Antibacterial activity of green tea leaves extracts against specific bacterial strains
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
There is an increase interest in the beneficial effects of green tea extracts which exhibits great activity against several diseases due to the presence of beneficial bioactive compounds. Green tea extracts were investigated for their anti microbial activity using specified Gram positive and Gram negative bacterial strains by specific methods. Therefore, this study aimed at evaluation of green tea aqueous, ethanol, and methanol extracts efficiency against potential gram positive and gram negative bacterial strains.
Methods
Fifty grams of dry leaves powder were used to prepare three different solutions with three different solvents; 80% ethanol, methanol, and sterile water; with a final concentration of 20% (g/ml). The flasks were placed for two days in a vibrator at 350 rpm at 15 °C. Four bacterial strains - two of them are gram positive and the other two are gram negative strains- were honored from King Fahd Educational Hospital. Qualitative phytochemical analysis technique was used to insure the presence of active compounds such as flavonoids, saponin, and tannins in plant extracts. Also, Gas chromatography mass spectrometry assay was used to determine extracts bioactive compounds. Seven types of antibiotics were used to determine minimum inhibitory concentration with two-fold dilution of plant extracts, by using agar plate method. While the minimum bactericidal concentration was performed using the pour plate technique.
Results
The three types of plant extracts showed high effectiveness against pathogenic bacterial strains. The highest value of inhibition was (4.3 ± 0.3) mm with ethanol extract against Bacillus subtilis. Gentamicin, Neomycin and Ciprofloxacin showed good values of inhibition against Gram-positive and gram-negative bacteria, while gram-negative bacteria showed resistance to Chloramphenicol, Methicillin, Vancomycin. On the other hand, Gram positive bacteria showed resistance to the Colistin only.
Conclusion
Green tea extracts showed effective anti microbial activity against different types of gram positive and negative bacterial strains as they have a good inhibitory and bactericidal activity due to the presence of special bioactive components such as ECGC and catechins.
Keywords
Antibacterial activity
Bactericidal
Green tea
Antibiotic
1 Introduction
In recent years, green tea plant (Camellia sinensis) which is belonging to family theaceae, has been considered as the most common beverge worldwide, due to its potential health benefits. It is consumed in different countries as green, black and Oolong tea (Sarwa et al., 2013). Previous studies have reported that green tea has great rule in the recovery from cancer, cardiovascular disease, obesity, diabetes, oral health, bone health. Moreover, it helps in clearing bad breath by killing bacterial living in throats. Green tea also improves healing from burns and wounds (Jigisha et al., 2012; Sarwa et al., 2013; Gupta et al., 2014; Pérez-Burillo et al., 2021). The composition of green tea leaves differ and vary with climate, season and the type of tea itself. The beneficial of green tea extracts are attributed due to the presence of polyphenolic compounds, polysaccharides, Vitamin B, C, E andamino acids (Sarwa et al., 2013).
Antibiotics provide the main basis for the therapy of microbial infections. Recently, antibiotic resistance is one of the most biggest issues in the world of antibiotics industry, different resistant strains emerged after the discovery of antibiotics more than 50 years ago (Chatterjee et al., 2009; Adil et al., 2018; Adel et al., 2019). The overuse of antibiotics leads to the emergence of multi-drug resistance of several microorganisms. Thus, in this increase of antibiotic resistance microbes, there is a big demand to find new antimicrobial agents. The use of herbal medicines is increased around the globe, as many humans now turn to these natural remidies to treat different infections (Organization, 2004). The pharmacological value of plants is because of some chemicals compounds that affect and have a specific physiological effect on the human body. Some of these biologically active components in plants are flavonoids, alkaloids, phenolic compounds and tannins, (Sarwa et al., 2013; Gupta et al., 2014; Pérez-Burillo et al., 2021). Ninety years ago, the antimicrobial activity of green tea was known, due to the presence of polyphenol main component (EGCG) (Fanaki et al., 2008). It was approved that extracts of green tea leaves have an antimicrobial strength that can kill many types of bacteria strains such as Salmonella spp., Enterococcus spp.,Escherichia coli, Staphylococcus aureus. As well as an antifungal effect for some fungals such as Candida albicans and an anti virus effect for many viruses such as HIV and herbes (Jigisha et al., 2012).
