Antibiogram and plasmid profiling of beta-lactamase producing multi drug resistant Staphylococcus aureus isolated from poultry litter
⁎Corresponding author. kthamaraiselvi@bdu.ac.in (Thamaraiselvi Kaliannan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The occurrence of antibiotic resistant microorganisms in poultry litter is due to the increasing use of antibiotics. Poultry litter is used as a fertilizer for its high nutrient content. The presence of antibiotic resistant microorganisms in poultry litter makes its handling, amendment and application as fertilizer a challenging task. This study is designed to evaluate the antibiotic susceptibility and plasmid profiling of beta-lactamase producing multi-drug resistant Staphylococcus aureus in poultry litter. Sample collected from a poultry farm was screened for the presence of Staphylococcus aureus strains. The in vitro antibiotic susceptibility test by disk diffusion method were carried out in all the isolates. All the isolates were highly susceptible to oxacillin and gentamycin and intermediate for erythromycin and were completely resistant to vancomycin and penicillin. The isolated Staphylococcus aureus strains showed beta-lactamase activity which is the basis for the resistance to beta-lactum antibiotics. These strains when exposed to curing agent namely ethidium bromide, the beta-lactamase activity was lost. Results indicate that Staphylococcus aureus isolated from poultry litter are beta-lactamase producers and relatively resistant to commonly used antibiotics. The resistance among the isolated strains is found to be plasmid-mediated. The outcome of our present study exercises both usefulness in shaping the existing antibiotics prescription and thus helps to reduce or prevent the development of multiple drug resistant microorganism.
Keywords
Antibiotic resistance
Beta-lactamase
Plasmid
Poultry litter
Staphylococcus aureus, Antibiotic sensitivity
1 Introduction
Over the last few years antibiotic use has become widespread and bacteria are steadily and routinely developing resistance. Especially in poor countries, the control of bacterial infections using general antibiotics are of concern, due to their resistance against them (Pereckaite et al., 2018; Shaikh et al., 2015). Antibiotics are also used in animal husbandry especially as therapeutic, control diseases such as prophylaxis and also as growth promotors (Rassow and Schaper, 1996; Van et al., 2019). During poultry meat production for commercial purpose, usually antibiotics are feed from a day old chick upto to its adult stage during their growth period (Chen et al., 2020; McDermott et al., 2002). Ultimately it causes to a selection of resistance in the animal’s native microbiome, pathogens and also in the environment through the feces (Inda et al., 2019; Sundin et al., 1995). Resistance developed in the bacteria from feces can be transferred to native bacteria by their sharing of extrachromosomal antibiotic resistant plasmids. The developed antibiotic resistance will be disseminated to the soil, water, animal and humans. This antibiotic resistance in microorganisms may interfere with the effective functioning of antibiotics, both in humans and animals by creating the microorganisms causing diseases to be unresponsive to antibiotics (Afewerki et al., 2019; Kolawole and Shittu, 1997). Staphylococcus aureus is a bacterium widely spread in the environment, such as air, dust and on the household items and has evolved resistance to all antibiotic classes used for its treatment through different mechanisms (Akindolire et al., 2015; Cockfield et al., 2007; Kiliç and Çirak, 2006). In addition, there are instances of occurrence of resistant Staphylococcus aureus in poultry litter (Amoako et al., 2019; Dhanarani et al., 2009). Beta-lactam antibiotics, the major class of antibiotics used against Staphylococcus aureus, develop resistance and are most often due to a plasmid-encoded penicillinase/beta-lactamase (Babic et al., 2006; Quinn et al., 2011).
Beta-lactamase production is the major resistance mechanism against penicillin and cephalosporin, which are the cheapest, easiest to produce and most effective antibiotic classes. Beta-lactamase inactivates the beta-lactam antibiotics (penicillin) by cleavage of the beta-lactam ring and it is produced by the Staphylococci that have bla genes (Kiliç and Çirak, 2006). Staphylococcal beta-lactamase is not chromosomal and is plasmid mediated and can be non-inducible or inducible with antibiotis (Maddux, 1991). Vancomycin is the drug of choice against Staphylococcus aureus when beta-lactams are inappropriate. The production of beta-lactamase and the resistance to varying range of antibiotics has increased the importance of Staphylococcus aureus infections. Not much information is available on the resistance pattern of Staphylococcus aureus in poultry litter and hence our focus was to investigate the antibiogram of the isolated Staphylococcus aureus from the poultry litter and to study the plasmid pattern responsible for beta-lactamase activity.
