol. 2019111667
- [Google Scholar]
- Isolation and characterization of antagonistic actinobacteria from mangrove soil. J. Biochem. Technol.. 2012;3(4):361-365.
- [Google Scholar]
- Antimicrobial activities of actinomycetes isolated from unexplored regions of Sundarbans mangrove ecosystem. BMC Microbiol.. 2015;15(1):170.
- [Google Scholar]
- Optimization of process variables for decolorization of Disperse Yellow 211 by Bacillus subtilis using Box-Behnken design. J. Hazard Mate.. 2009;164(2–3):1024-1039.
- [Google Scholar]
- Destination of aminoglycoside antibiotics in the ‘post-antibiotic era’. J. Antibiotic.. 2018;71(1):4-14.
- [Google Scholar]
- Influence of nutrition and culturing conditions for optimum growth and antimicrobial metabolite production by Streptomyces sp. 201. J. Mycolog. Medic.. 2009;19(3):161-167.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B 2019111670
- [Google Scholar]
- Application of response surface methodology to optimize the production of antimicrobial metabolites by Micromonospora Y15. Biotech. Biotech. Equip.. 2017;31(5):1016-1025.
- [Google Scholar]
- Optimization of fermentation medium for bacteriocin production of L. lactis KLDS4.0325 by response surface methodology. Sci. Technol. Food Ind.. 2016;37:137-142.
- [Google Scholar]
- Medium optimization for antifungal active substances production from a newly isolated Paenibacillus sp. using response surface methodology. Bioresour. Technol.. 2008;99(17):8245-8251.
- [Google Scholar]
- How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol.. 2001;176(5):386-390.
- [Google Scholar]
- A novel antibiotic produced by Streptomyces noursei Da07210. Antonie Van Leeuwenhoek. 2009;96(1):109-112.
- [Google Scholar]
- Three new compounds from soil actinomycete Streptomyces albospinus 15-4-2. J. A. Nat. Prod. Res.. 2011;13(10):901-906.
- [Google Scholar]
- Optimization of nutrients for dinactin production by a marine Streptomyces sp. from the high latitude Arctic. Biotech. Bioprocess Eng.. 2015;20(4):725-732.
- [Google Scholar]







