Translate this page into:
Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice
⁎Corresponding author. 18049105588@sina.cn (Jianhui Du)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lung inflammation is a common cause of health problem and death among adults and children. Antioxidant compounds have the capability to overcome this complication. This study targets to examine the anti-inflammatory and defensive role of D-carvone on lipopolysaccharide (LPS)-initiated lung damage in mice. Male BALB/c mice (n = 8) received intragastric feeding of D-carvone (25 and 50 mg/kg b.w.) or dexamethasone (5 mg/kg b.w.) an hour before intranasal instillation of LPS (20 µg). Seven hours after LPS instillation, the bronchoalveolar lavage fluid (BALF), blood serum, and tissue samples of lung were studied for the levels of inflammatory cells, antioxidant enzymes, pro-inflammatory cytokines, and histopathological changes. D-carvone significantly alleviated (p < 0.05) the lung damage caused by LPS by reducing the lung wet-to-dry (W/D) ratio along with the amount of total cells, macrophages, and neutrophils in BALF (p < 0.05). The serum TNF-α, IL-1β, and IL-6 were remarkably reduced (p < 0.05) in D-carvone treated mice. Histopathological derangements because of LPS-initiated lung damage were altered by D-carvone. The results of D-carvone were comparable with the positive control dexamethasone. Pre-treatment of D-carvone significantly provided anti-inflammatory and protective effect in LPS-instigated lung damage. Together, these findings acknowledge the use of D-carvone as preventive agent for lung damage and inflammation. Further research on the pharmacokinetics, pathway and mechanism of action of D-carvone are necessary to promote D-carvone as a commercial anti-inflammatory drug.
Keywords
D-carvone
Acute lung injury
Inflammation
Lipopolysaccharide
Histopathology
1 Introduction
Lung inflammation or acute lung injury is characterized with pulmonary edema, severe hypoxemia, overwhelming accumulation of neutrophils that leads to mortality caused by multiple organ failure (San et al., 2014). Overall incidence of mortality due to acute lung inflammation is increasing every year throughout the world (Li et al., 2019). The causes for lung inflammation and injury include bacterial infections, surgical defects, and uncontrolled infiltration of inflammatory cells (Lee et al., 2016). Lipopolysaccharide (LPS) is well-known to cause severe lung injury through activation IL-6, TNF-α, IL-1β, etc (Li et al., 2019; Ding et al., 2019). Inhibition and prevention of pro-inflammatory cytokines and their regulatory pathway has given tremendous results in modulating LPS-initiated lung inflammation (Ding et al., 2019; Huang et al., 2015). Antioxidant compounds have the ability to modulate inflammatory cytokine productions and control the overall progression of parenchymal inflammatory process in lung injury (Lee et al., 2018).
Carvone is an unsaturated monoterpenoid ketone, found as major phytochemical constituent in caraway seeds (Carumcarvi L.) as well in essential oil extracts of aromatic medicinal plants such as spearmint (Mentha spicata), dill (Anethum graveolens), and many more (Gopalakrishnan et al., 2019). Among carvone isomers, D-carvone has shown pharmaceutical values like anti-hyperlipidemic, anti-microbial, anti-carcinogenic, chemopreventive, anti-hypertensive, and anti-tumorigenic (Vinothkumar et al., 2013; Moro et al., 2018). Carvone and a few other monoterpenes have been reported to possess immunomodulatory and anti-inflammatory values (Rita De Cássia Da Silveira et al., 2013). However, there are no previous studies reported with specification to D-carvone on its modulating effects in lung inflammation. Hence, it was aimed to inspect the anti-inflammatory and protective role of D-carvone on lipopolysaccharide (LPS)-instigated lung damage in mice.
2 Materials and methods
2.1 Chemicals
D-carvone, LPS, and dexamethasone were bought from Sigma Aldrich, US. ELISA assay kits for inflammatory cytokine analysis and reagents for histopathological analysis were purchased from BD Biosciences, US. All chemicals used in the study are of purest grade.
2.2 Animals
Male BALB/c mice of 22–24 days old (28–30 g) were taken from the laboratory facility of The Second People’s Hospital of Liaocheng. Mice were acclimatized prior to experiment in groups of eight animals in cages, humidified and maintained room temperature with adequate water and food. Mice were treated with care following the ethical guidelines and approval was given by the ethical committee of The Second People’s Hospital of Liaocheng (ethical no.: JXC20171024). The experiment was conducted for eight months including analysis beginning in September 2018 at The Second People’s Hospital of Liaocheng, Shandong.
2.3 Experimental design
Animals (n = 8) were grouped into; Group I normal control received saline treatment; Group II model endured LPS (20 µg) through intranasal instillation to induce lung injury; Group III and IV were given 25 and 50 mg/kg b.w. of D-carvone via intragastric for three consecutive days and treated with LPS on the third day one hour after administration of D-carvone; Group V positive control received 5 mg/kg b.w. of dexamethasone through oral gavage for three consecutive days and treated with LPS on the third day an hour upon treatment of dexamethasone. Animals were anesthetized and sacrificed seven hours after treatment with LPS to obtain the blood serum and lungs for analysis. The experimental protocol was followed according to Lee et al. (2018).
