Translate this page into:
Anti-microbial and anti-cancer activity of gold nanoparticles phytofabricated using clerodin enriched clerodendrum ethanolic leaf extract
⁎Corresponding authors. mrganesh2000@hotmail.com (Ganesh Munuswamy-Ramanujam), rvenkatmpharm@gmail.com (Venkatalakshmi Ranganathan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Search for options that can overcome microorganism’s resistance to traditional drugs, has shifted the focus towards plant-based molecules and extracts. Latest developments in plant-based nanomaterials have further expanded the role of plant extracts since nano particle - plant extract combinations are shown to possess enhanced biological activity and reduced toxicity to human cells. In the present study, facile, one-pot synthesis of nearly monodispersed gold nanoparticles was developed using Clerodin enriched Clerodendrum infortunatum ethanolic leaf extract under ambient conditions. The synthesized gold nanoparticles were evaluated for broad spectrum anti-microbial activity as well as compatibility with human peripheral blood mononuclear cells.
Methods
Clerodin enriched fraction from C. infortunatum leaves ethanol extract was used to synthesize gold nanoparticle from chloroauric acid. Synthesized gold nanoparticles were characterized and tested for broad spectrum anti-microbial activity against Staphylococcus aureus, Bacillus cereus, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa using the standard broth dilution method. Cytotoxicity of the gold nanoparticles was evaluated against human monocyte leukemic cells (THP-I) and healthy peripheral blood mononuclear cells. MTT and FACS based Annexin V/FITC-PI assays were used to study its cytotoxic effect.
Results
TEM analysis showed an average diameter of 33 ± 5 nm for the synthesized nanoparticles. EDX validated the presence of elemental Au (98.8%) and confirmed its nano form. Antimicrobial assay of gold nanoparticles showed significant inhibitory effect in all the tested pathogens. Additionally, MTT and Annexin-V/ PI assay demonstrated the cytotoxic activity of the nanoparticles against THP-I cells. Interestingly, nanoparticles did not show cytotoxicity against healthy human peripheral blood mononuclear cells.
Conclusion
Gold nanoparticles generated in an eco-friendly process using Clerodin enriched fractions showed promising broad spectrum antibacterial activity. Simultaneously, they proved themselves to be benign against healthy human cells indicating potential therapeutic applications. Added benefit for the nanoparticles was, they had anti-proliferative effects against human leukemic monocyte cancer (THP-I) cells.
Keywords
Clerodin
Clerodendrum infortunatum
Gold nanoparticles
Antimicrobial activity
Cytotoxicity
- CGNPs
-
Clerodendrum Gold nanoparticles
- GNPs
-
Gold nanoparticles
- PI
-
Propidium Iodide
- FITC
-
Fluorescein isothiocyanate
- EDX
-
Energy dispersive X-Ray analysis
- TEM
-
Transmission electron microscopy
- CLSM
-
Confocal laser scanning microscopy
- FACS
-
Fluorescence-activated cell sorting
- PBMNCs
-
Peripheral Blood Mononuclear Cells
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- FBS
-
Fetal bovine serum
- DDW
-
Double-distilled water
- RPMI media
-
Roswell Park Memorial Institute media
- PBS
-
Phosphate- buffered saline
- DMSO
-
Dimethylsulfoxide
- HEPES
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- SAED
-
Selected Area Electron Diffraction
- PS
-
phosphatidylserine
- MIC
-
Minimum Inhibitory Concentration
Abbreviations
1 Introduction
Advances in nanotechnology have improved productivity and operation at the nanoscale. However, in terms of therapeutic options, nanotechnology is still in its nascent stage but gaining importance (Hornos Carneiro and Barbosa 2016, Chauhan et al., 2021). In this, metallic nanoparticles are of interest, since their size and shape can be tailored to influence their functionality. Literature reports indicate the ability of phytochemical extracts from plants to reduce metal ions from dissolved metal salts to generate nanoparticles (Das et al., 2013; Geetha et al., 2013; Phukan et al., 2016; Mashwani et al., 2016). Generating nanoparticles via these “green methods” has dual advantage. It adds bio value to the chosen plant extract as well as identifying novel methods for nanoparticle synthesis (Fu et al., 2017). Medicinal plant extracts are considered key candidates for such nanoparticle synthesis (Fu et al., 2017; Liu et al., 2019; Sengani et al., 2017). Since these nanoparticles have enhanced bioactivity with potential for numerous biomedical applications. Some of these biomedical applications that have been reported included bioimaging, targeted drug therapy, anti-cancer activity, anti-microbial activity, etc. (Ahmad et al., 2017; Fu et al., 2017; Katti et al., 2009; Park et al., 2011; Phukan et al., 2016; Rao et al., 2016). To be utilized as a treatment agent, the nanoparticle should be biocompatible and should be easily surface functionalized (Mitchell et al., 2021). Gold Nanoparticles (GNPs) are proven to have the above mentioned abilities, making them suitable drug candidates (Kong et al., 2017). GNPs have also showed increased bioavailability and aqueous solubility for the bioactive metabolites making them ideal candidates (Patra et al., 2018). Hence the objective of the current study is to generated GNPs using green synthesize methodology and study its bioactive profile.
