Translate this page into:
Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine
⁎Corresponding author at: Department of Biology, College of Science in Zulfi, Majmaah University, Majmaah 11952, Saudi Arabia; Microbiology and Immunology Department, Veterinary Research Division, National Research Center, 33 El-Buhouth St., Dokki, Giza 12622, Egypt. m.eraqi@mu.edu.sa (Mostafa M. Eraqi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Medicinal plants continue to gain popularity on a global scale. Besides, there is a need for discovering new antibacterial natural extracts that could be used as antibiotics alternatives against resistant bacteria. In this respect, the aim of this study was to determine the phytochemicals, antioxidant activity, calorific nutritional value and antibacterial potential of four traditionally used wild medicinal plants (Achillea fragrantissima (Delile) Hayne, Teucrium polium L., Peganum harmala L. and Solenostemma argel (Forssk.) Sch. Bip. grow in Saint Catherine Protectorate, South Sinai, Egypt.
Methods
Standard methods were applied to determine the proximate composition, calorific nutritional value, secondary metabolites (phenolics and flavonoids), antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH·), ferrous ion chelating (FIC), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS•+) and Ferric reducing antioxidant power (FRAP) techniques in addition to the antibacterial potential of the active aqueous extracts by broth dilution technique.
Results
The results obtained illustrated that T. polium recorded the highest nutritional calorific value (205.63 ± 5.76 calories/100 g dried plant), total phenolics (8.057 ± 0.322 g gallic acid equivalent/100 g dried plant), total flavonoids (2.013 ± 0.034 g catechin equivalent/ 100 g dried plant and antioxidant scavenging activity using DPPH (EC50 = 1.84 mg extract/g DPPH), FIC (IC50 = 0.068 mg extract/ml), ABTS (61.11%) and FRAP (2185.71 mmol Fe (II)/g extract) assays followed descendingly by A. fragrantissima, S. argel and P. harmala, respectively. There was direct relation between the flavonoids/ phenolics ratios and the radical scavenging activity among all extracts. Regarding the antibacterial potential, the extracts expressed broader antibacterial spectrum against Bacillus cereus (ATCC®11778™), Bacillus subtilis (ATCC®19659™), Escherichia coli (ATCC®10536™), klebsiella pneumoniae (ATCC®10031™), Listeria innocua (ATCC®33090™), Listeria monocytogenes (ATCC®19115™), Pseudomonas aeruginosa (ATCC®9027™), salmonella enterica (ATCC®15479™) and Salmonella typhimurium)ATCC®14028™(strains. The minimal inhibitory concentrations of the extracts estimated using broth dilution assay ranged from 0.049 to 1.56 mg/ml. T. polium extract possessed the highest activity among all other extracts.
Conclusions
In conclusion, the studied medicinal plants could be used as nutritional supplements, antioxidants and antibacterial botanicals for combating some of the pathogenic bacteria.
Keywords
Medicinal plants
Antibacterial potential
Nutritional
Phenolics
Flavonoids
Antioxidant activity
- ABTS
-
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid
- AE
-
antiradical efficiency
- Cfu
-
colony forming unit
- CAZ
-
ceftazidime antibiotic
- DPPH
-
2,2- diphenyl −1- picrylhydrazyl
- FIC
-
the ferrous ion chelating
- FRAP
-
ferric reducing antioxidant power
- MIC
-
minimal inhibitory concentration
- NCCLS
-
the national committee for clinical laboratory standards
- SAM
-
ampicillin-Sulbactam
- TPTZ
-
2, 4, 6 – tripyridyl - s - triazine
Abbreviations
1 Introduction
The Egyptian flora comprises about 2185 species that represent an important source of medically important compounds (Boulos, 2005). González-Tejero et al., (2008) concluded that the wild plant species, including the target species selected for the present study, play an important role in Bedouin‘s daily life and that their work in the Mediterranean region construct a base for consequent phytochemical and pharmacological researches that could lead to new therapeutic products. Traditionally, the herbal extracts in form of tea decoction of these species are used by local Bedouins in remote areas for the treatment of many illnesses like diabetes, allergy, kidney and respiratory and digestive problems (Baydoun et al., 2015).