2 Materials and methods
2.1 Sample collection
Poultry litter, a mixture of poultry manure, saw dust and rice hull bedding, was collected from a poultry farm in Namakkal District, Tamil Nadu, India. Samples were collected from the top and bottom. The composite samples were labelled and brought to the laboratory and stored at 4°C until use.
2.2 Isolation and identification of bacterial strains
The stored samples were thawed and brought to room temperature. Sample of about 1 g was weighed, mixed in 10 mL of 0.85% saline, serially diluted, spread plated onto nutrient agar plates and were incubated at 37°C for 24 h. From the plate of 10-5 dilution, discreet colonies were picked and maintained in nutrient agar slants.
The cultures were then inoculated onto Mannitol Salt Agar (MSA). The plates were incubated aerobically for 24 h at 37 °C and were examined for fermentation of mannitol which is indicated by color change of the medium around each colony from red to yellow. The organism from the fermented plates were identified by gram staining, colony morphology, hemolysis, catalase, oxidase, coagulase, IMViC, triple sugar iron agar test, urease test, Macconkey agar, DNase test using standardized protocol (Cowan, 1974). The 16S rRNA sequencing was carried out for further identification of isolates at species level.
2.3 Antibiotic susceptibility testing
Susceptibility testing for a total 10 Staphylococcus aureus strains to nine antibiotics were carried out by the agar disk diffusion method (Bauer et al., 1966). The isolates were inoculated into nutrient broth and incubated at 37°C for 24 h. Then the inoculum (approx. 0.1 mL) was evenly spread onto the surface of Mueller-Hinton (MH) agar plates and allowed to dry. Antibiotic impregnated filter paper disks (erythromycin (15 mcg), gentamycin (120 mcg), oxacillin (5 mcg), penicillin-G (10 units/disk), vancomycin (30 mcg), ciprofloxacin (10 mcg), streptomycin (10 mcg), chloramphenicol (30 mcg) and kanamycin (30 mcg)) were placed on the superficial layer of the agar and incubated at 37°C for 24 h. Antibiogram pattern was determined by measuring the zone size and were interpreted with the NCCLS standard.
2.4 Beta-lactamase activity
Beta-lactamase activities of the isolated 10 strains were checked by three Iodometric methods - Iodometric strip, iodometric agar plate and iodometric tube methods (Oberhofer and Towle, 1982; Perret, 1954; Sawai et al., 1978).
2.5 Isolation and electrophoretic pattern of plasmid DNA
Plasmid extraction was carried out by alkaline lysis method (Marko et al., 1982). Agarose gel electrophoresis of plasmid DNA from 10 multidrug resistant strains of Staphylococcus aureus was carried out according to the standard protocol (Sambrook and Russell, 2001).
2.6 Curing of the plasmid
All the beta-lactamase positive strains were incubated in Tryptic Soy Broth (TSB) containing acridine orange at 37°C for 24 h. After incubation, the strains were streaked onto medium consisting of 0.3% yeast extract, 3% of TSB and 1.5% agar-agar. After 24 h of incubation, the isolates were tested for beta-lactamase activity by iodometric techniques.
The susceptibility testing for cured strains were carried out on MH agar plates with and without penicillin (1%) and incubated for 48 h at 37°C and observed for growth. Antibiotic susceptibility test was performed with antibiotics that showed resistant previously with vancomycin, erythromycin, streptomycin, kanamycin and chloramphenicol. The plasmid profiling was again performed and checked for the presence of plasmid.
3 Results and discussion
Seventeen gram positive and catalase positive Staphylococcus strains were isolated from poultry litter of which only 10 strains were found to be Staphylococcus aureus based on their morphological, biochemical characteristics and 16S rRNA sequencing.