Compounds and extracts from plant sources are being constantly explored as viable antimicrobial and anticancer therapeutics (Álvarez-Martínez et al., 2021; Mitchell et al., 2021). Such explorations help in identifying novel bioactive lead molecules and drugs (AlSheikh et al., 2020). Factors like reduced side effects, enhanced host immunity and possible ability to prevent antibiotic resistance add to the attractiveness of plant based molecule (Vaou et al., 2021). There is also a strong belief that, generating drugs via “plant to patient route” would be more viable. Clerodendrum infortunatum is a widely used medicinal plant which contains an active component, clerodin. Studies show that C. infortunatum leaf extracts are primarily known to have antifeedant, cytotoxic, antioxidant, anti-inflammatory and antimalarial properties (Goswami et al., 1998; Kong et al., 2017). Existing reports suggest that Clerodanes isolated from various species of Clerodendrum have a wide range of bioactivity (Kong et al., 2017; Pallab Kar et al., 2014; Shendge et al., 2017). Clerodin (C24H3407) isolated from C. infortunatum, is a type of clerodane. It is a bicyclic terpenoid with a hydroxy group, an acetate functionality, a dihydrofuran ring and an epoxide functional group (Sindhu et al., 2020). Based on earlier literature reports, it would of interest to further mine clerodin or its enriched extract for other types of bioactivity-based applications. Since very little information is available in this regard, the current study focuses on utilizing Clerodin enriched extract to synthesize GNPs and study its impact on the overall bioactivity profile. It is expected that favorable findings from this study will add value to the Clerodendrum plant extract as a source for generating GNPs with therapeutic value.
Present study reports an easy, one-pot, sustainable synthesis of gold nanosuspension (CGNPs) using Clerodin enriched Clerodendrum ethanolic extract. The synthesized CGNPs were tested for broad spectrum anti-bacterial activity against gram-positive and gram-negative bacteria. Further, the CGNPS were also tested for their biocompatibility and anticancer activity against human PBMNCs and human leukemia cell lines, THP-1 respectively.
2 Materials and methods
2.1 Chemicals and reagents
Tetra chloroauric (III) acid, sodium hydroxide, Resazurin, Hydrogen peroxide, PI, MTT, and Fetal bovine serum (FBS) were procured from Sigma-Aldrich, USA. All other chemicals and reagents were from standard commercial sources and of the highest quality available. Annexin –V Fluorescein isothiocyanate (FITC) /PI staining kit-BD-pharmingen was procured from BD Biosciences, USA. Clerodin used in the study was isolated from C. infortunatum and characterized using spectroscopic techniques.
2.2 Preparing Clerodin enriched C. infortunatum Linn leaf extract
C. infortunatum leaves were shade dried, powdered and extracted with ethanol. Further, Ethanolic extract was dried and subjected to silica gel (60–120) column chromatography. The column was eluted first with Hexane, followed by 26%, 50% and 75% ethyl acetate in hexane. The eluted fraction was monitored with TLC along with standard clerodin (Fig.S1-S3). Fractions collected with 26% EtOAc/Hexane showed presence of clerodin and were subsequently used for GNPs synthesis.
2.3 Phyto fabrication of GNPs
Synthesis of CGNPs was done by mixing 9 ml aqueous solution of chloroauric acid (HAuCl4) (0.5 mM) and 1 ml aqueous solution (0.1 mg/mL − 1 mg/mL) of enriched ethanolic extract fractions. Extract suspension was added dropwise to the HAuCl4 solution under vigorous stirring at different temperatures (26 °C- 80 °C) and monitored for color change. The resulting mixture was centrifuged at 12000g for 15 min, washed with Double-distilled water (DDW) twice. The pellet was re-suspended in MilliQ water and used for further characterization and future assays.
2.4 Physical characterization of CGNPs
UV–Vis spectra of the synthesized nanosuspension were recorded using a spectrophotometer (Agilent Cary 3500) operated at a resolution of 1 nm. To evaluate the size and shape distribution of the particles, the samples were prepared as thin films in carbon-coated copper grids and analyzed using JEOL-JEM-2100 plus TEM (200Kv) equipped with high-resolution phosphor scintillator and CCD camera EMSIS. Elemental analysis was also carried out using TEM. Dynamic light scattering (DLS) measurements were carried out using Malvern Zetasizer v7.13. (Malvern Instruments Ltd, Malvern UK).
2.5 Resazurin assay
The antimicrobial activity of synthesized CGNPs was measured using the MIC Mcfarland standard broth dilution method (Elshikh et al., 2016). The MIC values of CGNPs, clerodin were determined based on a micro broth dilution method in 96 multi-well micro titer plates against Staphylococcus aureus (1189 ATCC), Bacillus cereus (CI 2106), Klebsiella pneumoniae (27736 ATCC), Escherichia coli (35218 ATCC) and Pseudomonas aeruginosa (1214 PTCC). 50 μl of nutrient broth and 50 μl of normal saline were added to each well of the plate. A volume of 100 μl of test materials was added into the first row of the plate. Serial dilutions were performed such that each well had total 100 μl of the test material in serially descending concentrations. 10 μl of resazurin indicator solution (prepared by dissolving a 270 mg tablet in 40 ml of sterile distilled water) was added to each well. Finally, 10 μl of bacterial suspension concentration of 2 × 106 CFU/ml was added to each well. The plate had a well with Chloramphenicol (0.5 – 16 μM) as positive control. The plates were placed in an incubator at 37 °C for 18 to 24 h. Any color change from purple to pink indicates growth of microbes. The highest dilution at which no color change occurred was taken as the MIC value of CGNPs and clerodin and was expressed in millimolar (mM). The experiment was performed in triplicates for statistical data analysis and to ensure reproducibility.