The environmental stresses in the natural habitats of wild plants enhance them to synthesize active ingredients with high therapeutic potential like phenolics and flavonoids that possess distinguished antioxidant activity and therapeutic effects that contributes to heart diseases, neurodegenerative diseases, cancer and aging process (El-Zayat et al., 2021; Kurutas, 2016; Tungmunnithum et al., 2018; Zargoosh et al., 2019).
The increased incidence of bacterial resistance to antibiotics, has forced the research towards alternatives, like traditionally used medicinal plants and combinational therapies (Cheesman et al., 2017; El-Shahaby et al., 2019; Thomford et al., 2018).
Therefore, based on local community surveys, this research aimed to evaluate the nutritional value, phytochemicals, antioxidant activity and antibacterial potential of four Egyptian medicinal plants used in folklore medicine at Saint Catherine Protectorate.
2 Materials and methods
2.1 Plants collection
The aerial parts of Solenostemma argel, Teucrium polium, Achillea fragrantissima and Peganum harmala at vegetative stage were gathered from Saint Catherine Protectorate, South Sinai, Egypt in the period from October to November 2018. They were taxonomically identified according to Boulos (1999–2002). The plants were air dried for 15 days, grinded and kept in dry containers (Table 1).
Botanical name
Family
Local name
Habitat
Part used
Traditional use
Mode of use
Solenostemma argel (Del)
Asclepiadaceae
Hargal
Sand plains & terraces
Air dried aerial parts
Cough, colds, gastro-intestinal cramps, stomachic, anti-colic, urinary tract and liver problems, syphilis
Tea decoction
Achillea fragrantissima (Forssk) Sch. Bip.
Asteraceae
Gysoume
Wadi beds & gorges
Stomach ache and stomach worms
Tea decoction
eye pain, and infected wounds.
cold infusion
Teucrium polium (L.)
Lamiaceae
Jaada
Rocks & cliffs, gorges and wadi beds
Allergy, stomach, colic pains and fattiness
Tea decoction
Peganum harmala (L.)
Zygophyllaceae
Harmal
Sand plains
Leaves and flowers are used as anti rheumatic, teeth pain & stomach problems.
Tea decoction
2.2 Extracts preparation
Aqueous extracts were prepared in the same manner of administration of plants in folklore medicine as tea decoction. 10 g of the dried plants were extracted in 100 ml distilled water using shaking water-bath at 65 °C for 30 min then evaporated until dryness using rotary evaporator.
2.3 Phytochemical analysis
2.3.1 Primary metabolic variables
The metabolic variables including fats, proteins and total carbohydrates content were estimated in the investigated plants on dry weight moisture free basis. Fats were determined according to Arlington (1995), proteins according to Bradford protein assay (Bradford, 1976) and the total carbohydrates according to Thayumanavan and Sadasivam, (1984).
The nutritional value was calculated according to the formula:
Nutritional calorific value = 4.1x proteins % + 9.2x fats % + 4.1x total carbohydrates %.
The energy produced expressed as calories /100 g dried plant (AOAC, 2016).
2.3.2 Secondary active compounds
2.3.2.1. The total phenolics were quantified using Folin Ciocalteu technique adopted by Wolfe et al (2003) and estimated as gm gallic acid equivalent / 100 gm dried plant concerning the standard curve (y = 0.0064x, r2 = 0.99).
2.3.2.2. The total flavonoids were quantified by AlCl3 colorimetric technique adopted by Zhishen et al (1999) and expressed as gm catechin equivalent /100 gm dried plant concerning the standard curve (y = 0.004 ×, r2 = 0.99).
2.3.3 Antioxidant scavenging activity
2.3.3.1.2,2- diphenyl −1- picrylhydrazyl (DPPH) assay was used for determination of the antioxidant scavenging activity as adopted by Kitts et al. (2000) and modified by Liyana Pathirana and Shahidi (2005). 1 ml of the aqueous extracts with different concentrations was introduced to 1 ml of 0.135 mM DPPH•. The absorbance was detected at 520 nm after 30 min at exclusion of light. The remaining DPPḢ % was calculated using the formula: and graphed against mg extract/ gm DPPḢ using exponential curve to estimate EC50 and against mg extract/ ml to estimate IC50 (The concentration of the extract capable of diminishing 50% of the initial DPPH concentration) (Sanchez Moreno et al., 1998). EC50 is inversely proportional to the antioxidant efficacy (AE = 1/ EC50) (Spranger et al., 2008). Gallic acid and ascorbic acid were employed as standards.