2.4 Bronchoalveolar lavage fluid (BALF) collection for inflammatory cell analysis
Lung tissues were rinsed with cold PBS using tracheal cannula to collect BALF following the method of Lee et al. (2018). The collected BALF suspension were centrifuged at 1500 rpm at 4 °C for 5 min and the aliquots were stored at −80 °C for biochemical analysis. The differential inflammatory cells in BALF were counted with automated cell counter.
2.5 Biochemical and ELISA assays
The activities of SOD, GSH, MDA, and CAT were estimated in the aliquots of BALF using commercially available diagnostic kits (Sigma Diagnostic Kits, St. Louis, US) following Jing et al. (2015). The serum IL-6, TNF-α, and IL-1β were determined using the commercially available ELISA assay kits (Shimadzu, Japan).
2.6 Histopathological studies
Portions of lung tissues were excised prior to collection of BALF and fixed in 10% neutral buffered formalin. The tissues were paraffin embedded, microtome to 4 µm and hematoxylin and eosin (H&E) stained to be analyzed under light microscope. The histopathological changes observed were photographed under microscope.
2.7 Statistical analysis
All experimental values were written as mean ± standard error of mean for eight mice. Mean differences were analyzed with ANOVA of windows SPSS 21.0, US and Tukey’s multiple comparisons test. Statistical significance was relevant at p < 0.05.
3 Results
3.1 Effects of D-carvone on lung wet-to-dry (W/D) ratio of LPS-induced mice
Relative lung weight W/D ratio of mice treated with LPS was measured to determine lung pulmonary edema. Sharp increase (p < 0.05) was noticed in the W/D ratio of lung of LPS-instigated mice (Fig. 1). Pre-treatment with D-carvone and dexamethasone brought down (p < 0.05) the W/D ratio of LPS-challenged mice.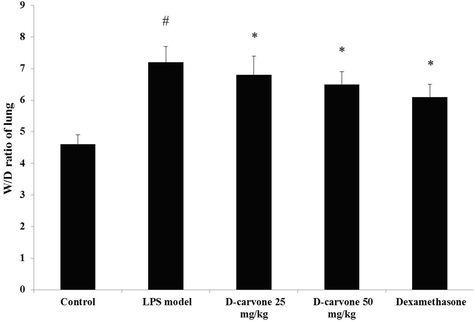
Effect of D-carvone on lung W/D ratio in LPS-induced mice. Values expressed as mean ± SEM (n = 8) of final body weight of mice. # p < 0.05 as compared to normal control group. * p < 0.05 as compared to LPS-induced acute lung injury model group.
3.2 Effects of D-carvone on accumulation of BALF inflammatory cells on LPS-instigated mice
Total inflammatory cells were counted in BALF of mice instigated with LPS and were found that the amount of lymphocytes, macrophages, neutrophils, and eosinophils were increased (p < 0.05) in contrast to the BALF of normal mice (Fig. 2). The amount of cells in the BALF of D-carvone treated mice were efficiently reduced (p < 0.05) compared with LPS-instigated model animals. Positive control dexamethasone treated mice also demonstrated decrease (p < 0.05) in the concentration of inflammatory cells in the BALF as compared to LPS-challenged model mice.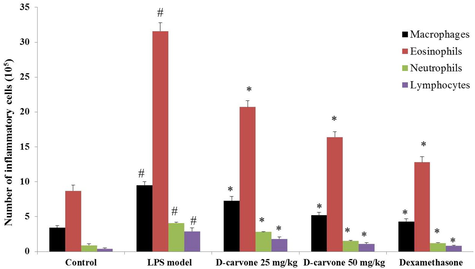
Effect of D-carvone on of inflammatory cell counts in BALF of LPS-induced mice. Values expressed as mean ± SEM (n = 8) of final body weight of mice. # p < 0.05 as compared to normal control group. * p < 0.05 as compared to LPS-induced acute lung injury model group.
3.3 Enhancing effects of D-carvone on the levels of GSH, MDA, and antioxidant enzymes in LPS-treated mice
Oxidative stress was observed in the LPS-initiated lung inflammation model group mice with reduction (p < 0.05) in the antioxidant GSH level, and SOD, CAT activities whereas the MDA levels were elevated (p < 0.05) from normal mice (Table 1). The levels of MDA were decreased (p < 0.05) by pre-treatment with D-carvone along with increase (p < 0.05) in SOD, CAT, and GSH from LPS-instigated model group. Pre-treatment with dexamethasone also increased SOD, CAT, GSH and decreased MDA (p < 0.05) against LPS-treated model animals. Values expressed as mean ± SEM (n = 8) of final body weight of mice.