2.6 Biocompatibility assay
The cytotoxic effect of CGNPs on healthy PBMNCs was studied using MTT assay. Blood sample (5 ml) was collected from one healthy male individual (∼39 yrs. old) after obtaining informed consent. Approval for collecting samples for ex vivo studies was obtained earlier (Ethical Clearance Number: 435/IEC/2013) from the SRM IST IEC (Institutional Ethical Committee). PBMNCs were isolated from peripheral blood sample by Ficoll‐Paque (specific gravity 1.077) gradient density method as described (Kuo et al., 2000). Isolated PBMNCs were cultured in RPMI with 10% heat-inactivated FBS, supplemented with 2 mM L-glutamine, 1% penicillin/streptomycin and 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) for 18 hrs. Earlier studies from our lab has proved 18 h incubation to be the most appropriate time point for measuring the cytotoxicity of the compound (Cheeran and Munuswamy-Ramanujam, 2020). Hence the same timepoint was used here. Moreover in vivo experiments show that gold nanoparticles usually clear the system by the 5 h time point in most cases. PBMNCs were counted and dispersed in 96 well plates (1 × 105 cells per well) and incubated with CGNPs for 18 h. After the treatment period, cytotoxicity was evaluated by MTT cell viability assay as mentioned below for the THP-I cells (Harish et al., 2020; Ramachandran et al., 2018).
Human monocytic leukaemia cell line THP-1(RRID: CVCL_0006), was procured from National Centre for Cell Sciences (NCCS), Pune, India. THP-1 cells were cultured for MTT assay as reported earlier (Cheeran and Munuswamy-Ramanujam 2020). Briefly, 104 cells per well were seeded in a 96-well tissue culture plate and treated with different concentrations of CGNPs and incubated for 18hrs. Clerodin treated and untreated THP-I cells were used for comparative studies with solvent treated cells being used as controls. After 18hrs, cells were washed with Phosphate-buffered saline (PBS) followed by incubation with MTT. Finally, absorbance was measured at 570 nm using Thermo Multiskan go 96 well plate reader after adding Dimethylsulfoxide (DMSO) to the cell pellet. The experiment was performed in triplicates for statistical data analysis and to ensure reproducibility.
2.7 FACS and CLSM based apoptosis assay
THP-1 cells were seeded as mentioned earlier and treated with IC50 concentration of CGNPs. The cells were incubated for 18 h, then washed with PBS and harvested from plates. Apoptotic THP-1 cells were determined with the Annexin V-FITC Apoptosis Detection Kit (BD pharmingen, BD Biosciences, USA.) following the manufacturer’s instructions. The data was acquired (10,000 cells/ sample) using fluorescence-activated cell sorting (FACS) (FACS Calibur Flow Cytometer; BD Biosciences, USA) and the percentage of apoptotic cells was determined using the BD Cell Quest™ Pro (BD Biosciences, USA).
The confirmation of the results from Annexin/PI assay was carried out by CLSM imaging using a LSM 700 using a 488 nm excitation followed by collecting the images using a 530 nm (Annexin V-FITC) filter and 670 LP filter (PI). All the images were acquired and processed using Zen 2010 software.
2.8 Statistical analysis
Statistical calculations were performed using origin pro 8.5 (Origin Lab Corp, Northampton, USA). Mean ± standard Deviation was used for data. The test performed was One-way ANOVA (p < 0.05) and the least significant differences.
3 Results and discussion
3.1 Standardization of condition for GNP synthesis
Stable Au nanosuspension was generated using environmentally benign Clerodin enriched ethanolic extract from C. infortunatum leaves (CE). Utilizing an enriched extract with established bioactivity profile has an advantage of generating NPs with promising bioactivity. It is well established that an effective control over the reductant concentration and temperature is an important aspect of NPs synthesis process. These factors affect the nanoparticle size and shape and ultimately its functionality (Boomi et al., 2020). Hence, careful standardization of the conditions is required to generate GNPs. Effective concentration of CE needed to generate GNP was identified by adding increasing concentration of CE (1 ml) to 9 ml of 0.5 mM chloroauric acid solution. This resulted in progressive darkening of the solution color from pale yellow to purple. This color change was used as a preliminary indicator. Along with this the samples were subjected to UV–Vis spectroscopy for spectroscopic confirmation. Maximum purple color intensity was observed after addition of 0.8 mg/ml of the CE. Further monitoring with UV–Vis absorption spectra showed a characteristic surface plasmon resonance peak at ∼ 552 nm providing further validation for the formation of Au nanosuspension (Fig-1A). Similarly, the ideal temperature for the synthesis of GNP was evaluated. GNP was synthesized using CE (0.8 mg/ml) at different temperatures (26 °C, 35 °C, 45 °C, 65 °C and 75 °C). Best results were observed at the room temperature of 26 °C.