2.3.3.1.2,2- diphenyl −1- picrylhydrazyl (DPPH) assay was adopted by Singh and Rajini (2004). 1 ml of 2 mM ferrous sulphate was added to 1 ml of the extracts and 1 ml 5 mM ferrozine then incubated for 10 min. The absorbance was estimaed at 562 nm. The concentration at which 50% of iron(II) chelated (IC50) was calculated where it is inversely proportional to the antioxidant power. Gallic acid and ascorbic acid were employed as standards.
2.3.3.3 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS·+) method adopted by Re et al. (1999) to measure the antioxidant scavenging activity of the extracts. ABTS·+ cation radical was released on basis of the reaction between 7 mM ABTS·+ and 2.45 mM K2S2O8 (1:1), kept in dark at room temperature for 16 h then diluted with methanol until reaching absorbance of 0.700 at 734 nm. 5 μl of each extract (contain 1.05 mg) was added to 3.995 ml ABTS·+ solution and left for 30 min. The absorbance inhibition percent was measured at 734 nm using the equation:
Where, AB is the absorbance of ABTS·+ and methanol; AA is the absorbance of ABTS·+ and extract.
Ascorbic acid and gallic acids were employed as standards.
2.3.3.4.Ferric reducing antioxidant power (FRAP) method was adopted by Benzie and Strain (1996) to determine the antioxidant potential of the extracts. This method aimed to reduce Fe3 + -TPTZ complex to Fe2+-tripyridyltriazine by the electron donating antioxidants. 300 mM acetate buffer with pH 3.6 were introduced to 10 mM TPTZ (2,4,6 – tripyridyl -s- triazine) and 20 mM FeCl3. 6 H2O in ratio of 10:1:1 to prepare FRAP solution. FeSO4 7H2O was used as Standard. 3.6 ml of FRAP solution were introduced to 0.395 ml water and left at 37˚ C for 5 min then mixed with 5 μl of the extract (contain 1.05 mg) and left for 10 min at 37˚ C. The absorbance was measured at 593 nm.
2.4 Determination of the antibacterial potential
2.4.1 Agar well diffusion technique
The antibacterial potential of the aqueous extracts was determined by the agar well diffusion technique according to the national committee for clinical laboratory standards (NCCLS, 1993; Valgas et al., 2007). The antibiotics, Ceftazidime and Ampicillin-Sulbactam were employed as positive controls.
2.4.2 Broth dilution technique
Stocks of the aqueous plant extracts were used to produce different dilutions in the range of 0.006 to 6.250 mg/ml. Each dilution was seeded with bacterial culture (106 cfu/ml) and incubated for 24 h at 37° C. The bacterial growth was observed as turbidity and the least concentration at which no turbidity was observed is the minimal inhibitory concentration (MIC) (NCCLS, 1993; Wiegand et al., 2008).
2.4.3 Bacterial strains
Bacillus cereus (ATCC®11778™), Bacillus subtilis (ATCC®19659™), Enterobacter cloacae (ATCC®23355™), Escherichia coli (ATCC®10536™), Klebsiella pneumonia (ATCC®10031™), Listeria innocua (ATCC®33090™), Listeria monocytogenes (ATCC®19115™), Proteus vulgaris (ATCC®49132™), Pseudomonas aeruginosa (ATCC®9027™), Salmonella enterica (ATCC®15479™), Salmonella typhimurium) ATCC®14028™ (, Staphylococcus aureus (ATCC®6538™), Staphylococcus epidermidis (ATCC®12228™) and Streptococcus pyogenes (ATCC®19615™).
The tested microorganisms were of animal origin and obtained from MERCIN center at Ain Shams University.