Varying range of resistance pattern was observed for each antibiotic. All the 10 isolates of Staphylococcus aureus strains showed resistance to penicillin and the polypeptide antibiotic vancomycin. On contrary all the strains showed sensitivity to semisynthetic antibiotic oxacillin. Intermediate sensitivity was seen in all the strains for the macrolide antibiotic, erythromycin. Nine strains showed sensitivity to the third generation cephalosporin, ciprofloxacin. One strain was found to show intermediate susceptibility to this antibiotic. Seven Staphylococcus aureus strains showed resistance to chloramphenicol, two strains showed sensitivity and one strain showed intermediate to the same antibiotic. Each aminoglycoside antibiotics such as erythromycin, gentamycin, oxacillin, penicillin-G, vancomycin, ciprofloxacin, streptomycin, chloramphenicol and kanamycin showed varying resistance pattern. All strains were sensitive to gentamycin. Seven strains showed resistance and three strains exhibited intermediate sensitivity to kanamycin. Two isolates were resistant, two strains showed intermediate sensitivity and 6 strains were sensitive towards streptomycin. The antibiotic susceptibility patterns are represented in Fig. 1.
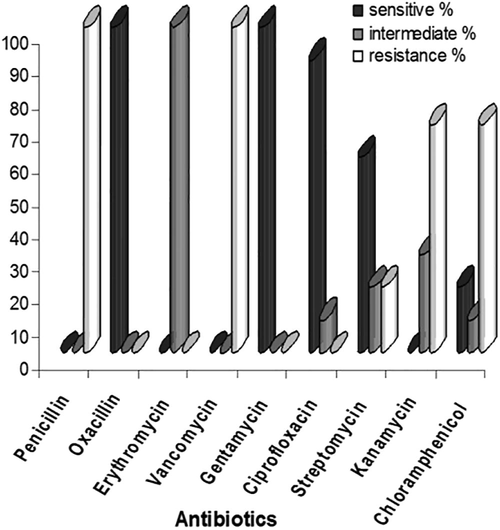
- Antibiotic susceptibility pattern of Staphylococcus aureus from poultry litter.
Multi drug resistant bacteria Staphylococci has been isolated from diverse environments, such as clinical, animal and food samples (Khan et al., 2005). Gram positive organisms were found majorly in chicken intestinal microflora namely Staphylococcus, Streptococcus and Micrococcus sp. (Dhanarani et al., 2009). In the present study seventeen gram positive and catalase positive bacterial strains were isolated out of which 10 strains were identified as Staphylococcus aureus. Other species such as Staphylococcus, Streptococcus, Micrococcus, Salmonella, Enterococci, E. coli, Aeromonas, and Pseudomonas have also been isolated from poultry litter (Dhanarani et al., 2009). Ten isolates of Staphylococcus aureus from poultry litter were subjected to in vitro antibiogram and they showed varying range of susceptibility pattern. Similarly E. coli was isolated from workers at the poultry farm also showed antibiotic resistance (Ojeniyi, 1989). Incongruous use of growth promoting antibiotics (Khachatourians, 1998) and also the selective pressure from antimicrobials is the driving force in the development of antibiotic resistance (Manie et al., 1998). Animal feed is progressively being supplemented with antibiotics to decline the risk of epidemics in animal husbandry (Khachatourians, 1998).
Penicillin inhibits the bacterial peptidoglycan synthesis which is the major cell wall component. It exhibits rigid mechanical stability due to its highly cross linked lattice wall structure in the bacterium. In our study 10 isolated Staphylococcus aureus strains from poultry litter were highly resistant to penicillin; and the resistance may be due to the excessive rational or irrational use of the penicillin. The inherent weakness of penicillin is because of the attack of ring nucleus by beta-lactamase produced in Staphylococcus aureus (Esimone et al., 2006).
In this study vancomycin was found to be resistant to all strains isolated from poultry litter. The acquirement of resistance occurs in different mechanisms such chromosomal mutations or through plasmids. Complete (100%) sensitivity to vancomycin and clindamycin in multidrug resistant Staphylococcus aureus have been reported. Staphylococcus aureus strains were found to be vancomycin resistant from clinical samples (Naseer and Jayaraj, 2010). But there have been no recorded history of vancomycin resistance in poultry. Though vancomycin is not used in poultry, resistance is shown due to the linkage of resistance genes from old antibiotics to the latest ones in market (Summers, 2002).