3.2 TEM, EDX and DLS analysis
To confirm and evaluate the morphology of CGNPs, TEM images were obtained. TEM images revealed monodispersed spherical shaped particles (Fig. 1B-C). No agglomerate was observed in the TEM images. Selected Area Electron Diffraction (SAED) pattern of CGNPs (Fig. 1D) confirmed their polycrystalline nature of the nano material, as indicated by the presence of the bright concentric rings. EDX analysis was carried out to confirm the elemental content of the synthesized CGNPs. EDX showed the presence of Au (98.8%) in the synthesized CGNPs (Fig. 1E). Overall, TEM data was consistent with previous results on GNPs obtained using similar studies (Sathiyaraj et al., 2021). Based on the TEM data the average size of CGNPs was identified as 33 ± 5 nm. This is within the optimum size range (20–100 nm) of nanomaterials for effective enhanced permeation and retention (EPR) (Fig-1F). EPR is a crucial factor to be consider for a nanoparticle with potential in vivo applications. Since this plays an important role in nanoparticles ability to reach the organs as well as the organism’s ability to clear the nanoparticle from the system (Blanco et al., 2015). The size distribution profile of synthesized CGNPs was also evaluated using DLS. The obtained DLS-numbers were close to TEM results. DLS-intensity doesn’t present a significant change in size distribution and it was found that measurements by DLS using number distribution presented accurate results (Fig-1F) These results confirm that CE can reduce the metallic gold to its nano form.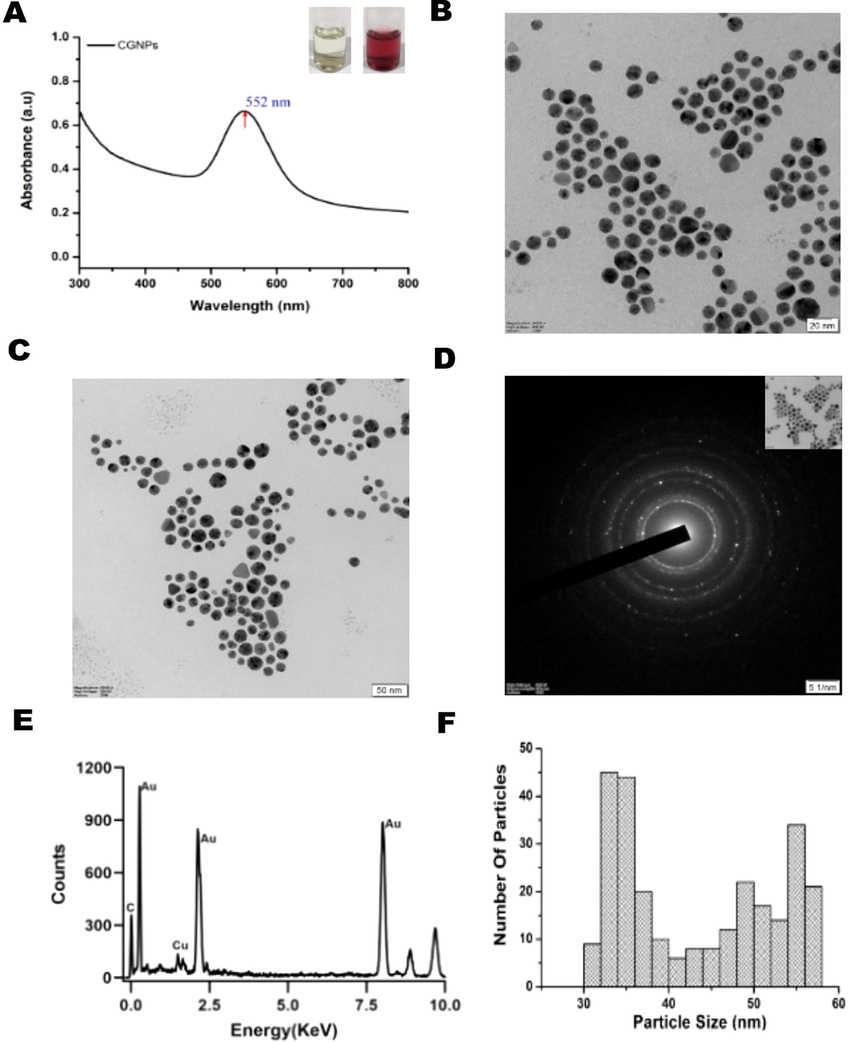
UV–Vis spectroscopy measurements of the synthesized nanosuspensions. Absorbance at 552 nm in UV–Vis spectroscopy confirms the presence of Au nanospheres (A) Transmission Electron Microscopy images of the CGNPs (B-C), Selective area diffraction pattern (D) EDX analysis of synthesized CGNPs (E) and size distribution profile of CGNPs (F) respectively.