3 Results
3.1 Metabolic variables
The proximate composition of the investigated plants is presented in Fig. 1. T. polium recorded the maximum content of crude fats and total carbohydrates, while S. argel recorded the maximum of proteins. In the mean time, P. harmala recorded the minimum content of crude fats and total carbohydrates. A. fragrantissima attained the minimum value of proteins among the investigated species. T. polium and S. arghel expressed the highest nutritive value followed by A. fragrantissima while that of P. harmala showed the least nutritive value as presented in Fig. 2.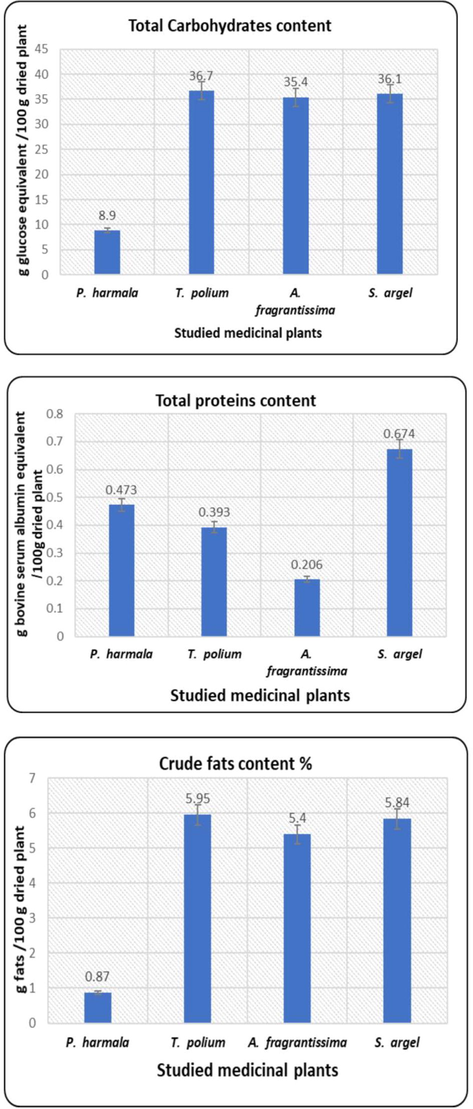
The estimated metabolic variables in the studied species.
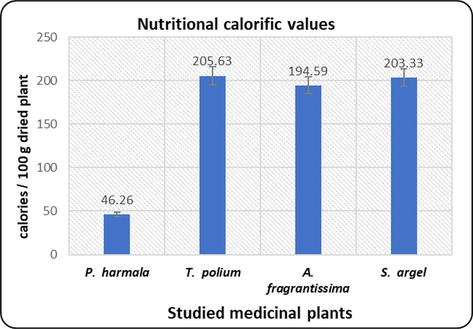
The nutritional calorific values of the studied plants.
3.2 Total phenolics and flavonoids
The values of phenolics varied from 3.34 ± 0.094 to 8.06 ± 0.322 gm gallic acid equivalent/100 gm dried plant while the flavonoids varied from 0.134 ± 0.004 to 2.013 ± 0.034 gm catechin equivalent/100 gm dried plant.
The highest phenolics content was in T. polium, followed by P. harmala, A. fragrantissima and S. argel, respectively. T. polium attained the highest flavonoids content, followed by A. fragrantissima, S. argel and P. harmala, respectively (Fig. 3).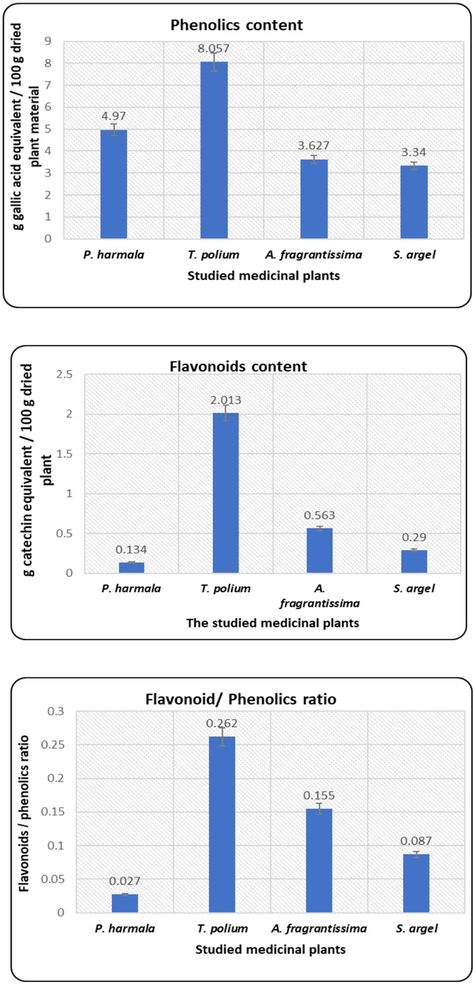
Total phenolics, flavonoids and flavonoids/ phenolics ratio in the extracts.