Ikigai et al, 1993 conducted a research study about using aqueous green tea extract against some of the Gram negative bacteria such as E. coli K-12 Strain G6 and Gram positive bacteria such as Staphylococcus aureus ATCC25932; they revealed that Gram positive strains were affected in a stronger way than Gram negative.
Cho et al, 2007 reported that the growth of E.coli can be inhibited green tea polyphenols compounds. The way of action of these compounds depends on the changing of cell membrane fatty acid composition. Araghizadeh et al, 2013 summarized that the activity of aqueous green tea extract on twenty isolated gum and teeth strains from each of the Streptococcus mutans, Aggreg actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia, showed that green tea extract had a strong anti-bacterial activity on S. mutans, A. actinomycetemcomitans, P. gingivalis and P. intermedia. Another research of Yang and Zhang, 2019, they studied the antimicrobial activity of aqueous and methanolic green tea extracts against E.coli, Bacillus and Proteus. They concluded that aqueous extract is less active against the six isolates of bacteria than the methanolic extract, which showed the maximum antibacterial activity. This study aimed at estimating the antimicrobial activity of green tea leaves crude extracts against selected bacterial strains using three different types of solvent for extraction.
2 Materials and methods
2.1 Sampling of green tea and crude extracts preparation
A healthy and mature Green tea leaves were sampled from a local shop in the city of Dammam-Saudi Arabia and were characterized using the Manual of Arriyadh Plants (Site, 2014). Collected leaves were washed, cleaned and dried at room temperature in the shade with continuous stirring to prevent them from rot. An electric grinder was used to obtain a fine powder. Fifty grams (50 g) of the dried powder were mixed in 100 ml of hot sterile water and other solvents (e.g. methanol, 80%ethanol and distilled water). A 250 ml of each solvent were put in a 500 ml conical flask until a final concentration of 20% (g/ml) was reached. The flasks were placed for two days in a vibrator at 350 rpm at 15 °C. Solutions were filtered by using sterile bacterial filters, and then 100 ml of each filtered extract were taken and placed in the oven at 80 °C to dry and then they were kept at 4 °C further for use. For the preparation of crude extract for antibacterial assay, sterile distilled water was used for aqueous extract and dimethyl Sulfoxxide (DMSO) for organic ones (Parvez and Shariare, 2019).
2.2 Test microorganisms
Four bacterial strains were honored from King Fahd Educational Hospital. They were: Bacillus subtilis and Staphylococcus aureus ATCC24213 Gram positive and Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC259 Gram negative.
2.3 Analyzing the presence of effective compounds qualitatively
According to (Edeoga et al., 2005), it was proved that green tea extract contains effective compounds; the flavonoids was detected by adding 2 ml of plant extract to 3 ml of 2% ammonia with 1 ml of concentrated sulfuric acid. The yellow precipitate insures a positive flavonoid. Saponins was revealed by adding 5 ml of plant extract with 3 ml of distilled water, with a continuous shaking for 5 min, the appearance of a 2 cm layer of foam is a positive result. The existence of tannins was reported by placing 3–5 drops of 0.1% ferric chloride solution into 3 ml of plant extracts. The brown-green precipitate is a positive one. Protein was detected by adding equal volumes of plant extracts with concentrated sulfuric acid (1:1v/v). A white precipitate insures the presence of protein. Carbohydrates were detected by mixing equal volumes of plant extract Benedict reagent. After boiling in a water bath, a brown–red color precipitate was appeared.
2.4 Gas Chromatography-Mass spectrometry analysis
According to the method described by Ababutain, 2015, bioactive compounds of green tea leaves were identified by using a QP2010 SE Spectrometer and 30 m, 0.25 mmID, 0.25-μm df dimethyl polysiloxane capillary column, 5 Sil MS 5% dipheny l/95% with 5 Sil MS 5% dipheny l/95% dimethyl polysiloxane capillary column (30 m, 0.25 mmID, 0.25-μm df).
2.5 Antibacterial susceptibility assay
Antibacterial sensitivity of the bioanalyse company was screened by using the following discs. Chloramphenicol (C30 mcg), Vancomycin(VA30 mcg), Ciprofloxacin (CIP5 mcg), Gentamicin (CN10 mcg), Methicillin (MET5 mcg), Neomycin (N10 mcg) and Colistin (CT10 mcg). The inhibition zone of each antibiotic was then measured by using a millimeter scale.