3.3 Antimicrobial effect of CGNPs
Antimicrobial resistance of microorganisms to traditional antibiotics has paved way for efforts to identify new lead molecules. Another approach is to identify a delivery system to overcome the pathogens defense mechanism. Phyto fabricated GNPs fit the dual role, since both the gold nanoparticle as well as the surrounding phytocompounds can show synergetic effect thereby exhibiting broad spectrum antibiotic effect (Mikhailova 2021). Antimicrobial activity of CGNPs was tested against pathogens S. aureus, B. cereus, K. pneumoniae, E. coli and P. aeruginosa. Resazurin assay was used to assess the MIC of CGNPs against the different microorganisms. Pure clerodin and Chloramphenicol was used as comparative controls. Chloramphenicol and Clerodin exhibited significant concentration dependent antimicrobial effect on all the tested microorganisms. Chloramphenicol showed inhibitory activity in the range of 30% − 100% for the tested concentrations (0.5 – 16 M) (Fig. 2). Clerodin showed a similar inhibition trend against all the tested microorganism, however needed higher concentrations (0.125 – 10 mM) compared to Chloramphenicol (Fig. 2). CGNPs also showed significant concentration dependent anti-microbial activity against all the tested microorganisms. CGNPs showed increasing inhibitory activity in the range of 28% − 98% for the tested concentrations (0.125 – 0.5 mM) (Fig. 2B). Results indicate that CGNPs were able to inhibit the microorganisms at much lower concentrations (∼20 fold) compared to pure clerodin. This could be the result of increased surface area of the nanoparticle which resulted in enhanced antimicrobial activity of the CGNPs.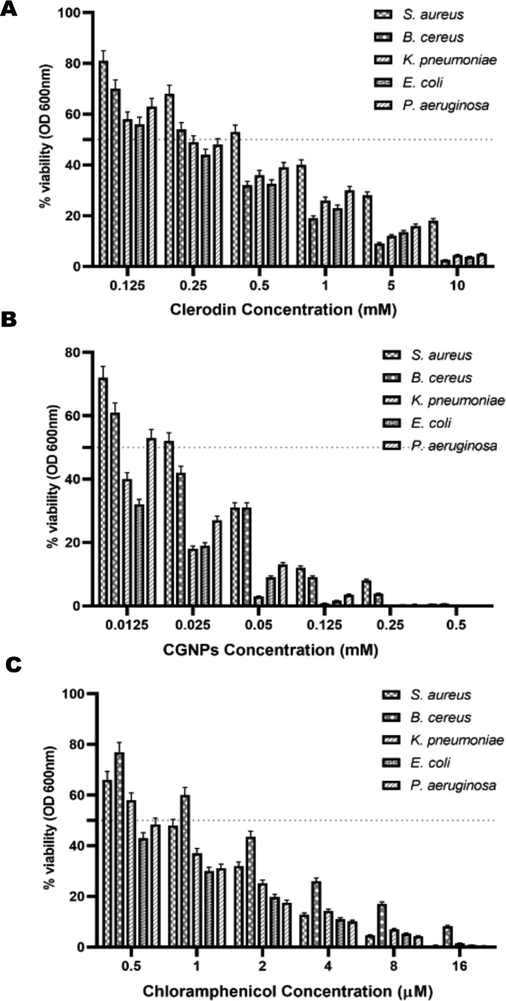
Determination of the MIC by Resazurin aided microdilution method of CGNPs and Clerodin against microbial strains. Chloramphenicol was used as positive control.
3.4 Effect of CGNPs on THP-1 cell viability
Ability to inhibit proliferation or induce death in the cancer cell is a widely studied application of GNPs. The NPs salient features like biocompatibility, cell permeability and retention time makes it an feasible candidate for anti-cancer studies (Chauhan et al., 2021). As discussed earlier the size of the generated CGNPs fell in the ideal range for EPR. Thus, if proven to possess cytotoxicity against cancer cells, CGNPs could be valuable in cancer theapy. MTT assay was carried out on human monocytic leukaemia (THP-1) cell line to evaluate the effect of CGNPs on the leukaemia cell viability. MTT results showed a direct concentration-dependent reduction in cell viability by CGNPs. The results obtained after 18 h treatment of THP-I cells with CGNPs showed a significant decrease in cell survival (5% − 79%) with increasing concentration (0.0125–0.5 mM) of CGNPs (Fig. 3). Pure Clerodin also showed a similar cytotoxicity trend against THP-I cells (Fig. 3). However, the concentration of CGNPs required to reduce the cell viability of THP-1 to a similar level as pure Clerodin was significantly lower (∼10 fold) than the pure compound. The results indicate a strong in vitro cytotoxic effect of CGNPs against human leukemia cells.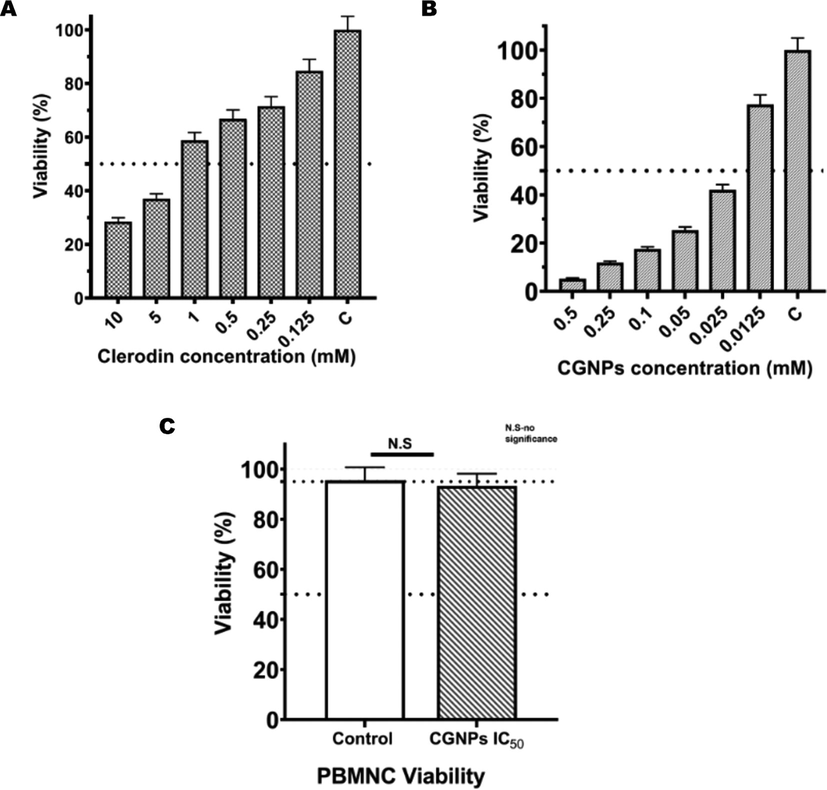
Invitro cytotoxicity evaluations of Clerodin and CGNPs by MTT assay in THP-1 cells. Clerodin (Fig. 3A) and CGNPs (Fig. 3B) both exhibited concentration dependent cytotoxicity in THP-1 cells. Significantly lower concentration of CGNPs was needed to produce the same cytotoxic effect compared to pure clerodin. PBMNCs treated with CGNPs IC50 for 18 h and subjected to MTT assay. MTT assay showed no significant change in viability in CGNPs on healthy PBMNCs. The error bars indicate the standard deviation p < 0.05.