3.3 Evaluation of the antioxidant activity
The antioxidant scavenging activity of the extracts was determined using different assays as presented in (Table 2).
Plant Species
DPPH
FIC
ABTS
FRAP
IC50 (mg extract/ml)
EC50 (mg extract/gm DPPH)
AE
IC50 (mg extract/ml
% of Inhibition
mmol Fe (II)/gm extract
S. argel
1.20
35.25
0.028
1.27
23.77%
112.5
A. fragrantissima
0.15
4.13
0.242
0.149
73.54%
972.32
T. polium
0.07
1.84
0.544
0.068
81.11%
2185.71
P. harmala
4.70
130.73
0.008
4.706
7.81%
32.14
Ascorbic acid
0.02
0.610
1.64
0.022
88.86%
6589.29
Gallic acid
0.02
0.525
1.91
0.019
90.18%
7664.11
DPPH and FIC assays illustrated that T. polium extract expressed the highest antioxidant scavenging activity followed by A. fragrantissima while S. argel showed moderate activity and Peganum harmala found to be the lowest.
ABTS·+ radical cation decolorization assay showed that the aqueous extract of T. polium was the most active as it nearly scavenged 81.11% ABTS·+ followed by that of A. fragrantissima (73.54%), S. argel (23.77%) and P. harmala (7.81%), respectively.
Regarding the FRAP assay the antioxidant potential of each extract was evaluated on the basis of their ability to the reduction of TPTZ-Fe3+ complex to TPTZ-Fe2+ complex. The antioxidant potential of T. polium (2185.71 mmol Fe (II)/g extract) was higher than that of A. fragrantissima, S. argel and P. harmala, respectively.
The results revealed that the total flavonoids/ total phenolics ratio is directly proportional to the free radical scavenging activity of the infusions (Fig. 4).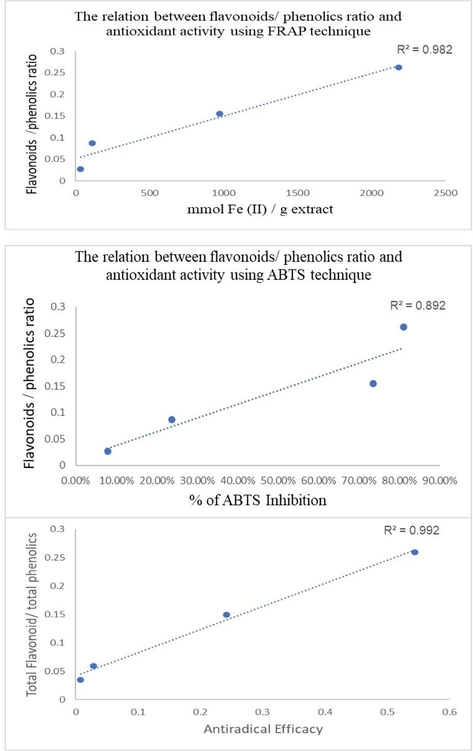
The relation between flavonoids/ phenolics ratio and antioxidant activity.
3.4 Antibacterial activity
T. polium exhibited the broadest antimicrobial spectrum against 64.28% of the tested pathogens followed by S. argel (57.24%), A. fragrantissma (50%) while the extract of P. harmal exhibited the least antimicrobial spectrum (42.86%) (Fig. 5). None of the extracts exhibited activity against S. aureus, S. epidermidis, E. cloacae, P. vulgaris and S. pyogenes (Table 3). *(CAZ): Ceftazidime, (SAM): Ampicillin/Sulbactam, R: Resistance, the diameter of the well (8.0 mm) is included in the measured zone of inhibition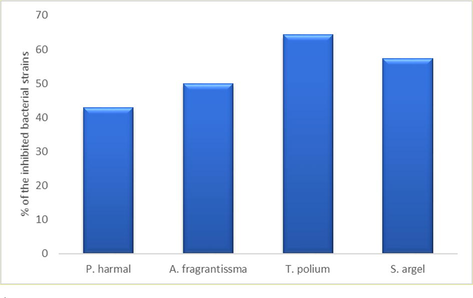
% of the inhibited bacterial strains by each of the tested extracts.