2.6 Screening for antibacterial activities
According to National Committee for Clinical Laboratory Standards, the agar well diffusion method was used to investigate the antibacterial effects of green tea extract (Ferraro, 2000). After the preparation of media, 1 mm of bacteria was implanted in sterile plates and left for (18–24) hours, then a 50 μL of plant extract were poured into a number of previously made 5 mm diameter holes. The inoculated agar plates were incubated at 37 °C for 2 days. Negative controls were made by using solvents without plant material, methanol, water, and ethanol. The antibacterial ampicillin (AML 10 mcg) was used as positive control. Then clear zones around the holes were measured in millimeters. Each experiment was repeated three times for confirmation.
2.7 Measuring the minimum inhibitory concentration (MIC)
According to (Khan and Rosina et al., 2012), two fold dilution methods were used to measure the minimum inhibitory concentration (MIC) using 96 microtitre plates. The plates were incubatioed at 37 °C and the absorbance was read at 600 nm wavelength,. The lowest reading concentration was recorded as a MIC. Each experiment was repeated three times.
2.8 Measuring the minimum bactericidal concentration (MBC)
As described in NCCLS, the pour plate technique was used to measure the concentrations of plant extracts using a zero turbidity of MBC, The plant extract concentrations of MIC and higher concentrations were sub-cultured one at the time in petri plates. A 12 ml of the dissolved nutrient agar media was poured over test inoculums with gentle mixing. Then it was placed in the incubator for a day at 37 °C. The lowest concentration without colonies was recorded as MBC. All trials were conducted in triple.
2.9 Statistical analysis
SPSS, 2007 (Ver. 17.0) was used in calculating the ANOVA. To investigate the ability of plant extract to inhibit selected bacterial strains, and significance value was measured at p < 0.01.
3 Results and discussion
3.1 Plant yields
Table 1 showed that solvent type has an effect on plant yields. This agreed with (Padalia and Chanda, 2015). The results revealed that organic solvents were the most effective, the plant yield was highest with a value of 18.32 g, followed by the water solvent with a value of 13.68 g, the results agree with the study of (Do et al., 2014), in that the ethanol extract was the highest solvent effectiveness in plant extraction.
3.2 Qualitative phytochemical analysis
Table 2 showed the qualitative phytochemical analysis of green tea leaves extracts. The results showed that carbohydrate compounds were present in all type of extracts. While, there was no extract have Saponin compounds. The tannins complex did not appear in the aqueous extract. Whereas, the protein was present only in the aqueous extract. Flavonoids appeared in all extracts except in the methanol extract. We noted that ethanol and water are the best solvents in extraction of effective compounds which in agree with the study of (Dailey and Vuong, 2015), who stated that the type of solvent has a real effect on the extracted compounds and recommended the use of ethanol alcohol in the extraction method. As mentioned before, green tea plant contains tannins, flavonoids, fluoride, vitamins and other metals (Bérubé-Parent et al., 2005; du Toit et al., 2001). Tannins are synthetic materials that have a strong antibacterial effect (Barel et al., 2014).
| Vitex extract solvent | Saponins | Tannins | Flavonoids | Carbohydrate | Protein |
|---|---|---|---|---|---|
| Ethanol | – | + | + | + | – |
| Water | – | – | + | + | + |
| Methanol | – | + | – | + | – |
-, not exist. +, exist. ++, highly exist.
3.2.1 Gas Chromatography mass (GC-MSs) analysis
The results of Gas Chromatography mass analysis of ethanol, methanol, and water extracts of green tea showed differences in the number of chemical components (Table 3 and Figs. 1-3). The highest number of the chemical compounds achieved by the ethanol extract was 112, followed by the methanol extract at 82 and the last least aqueous extract with 50 compounds, which is compatible with the study of (Ababutain, 2015; Dailey & Vuong, 2015). The results proved that those extracts contain other compounds as shown in Table 3. Those compounds proved their medical importance in other studies, such as D-Allose, Maltol, Vitamin E, Oleic Acid, Benzoic acid Hexadecanoic acid, ethyl ester, Stigmasterol, Phytol, n-Hexadecanoic acid.