In our present investigation, all the isolated strains showed high sensitivity to oxacillin, which is a cell wall inhibitor, whereas seven strains showed resistance to kanamycin and chloramphenicol. S. aureus has been shown to be resistant to oxacillin in other studies (Salimnia and Brown, 2005). Kanamycin resistance was proved to be due to a plasmid encoded determinant (Storrs et al., 1988). In a previous report erythromycin and tetracycline showed to be effective antibiotics against Staphylococcus aureus (Hassam et al., 1978). But in our study erythromycin intermediate strains were observed making the treatment difficult.
All the strains of Staphylococcus aureus studied for beta-lactamase production showed positive results for beta-lactamase activity in all the three iodometric methods. Beta-lactamase production is a very important cause for antimicrobial resistance in bacteria. The present study shows the presence of beta-lactamase activity in all the strains and it was checked and confirmed using three iodometric methods. Beta-lactamase producing Staphylococcus aureus has also been isolated from humans, animals and various organs or tissues in chicken (Mamza et al., 2010). Staphylococcal beta-lactamase is present in plasmids and may be non-inducible or inducible in the presence of antibiotics (Maddux, 1991). Beta-lactamase activity in S. aureus and E. coli isolated from chicken were found to be 8.8% and 11%, respectively (Mamza et al., 2010). Reports showed that S. aureus from various samples had beta-lactamase production (Salimnia and Brown, 2005) and also showed multiple drug resistance (Mamza et al., 2010).
Electrophoretic pattern of plasmid isolated from the 10 isolated Staphylococcus aureus strains, which indicates that all the strains are encoded by plasmid. The molecular size of the plasmid DNA was calculated to be ∼13 kb. Here, λ DNA (Eco RI /Hind III double digest) was used as marker DNA.
The cured strains subjected to iodometric tube method, showed no color change after the addition of iodine confirming the absence of beta-lactamase activity. The cured strains inoculated in MHA without penicillin showed growth and MHA with penicillin showed no growth. Antibiotic susceptibility tests for the cured strains were performed with the antibiotics (Vancomycin, Erythromycin, Streptomycin, Kanamycin and Chloroamphenicol) and found that all those antibiotics for which the strains showed resistance before curing, showed high susceptibility (Fig. 2). This proved that beta-lactam resistance was lost during curing process and confirmed the beta-lactamase production is plasmid mediated. The resistance patterns confirmed the presence of multi drug resistant plasmid in the bacterial isolates. Plasmid isolation and agarose gel electrophoresis for the cured strains also indicated that the strains had lost their plasmid during curing.
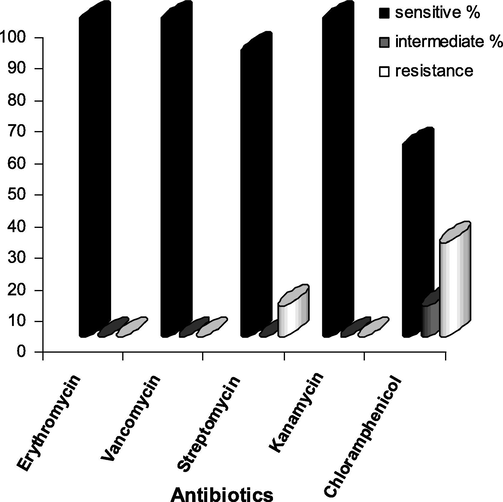
- Antibiotic susceptibility pattern of Staphylococcus aureus after plasmid curing.
Different strains of Staphylococcus aureus possessed plasmids of different molecular weight. In our study the isolated plasmid was ∼13 kb. Presence of plasmids indicates that the antibiotic resistance is plasmid mediated. Complete plasmid genome sequencing will help in further elucidating the actual mechanism involved. In previous studies multidrug resistant S. aureus isolate possessed 23 kb plasmid (Rahman et al., 2005).
Plasmid curing was carried out by acridine orange for the isolated Staphylococcus aureus strains. Similar to our study, Streptococcus and Micrococcus plasmids were cured by acridine orange (Dhanarani et al., 2009). The loss of antibiotic resistance and plasmid is interrelated. The loss of 6.4- and 3.4-kb plasmid in Bacteroide fragilis C68c was associated to antibiotic resistance (Nakano et al., 2004). Non-existence of plasmid related to chromosomally mediated resistance has also been reported (Pestana et al., 1999).