3.5 Biocompatibility of CGNPS
GNPs are preferred for novel biomedical applications, since they have established a track record of being biocompatible to cells and tissues(Mario D'Acunto 2021). Results from the previous experiments proved CGNPs potential as a therapeutic drug with multiple treatment targets. Its ability to inhibit microorganisms projected it as a broad-spectrum antibiotic agent. Simultaneously, CGNPs cytotoxicity to human monocyte leukemic (THP-1) cells made it a promising anticancer agent. In spite of these significant bioactivities, for the CGNPs to be effectively used for treating patients, it should be biocompatible. PBMNCs from healthy donor was taken up for biocompatibility studies for two reasons. First, a preliminary contact for the in vivo drug is usually with the vasculature. Which consists of various immune cells including monocytes. Any adverse reaction caused by a drug in the blood PBMNCs will usually result in the drug being incompatible with the in vivo system. This in turn will lead to failure of the drug as treatment option. Second, the generated CGNPs showed cytotoxicity against THP-I cells. These cell lines mimic human monocytes and has been used as such for in vitro inflammatory and anti-cancer experiments. Hence, it becomes important to study the effect CGNPs have on healthy PBMNCs before considering them for drug related applications. Thus, MTT assay was carried out in healthy PBMNCs to study the biocompatibility of CGNPs. Healthy PBMNCs were treated with IC50 (25 μM) concentration of CGNPs and untreated samples were used as control. CGNPs did not show any significant cytotoxicity against healthy human PBMNCs indicating their biocompatibility. The results confirm the potential of CGNPs as treatment options.
3.6 Cgnps nanosuspension promotes apoptosis
Regulation of apoptosis machinery in a cancer cell is altered, leading to uncontrolled proliferation and disease severity. One of the key features for an effective anti-cancer drug is to induce apoptosis in the cancer cell (Pfeffer and Singh 2018). MTT results obtained with THP-1 cell lines indicated the ability of CGNPs to control cancer cell proliferation. Hence, FITC-Annexin V-PI assay was carried out using FACS to understand the anticancer potential of CGNPs. In the early stages of apoptosis phosphatidylserine (PS) of apoptotic cells is translocated from the cytosolic side of the plasma membrane to the cell surface where it binds with Annexin V and thus gets stained with Annexin V FITC antibodies. However, cells at the late stage of apoptosis exhibit cell membrane compromise and gets stained for both PI and Annexin FITC. The necrotic cells on the other hand will not be stained for Annexin and will uptake PI due to the compromised membrane. Hence, the Annexin V FITC- PI assay was carried out using IC50 concentration of CGNPs, to evaluate its apoptotic potential in THP-I cells. FACS analysis of the FITC-Annexin V-PI-stained cells revealed the initiation of apoptosis by CGNPs in THP-I cells. Results showed a significant increase in apoptotic THP-1 cells treated with CGNPs compared to the control untreated group (Fig. 4A-C). At IC50 (25 μM) CGNPs exerts 28.6 % early-stage apoptosis and 58% of late-stage apoptosis. It is also evident from the data that the necrotic population (∼6 %) did not increase significantly followed by CGNPs treatment (Fig. 4B). Notably, the potential of CGNPs to induce apoptosis is an ideal property for cancer therapy-related drugs. The results obtained from FACS were confirmed by using CLSM imaging of the THP-I cells. Cells at the stage of early apoptosis stained positive for Annexin V FITC (Green fluorescence) alone. Cells in the later stages of apoptosis stained positive for both FITC (Green) and PI (red fluorescence), while the necrotic cells stained only for PI (red fluorescence) (Fig. 4D).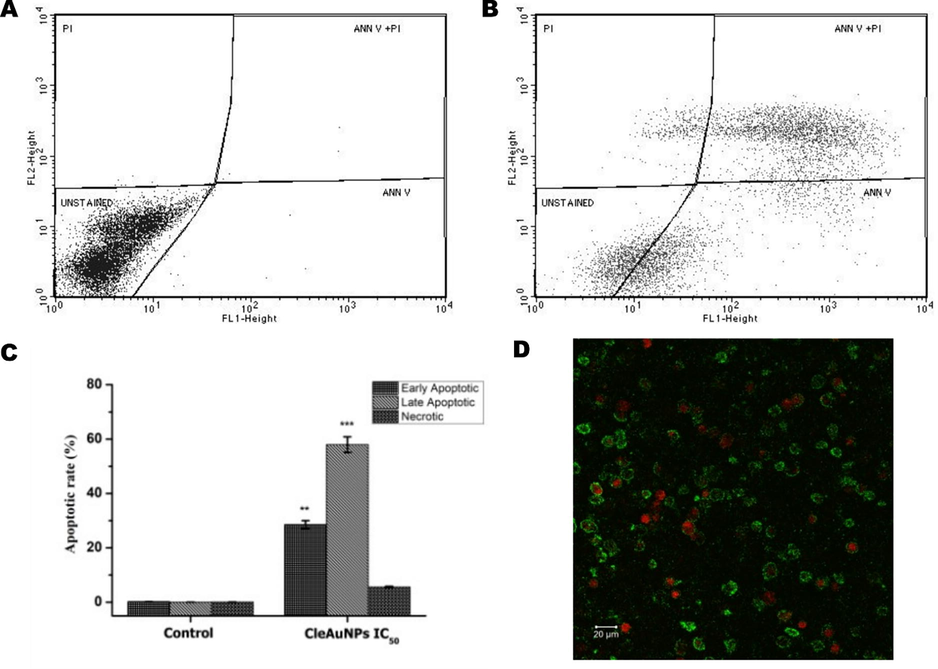