| No | Compound name | Peak Area% | Biological activity | ||
|---|---|---|---|---|---|
| EL | ML | WL | |||
| 1 | Vitamin E |
– | – | 21.178 | Improve cell immunity (Gay & Meydani, 2001) |
| 2 | 4,5-Dichloro-1,3-dioxolan-2-one | 7.111 | 7.018 | 7.016 | No activity reported or found |
| 3 | Maltol | 8.827 | – | 8.274 | Antimicrobial (Jay & Rivers, 1984) |
| 4 | Oleic Acid | 20.644 | – | – | A weak effect on some bacteria (Marounek et al., 2002) |
| 5 | Benzoic acid | – | 14.491 | 9.054 | Increase in soil bacterial and fungal (Liu, Li, Jia, Zhang, & Wang, 2017) |
| 6 | Hexadecanoic acid-ethyl ester | 19.600 | – | – | Antioxidant, Flavor, Anti-androgenic, Nematicide, Hemolytic, Hypocholesterolemic (Tyagi & Agarwal, 2017). |
| 7 | Linalyl acetate | 10.722 | – | 9.103 | No activity reported or found |
| 8 | Stigmasterol | – | – | 26.208 | Antimicrobial activity(Yinusa et al., 2014) |
| 9 | Erythritol | 8.057 | – | – | No activity reported or found |
| 10 | Phenylethyl Alcohol | – | 8.357 | No activity reported or found | |
| 11 | D-Allose | – | 9.990 | – | Anti-oxidative activity (Ishihara et al., 2011) |
| 12 | Linalyl acetate | 10.722 | – | – | No activity reported or found |
| 13 | Bornyl acetate | 11.329 | – | – | No activity reported or found |
| 14 | Geranyl acetate | 12.533 | – | – | No activity reported or found |
| 15 | Homoserine | 14.491 | 8.874 | No activity reported or found | |
| 17 | Methyleugenol | 12.854 | – | – | No activity reported or found |
| 18 | Caryophyllene | 13.319 | – | – | No activity reported or found |
| 19 | Phytol | 20.747 | 12.088 | – | Antimicrobial, Anticancer, Anti-inflammatory(Tyagi & Agarwal, 2017). |
| 20 | n-Hexadecanoic acid |
19.279 | 11.716 | – | Antioxidant, Nematicide, Hypochloesterolemi, Antiandrogenic, pesticide (Tyagi & Agarwal, 2017). |
| 21 | Humulene | 13.389 | – | – | No activity reported or found |
| 22 | Ferruginol | 22.857 | – | – | No activity reported or found |
| 23 | Succinimide | – | 14.305 | 8.758 | No activity reported or found |
EL, Ethanol green tea leaves extract. ML, Methanol green tea leaves extract. WL, Water green tea leaves extract.
- GC/MS chromatogram of ethanol leaves extract of Green tea.
- GC/MS chromatogram of Methanol leaves extract of Green tea.
- GC/MS chromatogram of water leaves extract of Green tea.
3.3 Antibacterial susceptibility assay
As showed in Table 4, all Gram-positive bacteria were affected by all antibiotics used in the study but were not affected by Colistin (CT10 mcg), on the other hand all Gram-negative bacteria were affected by all antibiotics except Chloramphenicol (C30 mcg), Methicillin (MET5) mcg), Vancomycin (VA30 mcg).
| No. | Antibacterial discs | Mode of action | Zone of inhibition (mm) ± Standard Deviation | |||
|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram postive Bacteria | |||||
| E.coli | P.aeruginosa | S. aureus | B. subtilis | |||
| 1 | CN | Target protein synthesis 30S inhibition | 17.0 ± 0.6 | 15.0 ± 0.7 | 15.7 ± 0.8 | 13.5 ± 0.5 |
| 2 | N | Damaging bacterial DNA | 12.5 ± 0.5 | 14.5 ± 0.5 | 13.0 ± 0.7 | 14.0 ± 0.2 |
| 3 | CIP | Target DNA gyrase | 28.5 ± 0.5 | 29.0 ± 0.2 | 24.0 ± 0.4 | 29.5 ± 0.5 |
| 4 | C | Target protein synthesis 50S inhibition | R | R | 21.6 ± 0.7 | 27.0 ± 0.2 |
| 5 | MET | Inhibit cell wall synthesis | R | R | 19.6 ± 0.8 | 31.0 ± 0.9 |
| 6 | VA | Inhibit cell wall synthesis | R | R | 15.0 ± 0.8 | 20.4 ± 0.4 |
| 7 | CT | Target membrane function | 9.5 ± 0.5 | 17.0 ± 0.5 | R | R |
R: resistance, Gentamicin (CN 10 mg), Neomycin (N10 mcg), Ciprofloxacin (CIP5 mcg), Chloramphenicol (C30 mcg), Methicillin (MET5 mcg), Vancomycin (VA30 mcg), Colistin (CT10 mcg).