Cured strains of Staphylococcus aureus showed negative result for beta-lactamase activity. Antibiotics which were resistant previously showed sensitive pattern after the strains were cured. The cured plasmid strains failed to grow on media containing penicillin. Plasmid pattern of the cured strains showed loss of plasmid. All the experiments proved that a factor which was present in the strains before curing, was the reason for the strains to exhibit multidrug resistance, was removed after curing. Hence, it was concluded that this factor, the antibiotic resistance, was plasmid mediated.
On the whole, Staphylococcus aureus was able to overcome the antibiotics developed against it and will also acquire resistance to any new drugs formulated for it. Hence lot of attention should be given while developing new drugs. It is necessary to control the usage of antibiotics as a growth promoter in the poultry. Government and health care authorities should implement educational awareness to farmers, encourage better infection control and prevent antimicrobial usage.
4 Conclusion
The present study indicates that the Staphylococcus aureus strains isolated from poultry litter is found to be multi-drug resistant and is plasmid encoded. The antibiotic resistant organisms spread from environment to different biospheres and finally reaches human. Plasmid mediated antibiotic resistance is a worldwide problem as it spreads from one host to the other by horizontal transfer hence treatment for these opportunistic pathogens becomes difficult. Vancomycin the last drug preferred in all the Staphylococcal infection also showed high resistance, making the Staphylococcus aureus infection as a completely incurable infection. Hence the unnecessary usage of antibiotics for the cause of increased profit and high market prices of poultry must be controlled to save humans from becoming endangered.
Acknowledgement
The authors extent their gratitude to the Council of Scientific and Industrial Research (CSIR) for providing financial support through the sponsored project (38(1295)/11/EMR-II). Also the authors would like to thank the DST for providing FIST scheme (FST: SR/FST/LSI-687/2016) and UGC for approving SAP (UGC-SAP: No. F.5-4/2016/DRS-1 (SAP-11)) to the Department of Environmental Biotechnology, Bharathidasan University, Tiruchirappalli, India. The authors NAA-D, MVA and VD would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for the funding of this research through the Research Group Project No. RG-1435-071.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopedic applications. Nanomed. Nanotechnol. Biol. Med. 2019:102143.
- [Google Scholar]
- Genetic characterization of antibiotic-resistant Staphylococcus aureus from milk in the North-West Province, South Africa. Saudi J. Biol. Sci.. 2015;25(7):1348-1355.
- [Google Scholar]
- Genomic analysis of methicillin-resistant Staphylococcus aureus isolated from poultry and occupational farm workers in Umgungundlovu District, South Africa. Sci. Total Environ.. 2019;670:704-716.
- [Google Scholar]
- What's new in antibiotic resistance? Focus on beta-lactamases. Drug Resistance Updates. 2006;9(3):142-156.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45(4_ts):493-496.
- [Google Scholar]
- Effect of peracetic acid on Campylobacter in food matrices mimicking commercial poultry processing. Food Control 2020107185
- [Google Scholar]
- Rapid determination of hospital-acquired meticillin-resistant Staphylococcus aureus lineages. J. Med. Microbiol.. 2007;56(5):614-619.
- [Google Scholar]
- Cowan and Steel manual for identification of medical bacteria (2nd ed.). New York: Cambridge University Press; 1974.
- Study on acquisition of bacterial antibiotic resistance determinants in poultry litter. Poul. Sci.. 2009;88(7):1381-1387.
- [Google Scholar]
- In vitro evaluation of the interaction between tea extracts and penicillin G against Staphylococcus aureus. Afr. J. Biotechnol.. 2006;5(11)
- [Google Scholar]
- Changes in antibiotic sensitivity in strains of Staphylococcus aureus, 1952–78. Br. Med. J.. 1978;2(6136):536-537.
- [Google Scholar]
- Emerging frontiers in microbiome engineering. Trends Immunol.. 2019;40(10):952-973.
- [Google Scholar]
- Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Cmaj. 1998;159(9):1129-1136.
- [Google Scholar]
- Molecular characterization of multidrug-resistant Enterococcus spp. from poultry and dairy farms: detection of virulence and vancomycin resistance gene markers by PCR. Mol. Cell. Probes. 2005;19(1):27-34.
- [Google Scholar]
- Comparison of staphylococcal beta-lactamase detection methods. Fabad J. Pharmaceut. Sci.. 2006;31(2):79.