Pathogenic bacteria
Inhibition zones in millimeters
Standard Antibiotic
P. harmal
A. fragrantissma
T. polium
S. argel
SAM
CAZ
Gram negative bacteria
S. typhimurium
10
–
15
20
15
20
P. aeruginosa
14
14
19
15
R
19
E. cloacae
–
–
–
–
10
20
K. pneumoniae
15
16
17
15
15
28
P. vulgaris
–
–
–
–
12
25
E. coli
12
14
23
14
15
24
S. enterica
20
20
28
17
R
28
Gram positive bacteria
S. epidermidis
–
–
–
–
15
8
B. subtilis
–
18
25
20
20
24
B. cereus
–
15
20
12
13
7
S. aureus
–
–
–
–
12
20
S. pyogenes
–
–
–
–
R
25
L. innocua
11
13
17
14
17
18
L. monocytogenes
–
–
19
–
34
13
The MIC values < 100 μg/ml have been proposed to be highly active, 100–500 μg/ml active, 500–1000 μg/ml moderately active and 1000–2000 μg/ml less active (Silva et al., 2013). Accordingly, T. polium extract was highly active against S. enterica, B. subtilis, B. cereus and L. monocytogenes, active against E. coli, L. innocua and P. aeruginosa and moderately active against K. pneumoniae. A. fragrantissma was highly active against S. enterica and B. subtilis and active against E. coli, K. pneumoniae, B. cereus, L. innocua and P. aeruginosa. S. argel extract was highly active against S. typhimurium and B. subtilis, active against S. enterica and E. coli, moderately active against P. aeruginosa and L. innocua and with very low activity against B. cereus. P. harmal extract was active against K. pneumoniae and S. enterica as presented in Table 4.
Plant species
P. harmal
A. fragrantissma
T. polium
S. argel
Bacterial strains
MIC (mg sample/ml)
Gram negative bacteria
S. typhimurium
1.56
–
0.781
0.097
P. aeruginosa
1.56
0.390
0.390
0.781
K. pneumoniae
0.195
0.195
0.781
1.56
E. coli
1.56
0.390
0.195
0.390
S. enterica
0.390
0.049
0.097
0.195
Gram positive bacteria
B. subtilis
–
0.097
0.049
0.097
B. cereus
–
0.195
0.097
3.125
L. innocua
1.56
0.195
0.195
0.781
L. monocytogenes
–
–
0.097
–
4 Discussion
Herbal medicines and their use in treating and preventing certain diseases worldwide and in Egypt have increased potentially (Shaito et al., 2020). The primary metabolites estimated in the studied plants are essentially required due to their nutritional calorific value. The obtained results illustrated that T. polium, S. argel and A. fragrantissima contain appreciable levels of nutritive content within appropriate limits of using in feeding stuffs. Primary metabolites are essential for plant growth and development, besides their role in determining the nutritional quality (Sagwan et al, 2010; Tungmunnithum et al., 2018; Zargoosh et al., 2019).
T. polium expressed the highest antioxidant activity, phenolics and flavonoids content, followed by A. fragrantissima, S. argel and P. harmal, respectively. The therapeutic benefits of medicinal plants are often related to their antioxidant properties that attributed to the phenolics and flavonoids content in the extracts (Esmaeili et al., 2015). It was also clear from the results that, flavonoids/ phenolics ratio is directly proportional to the antioxidant activity of the extracts. Khazaei et al. (2018) reported that T. polium possesses antioxidant and free radical scavenging activity in addition to its effect against peroxidation.
The misuse of antibiotics in addition to the lack of developing new medications has forced the search for antimicrobials of natural origin (Othman et al, 2019). Medicinal plants possess potent pharmacological properties, lower toxicity, and economic potentiality due to the presence of phenolics, flavonoids, tannins and alkaloids (Shakya, 2016; Atef et al., 2019). Phytoactive components solely or in combination with antibiotics may be considered effective antimicrobials (Gupta and Birdi, 2017). The results proved variable antibacterial spectrum of the studied extracts with remarkable MIC values as T. polium exhibited the broadest spectrum followed by S. argel, A. fragrantissima and P. harmal, respectively. Accordingly, the activities observed in this study are. The results agree with that reported by Khazaei et al. (2018) that T. polium aqueous extract possesses broad antibacterial potential.