3.4 Screening for antibacterial activities
In this study, the effect of green tea leaves extracts were tested by measuring the non-growth zone of Gram -ve bacteria (E. coli ATCC25922 and P. aeruginosa ATCC27853) and Gram + ve bacteria (S. aureus ATCC24213 and B. subtilis). The data presented that the methanolic extract gave the highest zone of inhibition against P. aeruginosa ATCC27853 (1.7 ± 0.2) and E. coli ATCC25922 (1.3 ± 0.2). While, methanolic and ethanolic extracts showed the highest activity against B. subtilis strains with zone of inhibition measured about (3.7 ± 0.2 and 4.3 ± 0.3) each solvent respectively. This is consistent with the results obtained by Dailey and Vuong, 2015 and the study of (Ikigai et al., 1993), that green tea extracts has more activity against + ve Grams than Gram -ve bacteria membranes. Data obtained were compatible too with Ababutain, 2015; Arokiyaraj et al., 2009 as well as the solvent type is an important factor which affects the antimicrobial effect of the plant extract (Table 5 and Fig. 4).
| Test Microbes | Zone of inhibition (mm) ± Standard Deviation | ||||||
|---|---|---|---|---|---|---|---|
| Ethanol extract | ENC* | Water extract | ANC* | Methanol extract | MNC* | Ampicillin (10mcg) ** | |
| G-ve bacteria | |||||||
| E. coli ATCC25922 | 0.5 ± 0 | 0 | 1.4 ± 0.1 | 0 | 1.3 ± 0.2 | 0 | R |
| P. aeruginosa ATCC27853 | 1.5 ± 0.3 | 0 | 1 ± 0.3 | 0 | 1.7 ± 0.2 | 0 | R |
| G + ve bacteria | |||||||
| S. aureus ATCC24213 | 1.6 ± 0 | 0 | 1 ± 0.1 | 0 | 1.5 ± 0.3 | 0 | 12 |
| B. subtilis | 4.3 ± 0.3 | 0 | 1 ± 0 | 0 | 3.7 ± 0.2 | 0 | 18 |
| Significance (p ≤ 0.01) | 0.000 | – | 0.003 | – | 0.000 | – | – |
*ENC, Ethanol negative control. MNC, Methanol negative control.ANC, Water negative control. **, Positive Control. R, Resistant.
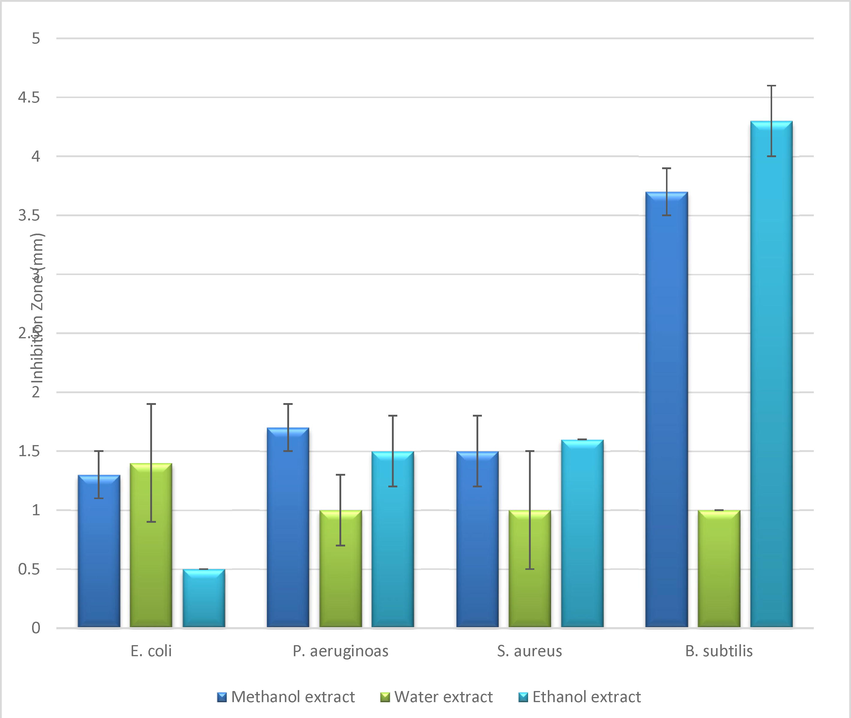
- Antibacterial activity of Green tea leaves extracts at concentration of 20% by using well diffusion assay.