- [Google Scholar]
- Unusual recovery of animal staphylococci from septic wounds of hospital patients in Ile-Ife, Nigeria. Lett. Appl. Microbiol.. 1997;24(2):87-90.
- [Google Scholar]
- Effects of ß-lactamase-mediated antimicrobial resistance: the role of ß-lactamase inhibitors. Pharmacother. J. Human Pharmacol. Drug Therapy. 1991;11(2P2):40S-50S.
- [Google Scholar]
- Beta-lactamase Escherichia coli and Staphylococcus aureus isolated from chickens in Nigeria. Veterinaria italiana. 2010;46(2):155-165.
- [Google Scholar]
- Antimicrobial resistance of bacteria isolated from slaughtered and retail chickens in South Africa. Lett. Appl. Microbiol.. 1998;26(4):253-258.
- [Google Scholar]
- A procedure for the large-scale isolation of highly purified plasmid DNA using alkaline extraction and binding to glass powder. Anal. Biochem.. 1982;121(2):382-387.
- [Google Scholar]
- Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Diseases. 2002;185(6):837-840.
- [Google Scholar]
- Nakano, V., Padilla, G., do Valle Marques, M., Avila-Campos, M.J. 2004. Plasmid-related β-lactamase production in Bacteroides fragilis strains. Res. Microbiol., 155(10), 843–846
- Identification of multiresistant Staphylococcus aureus in clinical specimens. J. Res. Med. Sci.. 2010;4:204-207.
- [Google Scholar]
- Evaluation of the rapid penicillinase paper strip test for detection of beta-lactamase. J. Clin. Microbiol.. 1982;15(2):196-199.
- [Google Scholar]
- Direct transmission of Escherichia coli from poultry to humans. Epidemiol. Infect.. 1989;103(3):513-522.
- [Google Scholar]
- Current antimicrobial susceptibility testing for beta-lactamase-producing Enterobacteriaceae in clinical settings. J. Microbiol. Methods. 2018;152:154-164.
- [Google Scholar]
- Plasmid-related resistance to clindamycin and penicillin G in a Bacteroides vulgatus strain. Anaerobe. 1999;3(5):447-449.
- [Google Scholar]
- Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S., Fitzpatrick, E.i., 2011. Veterinary Microbiology and Microbial Disease, second ed., Veterinary Microbiology and Microbial Disease. John Wiley & Sons.
- Antibiotic susceptibility and R-plasmid mediated drug resistance in Staphylococcus aureus. Med. J. Islamic World Acad. Sci.. 2005;15(3):111-116.
- [Google Scholar]
- The use of feed medications in swine and poultry facilities in the Weser-Ems region. Deutsche Tieraerztliche Wochenschrift. 1996;103(7):244-249.
- [Google Scholar]
- Detection of oxacillin resistance in Staphylococcus aureus: comparison of phoenix oxacillin and cefoxitin MICs, microscan oxacillin MIC, oxacillin and cefoxitin disk diffusion, and mecA gene detection. ICAAC 2005:12-20.
- [Google Scholar]
- Sambrook, J., Russell, D. 2001. Molecular cloning: A laboratory manual, the third edition, Cold spring harbor laboratory press, cold spring harbor, New York
- Iodometric assay method for beta-lactamase with various beta-lactam antibiotics as substrates. Antimicrob. Agents Chemother.. 1978;13(6):910-913.
- [Google Scholar]
- Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J. Biol. Sci.. 2015;22(1):90-101.
- [Google Scholar]
- Genetic analysis of gentamicin resistance in methicillin-and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals. Antimicrob. Agents Chemotherapy. 1988;32(8):1174-1181.
- [Google Scholar]
- Generally overlooked fundamentals of bacterial genetics and ecology. Clin. Infect. Diseases. 2002;34(Supplement_3):S85-S92.
- [Google Scholar]
- Distribution of the streptomycin-resistance transposon Tn 5393 among phylloplane and soil bacteria from managed agricultural habitats. Can. J. Microbiol.. 1995;41(9):792-799.
- [Google Scholar]
- Antibiotic use in food animals in the world with focus on africa: pluses and minuses. J. Global Antimicrob. Resistance. 2019;20:170-177.
- [Google Scholar]