FACS dot plot of Annexin V-FITC/PI analysis (Fig. 4A-B) in THP-1 cells. THP-1 cells treated with CGNPs IC50 18 h and stained with Annexin V-FITC/PI showed induction of apoptosis. Unstained – viable cells, Ann V – Early apoptotic cells, Ann V + PI – late apoptotic cells and PI – Necrotic cells. CLSM images (20X) of THP-1 cells treated with CGNPs IC50 for 18 h and stained using Annexin-V-FITC/PI (Fig. 4D) confirmed the FACS results (scale bars represent 20 μm). Early-stage apoptosis - Annexin V FITC + ve (Green fluorescence), Necrotic cells – PI + ve (Red fluorescence).
The overall bioactivity results confirm the potential of CGNPs as an anticancer agent with the ability to induce apoptosis in human leukemic THP-1 cell lines. Confocal microscopy and flow cytometry gave conclusive evidence for damage to the nuclear material and initiation of apoptosis (programmed cell death).
4 Conclusion
Constant requirement for developing novel drugs with multiple utility has shifted the focus to therapeutic nano materials. Additionally, focus is also to generate such materials in a sustainable, environmentally friendly method. Green synthesis method generally utilizes plant products or other biomaterials to generate nanoparticles. When a bioactive plant material is used for nanomaterial synthesize, it has a dual advantage of being a green synthesis process, along with generating a novel bioactive material. Current study identified for the first time; a broad-spectrum antimicrobial ability of gold nanoparticles generated through clerodin enriched Clerodendrum leaf extract. The study also proved the benign nature of the nanoparticle to healthy human peripheral blood mononuclear cells. Additionally, the plant extracts incorporated gold nanoparticle showed cytotoxicity against leukemia monocyte cells. This indicated an added therapeutic benefit for the nanomaterial. These types of studies could potentially generate plant incorporated gold nano materials that are effective against a broad spectrum of pathogens and cancer cells while simultaneously being host friendly.
Acknowledgements
TEM analysis was carried out at HRTEM FACILITY at SRMIST set up with support from MNRE (Project No. 31/03/2014-15/PVSE-R&D), Government of India. The authors extend their appreciation to the Researchers supporting project number (RSP-2022R479) King Saud University, Riyadh, Saudi Arabia. The research reported in this publication was supported by CSIR -Senior Research Fellow (Direct) Award Number-[09/1045(0032)-2k19-EMR-I]. The corresponding author Dr. M.R. Ganesh thanks DBT India for support (BT/PR9930/NDB/39/457/2013).
Funding
The research reported in this publication was supported by CSIR -Senior Research Fellow (Direct) Award Number-[09/1045(0032)-2k19-EMR-I]. The corresponding author Dr. M.R. Ganesh thanks DBT India for support (BT/PR9930/NDB/39/457/2013). The authors extend their appreciation to the Researchers supporting project number (RSP-2022R479) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of phytochemicals-stabilized gold nanoparticles and their biological activities against bacteria and Leishmania. Microbial Pathogenesis. 2017;110:304-312.