3.5 Measuring the minimum inhibitory concentration (MIC)
Table 5 showed the MIC of the plant extracts against tested bacterial strains. Results showed that minimum inhibitory concentration of all extract against Gram negative bacteria was 12.5 µg|ml except for ethanol extract against P. aeruginoas, it was 25 µg| ml. On the other hand, the minimum inhibitory concentration of methanol extract against Staphylococcus aureus was the least (3.125 µg|ml) and the highest concentration of inhibition was for Bacillus subtilis (25 µg|ml). Results revealed that green tea extracts with minimum inhibitory concentration work effectively against Gram negative bacterial strains which is consistent with Reygaert, 2014.
3.6 Measuring the minimum bactericidal concentration (MBC)
Table 5 shows that the plant extracts have an effect on all bacterial strains with MBC values ranging from 12.5 to 50 µg| ml. The effect of the extract of ethanol and methanol is less than the effect of the aqueous extract which exhibits the highest MBC with 50 µg| ml, while the values for the ethanol and methanol extract ranged between 12.5 and 25 µg|ml. This is because of the compounds extracted according to the solvents used (Ababutain, 2015; Dailey and Vuong, 2015).According to Gopal et al, 2016 study, green tea has an active antibacterial compound, the EGCG, which plays an effective role in HIV and Staphyloccocus aureus restriction. The dry weight of greens tea contains about 25–35% of catechins. They consist of two benzene rings A and B, where they play a great role in damaging the bacterial cell membrane. In E.coli, ECGC compound can improve biofilms destruction by interrupting the bacterial cell membrane and degrading its exopolysaccarides (Table 6).
| Test Microbes* | Ethanol extract | Water extract | Methanol extract | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| G-ve bacteria | ||||||
| 1 | 12.5 | 25 | 12.5 | 50 | 12.5 | 50 |
| 2 | 25 | 50 | 12.5 | 25 | 12.5 | 25 |
| G+ve bacteria | ||||||
| 3 | 6.25 | 12.5 | 12.5 | 50 | 3.125 | 12.5 |
| 4 | 6.25 | 12.5 | 25 | 50 | 6.25 | 25 |
4 Conclusion
The study showed that green tea plant extracts with ethanol, methanol, and water solvents have a role in eliminating gram-positive and gram-negative bacteria under study by using the agar well diffusion technique, the MIC method and the MBC method. In the study, the presence of many compounds in green tea extract was also detected. While there are a group of compounds that have not been studied or researched and are considered new compounds. Therefore we recommend using green tea and drinking it after eating in order to eliminate the microbes in the food. Especially that in our study we proved the role of green tea against some positive and negative bacteria.
Acknowledgement
In this study, I would like to express my special thanks to everyone who helped me completing this work in Al-Rayyan Campus - College of Science- in Imam Abdulrahman bin Faisal University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of solvent type on antibacterial activities of Lawsonia inermis leaves. J. Food Agric. Environ.. 2015;13(1):51-53.
- [Google Scholar]
- Impact of citral and phloretin, alone and in combination, on major virulence traits of Streptococcus pyogenes. Molecules. 2019;24(23):4237.
- [Google Scholar]
- Application of medicinal plants as a source for therapeutic agents against Streptococcus pyogenes infections. Curr. Drug Metab.. 2018;19(8):695-703.
- [Google Scholar]
- Inhibitory activity of green tea (Camellia sinensis) extract on some clinically isolated cariogenic and periodontopathic bacteria. Med. Princ. Pract.. 2013;22(4):368-372.
- [Google Scholar]
- Phytochemical analysis and antibacterial activity of Vitex agnus-castus. Int. J. Green Pharm. (IJGP). 2009;3:(2).
- [Google Scholar]
- Handbook of cosmetic science and technology. CRC Press; 2014.
- Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br. J. Nutr.. 2005;94(3):432-436.
- [Google Scholar]
- In vitro synergistic effect of doxycycline & ofloxacin in combination with ethanolic leaf extract of Vangueria spinosa against four pathogenic bacteria. Indian J. Med. Res.. 2009;130(4):475.
- [Google Scholar]
- Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol.. 2007;55(6):501-506.
- [Google Scholar]
- Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric.. 2015;1(1):1115646.
- [Google Scholar]
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22(3):296-302.
- [Google Scholar]
- Comparison of the antioxidant content of fruits, vegetables and teas measured as vitamin C equivalents. Toxicology. 2001;166(1–2):63-69.
- [Google Scholar]
- Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol.. 2005;4(7):685-688.
- [Google Scholar]
- Influence of Aqueous green tea extract on the antimicrobial activity of some antibiotics against multiresistant clinical isolates. J. Med. Microbiol.. 2008;17(3)
- [Google Scholar]
- Ferraro, M.J.,2000. Performance standards for antimicrobial disk susceptibility tests: NCCLS.
- The effects of vitamin E, vitamin B6, and vitamin B12 on immune function. Nutr. Clin. Care. 2001;4(4):188-198.
- [Google Scholar]
- Bactericidal activity of green tea extracts: the importance of catechin containg nano particles. Sci. Rep.. 2016;6:19710.
- [CrossRef] [Google Scholar]
- Green tea: a review on its natural anti-oxidant therapy and cariostatic benefits. Biol. Sci. Pharm. Res. 2014;2:8-12.
- [Google Scholar]
- Bactericidal catechins damage the lipid bilayer. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1993;1147(1):132-136.
- [Google Scholar]
- Antioxidant properties of rare sugar D-allose: effects on mitochondrial reactive oxygen species production in Neuro2A cells. J. Biosci. Bioeng.. 2011;112(6):638-642.
- [Google Scholar]
- Antimicrobial activity of some food flavoring compounds. J. Food Saf.. 1984;6(2):129-139.
- [Google Scholar]
- Green tea: a magical herb with miraculous outcomes. Int. Res. J. Pharm. 2012;3(5):139-148.
- [Google Scholar]
- Khan, Rosina, et al., 2012. In vitro and in vivo inhibition of Streptococcus mutans biofilm by Trachyspermumammi seeds: an approach of alternative medicine. Phytomedicine 19(8-9) 747-755 Bandaranayake, W.M., 2006. Quality control, screening, toxicity, and regulation of herbal drugs. Modern Phytomed. 1, 25-57.
- Effect of benzoic acid on soil microbial communities associated with soilborne peanut diseases. Appl. Soil Ecol.. 2017;110:34-42.
- [Google Scholar]
- Effect of caprylic, capric and oleic acid on growth of rumen and rabbit caecal bacteria. J. Anim. Feed Sci.. 2002;11(3):507-516.
- [Google Scholar]
- Organization, W.H., 2004. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems: World Health Organization.
- Antimicrobial efficacy of different solvent extracts of Tagetes erecta L. flower, alone and in combination with antibiotics. Appl. Microbiol.: open access. 2015;1(1)
- [Google Scholar]
- Antibacterial activities of green tea crude extraxts and synergistic effects of epigallocaechingallate (ECGC) with gentamicin against MDR pathogens. Heliyon. 2019;7(5)
- [Google Scholar]
- Extraction of green tea leaves: the use of different methods, their optimization and comparative evaluation. Biosci. Biotechnol. Res. Asia. 2013;10(1):383-386.
- [Google Scholar]
- Site, A.-R.C.W., 2014. High Commission for the Development of Ar-Riyadh.
- SPSS, 2007. SPSS for Windows, Base System User's Guide, Release 17.0. University of Sussex, USA., Page: 224. 26.
- Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacognosy Phytochem.. 2017;6(1):195-206.
- [Google Scholar]
- Antimicrobial activities of tea polyphenol on phytopathogens: a review. Molecules. 2019;24(4):816.
- [Google Scholar]
- Bioactivity of stigmasterol isolated from the aerial part of Spillanthes acmella (Murr) on selected microorganism. Int. J. Curr. Microbiol. App. Sci.. 2014;3(2):475-479.
- [Google Scholar]