5 Conclusion
In conclusion, the studied medicinal plants could be used as source of nutritional supplements, antioxidants, drugs and antibacterial botanicals for combating of the studied pathogenic bacteria. The studied extracts could be used as food preservatives and as antibacterial that protect animals and human from pathogenic bacterial diseases.
Acknowledgement
The authors would like to thank Deanship of Scientific Research at Majmaah University, Kingdom of Saudi Arabia for supporting this work under Project Number (R-2021-81).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AOAC., 2016. Association of official analytical chemists. Official Methods of Analysis of AOAC International - 20th Edition, Editor: Dr. George W. Latimer, Jr Published by AOAC international suit 300 2275 research Blvd Rockville, Maryland 2850–3250, USA.
- Arlington, V.A., 1995. Oil in cereal adjuncts: petroleum ether extraction method. Association of Official Analytical Chemists, Method 945.16, in AOAC Official Methods of Analysis, 16th ed. AOAC.
- Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull. Natl. Res. Cent.. 2019;43(1)
- [CrossRef] [Google Scholar]
- Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol.. 2015;173:139-156.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Boulos, L., 1999-2002. Flora of Egypt. volums1-3. Al Hadara Publishing, Cairo, Egypt.
- Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev.. 2017;11(22):57-72.
- [Google Scholar]
- Evaluation of the biological activity of Capparis spinosa var. aegyptiaca essential oils and fatty constituents as Anticipated Antioxidant and Antimicrobial Agents. Prog. Chem. Biochem. Res.. 2019;2:211-221.
- [CrossRef] [Google Scholar]
- The antimicrobial, antioxidant, and anticancer activity of greenly synthesized selenium and zinc composite nanoparticles using Ephedra aphylla extract. Biomolecules. 2021;11(3):470.
- [CrossRef] [Google Scholar]
- Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol.. 2008;116(2):341-357.
- [Google Scholar]
- Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med.. 2017;8(4):266-275.
- [Google Scholar]
- Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem.. 2000;203:1-10.
- [Google Scholar]
- Review on Teucrium polium biological activities and medical characteristics against different pathologic situations. J. Contemp. Med. Sci.. 2018;4(1)
- [Google Scholar]
- Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover) Biomed Res. Int.. 2015;2015:1-11.
- [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J.. 2016;15(1):71.
- [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards), 1993. Performance Standard for Antimicrobial Disc Susceptibility Tests. Approved Standard. National Committee for Clinical Laboratory Standards, Villanova, P.A. Publication; M2-A5. USA.
- Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol.. 2019;10:911.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26(9-10):1231-1237.
- [Google Scholar]
- Biochemical estimation of primary metabolites from Pongamia pinnata (L.): an important biodiesel plant. Int. J. Pharm. Sci. Rev. Res.. 2010;5(1)
- [Google Scholar]
- A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric.. 1998;76(2):270-276.
- [Google Scholar]
- Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol.. 2020;11:422.
- [CrossRef] [Google Scholar]
- Silva, A.C.O., Santana, E.F., Saraiva, A.M., Coutinho, F.N., Castro, R.H.A., Pisciottano, M.N.C., Amorim, E.L.C., Albuquerque, U.P. 2013. Which approach is more effective in the selection of plants with antimicrobial activity? Evid.-Based Complementary Altern. Med. ECAM.; 2013:308980. doi: 10.1155/2013/308980.
- Free radical scavenging activity of an aqueous extract of potato peel. Food Chem.. 2004;85(4):611-616.
- [Google Scholar]
- Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. J. Food Chem.. 2008;108(2):519-532.
- [Google Scholar]
- Physicochemical basis for preferential uses of certain rice varieties. Qual. Plant Foods Hum. Nutr.. 1984;34:253-259.
- [Google Scholar]
- Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci.. 2018;19(6):1578.
- [Google Scholar]
- Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines (Basel, Switzerland). 2018;5(3):93.
- [Google Scholar]
- Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol.. 2007;38(2):369-380.
- [Google Scholar]
- Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc.. 2008;3(2):163-175.
- [Google Scholar]
- Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep.. 2019;9(1)
- [CrossRef] [Google Scholar]
- Research on antioxidant activity of flavonoids from natural materials. Food Chem.. 1999;64:555-559.
- [Google Scholar]