- [Google Scholar]
- Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics. 2020;9(8)
- [Google Scholar]
- The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Scientific Reports. 2021;11(1):588.
- [Google Scholar]
- Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology. 2015;33(9):941-951.
- [Google Scholar]
- Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. International journal of nanomedicine. 2020;15:7553-7568.
- [Google Scholar]
- Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. International Journal of Molecular Sciences. 2021;22(15)
- [Google Scholar]
- Sesquiterpene lactone Zaluzanin D alters MMP-9 promoter methylation in differentiated THP-1 monocytic cells and down regulates inflammatory cytokines IL-1β and TNF-α. International Immunopharmacology. 2020;87:106803
- [Google Scholar]
- One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids and Surfaces B: Biointerfaces. 2013;107:27-34.
- [Google Scholar]
- Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnology letters. 2016;38(6):1015-1019.
- [Google Scholar]
- Synthesis of Gold Nanoparticles and Their Applications in Drug Delivery. Metal Nanoparticles in Pharma. Springer International Publishing:; 2017. p. :155-191.
- Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnology. 2013;4(4):91-98.
- [Google Scholar]
- Antimalarial trials on herbal extracts. I. Clerodendron infortunatum L. Bionature. 1998;18(2):45-49.
- [Google Scholar]
- Cytotoxicity assessment of chitosan coated CdS nanoparticles for bio-imaging applications. Applied Surface Science. 2020;499:143817
- [Google Scholar]
- Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. Journal of Toxicology and Environmental Health, Part B. 2016;19(3–4):129-148.
- [Google Scholar]
- Green Nanotechnology from Cumin Phytochemicals: Generation of Biocompatible Gold Nanoparticles. International journal of green nanotechnology. Biomedicine. 2009;1(1):B39-B52.
- [Google Scholar]
- Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules. 2017;22(9)
- [Google Scholar]
- Regulation of Cell Proliferation, Gene Expression, Production of Cytokines, and Cell Cycle Progression in Primary Human T Lymphocytes by Piperlactam S Isolated from <em>Piper kadsura</em>. Molecular Pharmacology. 2000;58(5):1057.
- [Google Scholar]
- A novel, rapid, seedless, in situ synthesis method of shape and size controllable gold nanoparticles using phosphates. Scientific Reports. 2019;9(1):7421.
- [Google Scholar]
- Mario D'Acunto, P. C., Edi Gabellieri1 and Gianluca Presciuttinis (2021). “Exploiting gold nanoparticles for diagnosis and cancer treatments.” Nanotechnology 32(19).
- Mashwani, Z.-u.-R., M. A. Khan, T. Khan and A. Nadhman (2016). “Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles.” Advances in Colloid and Interface Science 234: 132-141.
- Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. Journal of Functional Biomaterials. 2021;12(4)
- [Google Scholar]
- Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery. 2021;20(2):101-124.
- [Google Scholar]
- Antioxidant and pharmaceutical potential of Clerodendrum L.: An overview. International Journal of Green Pharmacy. 2014;8(4):210-216.
- [Google Scholar]
- Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnology. 2011;5(3):69-78.
- [Google Scholar]
- J.K. Patra G. Das L.F. Fraceto E.V.R. Campos M. d. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H.-S. Shin, Nano based drug delivery systems: recent developments and future prospects Journal of Nanobiotechnology 16 1 2018 71.
- Apoptosis: A Target for Anticancer Therapy. International Journal of Molecular Sciences. 2018;19(2)
- [Google Scholar]
- Phytochemical assisted synthesis of size and shape tunable gold nanoparticles and assessment of their catalytic activities. RSC Advances. 2016;6(55):49307-49316.
- [Google Scholar]
- Forskolin attenuates doxorubicin-induced accumulation of asymmetric dimethylarginine and s-adenosylhomocysteine via methyltransferase activity in leukemic monocytes. Leukemia Research Reports. 2018;9:28-35.
- [Google Scholar]
- P.V. Rao D. Nallappan K. Madhavi S. Rahman L. Jun Wei S.H. Gan Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents Oxidative medicine and cellular longevity 2016 2016 3685671 3685671.
- Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. Journal of Infection and Public Health. 2021;14(12):1842-1847.
- [Google Scholar]
- Recent trends and methodologies in gold nanoparticle synthesis – A prospective review on drug delivery aspect. OpenNano. 2017;2:37-46.
- [Google Scholar]
- In vitro antioxidant and antiproliferative activities of various solvent fractions from Clerodendrum viscosum leaves. Pharmacogn Mag. 2017;13(50):344-353.
- [Google Scholar]
- Sindhu. T. J, A. K. J., Anju. Jose, Binsiya K. P, Blessy Thomas, Elizabeth Wilson (2020). “Clerodin (C24H3407), a type of clerodane, is a bicyclic terpenoid which contains a hydroxy group, an acetate functionality, a dihydrofuran ring.” Asian Journal of Research in Chemistry 13(2): 128-132.
- Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms. 2021;9(10)
- [Google Scholar]