Groups
GSH (nmol/mg protein)
MDA (nmol/mg protein
SOD (U/mg protein)
CAT (U/mg protein)
Normal control
16.88 ± 2.52
7.72 ± 0.31
7.58 ± 0.72
5.88 ± 0.42
LPS-induced model group
11.53 ± 2.86#
14.51 ± 0.88#
4.49 ± 0.81#
3.62 ± 0.39#
D-carvone 25 mg/kg b.w. + LPS
14.72 ± 3.18*
9.98 ± 0.42*
6.52 ± 0.32*
4.91 ± 0.51*
D-carvone 50 mg/kg b.w. + LPS
15.86 ± 2.68*
8.32 ± 0.46*
7.16 ± 0.56*
5.18 ± 0.45*
Dexamethasone 5 mg/kg b.w. + LPS
15.92 ± 2.50*
8.18 ± 0.22*
7.42 ± 0.65*
5.31 ± 0.82*
3.4 Modulating effects of D-carvone on the levels of pro-inflammatory cytokines in LPS-instigated mice
ELISA assay performed on the pro-inflammatory cytokine productions in serum of LPS-challenged model mice showed a significant upsurge (p < 0.05) in IL-6, TNF-α, and IL-1β compared to normal control (Table 2). D-carvone treatment modulated (p < 0.05) the pro-inflammatory cytokine productions with downregulation of IL-6, IL-1β, and TNF-α from LPS-instigated model animals. Dexamethasone treatment also downregulated (p < 0.05) TNF-α, IL-6, and IL-1β in contrast to LPS-instigated model animals. Values expressed as mean ± SEM (n = 8) of final body weight of mice.
Groups
TNF-α (pg/mL)
IL-1β (pg/mL)
IL-6 (pg/mL)
Normal control
126.95 ± 17.37
138.26 ± 12.34
149.25 ± 17.27
LPS-induced model group
268.31 ± 26.46#
265.53 ± 24.68#
227.59 ± 14.28#
D-carvone 25 mg/kg b.w. + LPS
191.48 ± 22.42*
184.26 ± 27.15*
161.63 ± 18.68*
D-carvone 50 mg/kg b.w. + LPS
152.84 ± 13.41*
158.57 ± 22.16*
157.74 ± 16.14*
Dexamethasone 5 mg/kg b.w. + LPS
139.62 ± 17.44*
148.42 ± 20.46*
153.81 ± 16.28*
3.5 Histopathological modifications of D-carvone in LPS-instigated mice
Observation of lung histopathology of normal control mice showed no changes and healthy arrangements of alveolar spaces (Fig. 3). In contrast, the LPS-challenged model mice demonstrated lung edema, infiltration of inflammatory cells, distorted alveolar spaces and hemorrhage. Pre-treatment with D-carvone significantly attenuated the histopathological changes caused by LPS in a dose-dependent manner. Positive control drug dexamethasone also prevented the histopathological modifications induced by LPS.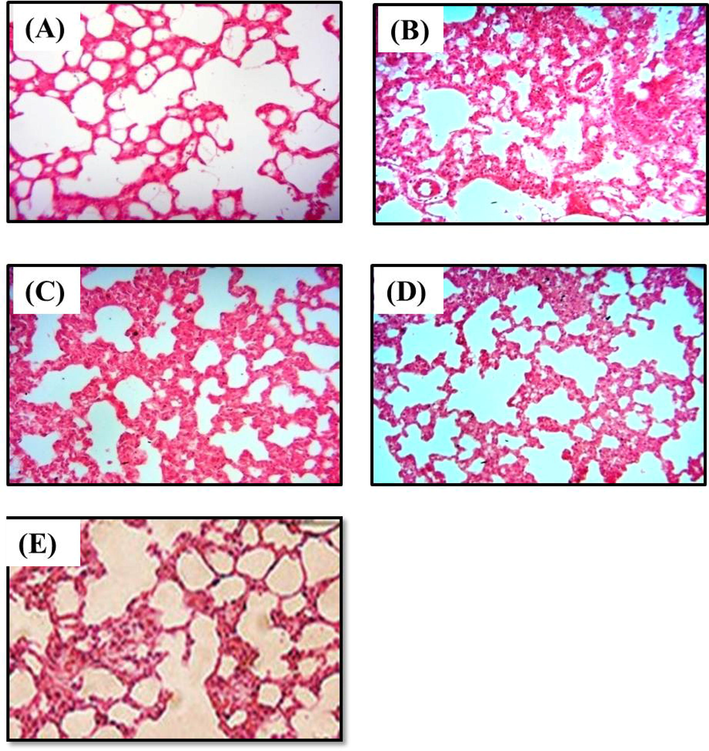
Histopathological alterations due to D-carvone administration in LPS-induced mice. H&E staining at 400 × magnification. (A) Normal control lung showing no pathological changes with healthy arrangements of alveolar spaces; (B) LPS-challenged model lung demonstrating thickening of alveolar wall, infiltration of inflammatory cells, lung edema, distorted alveolar spaces and hemorrhage; (C) & (D) Lung pre-treated with D-carvone at 25 and 50 mg/kg b.w. shows signs of recovery and reduced inflammation; (E) Lung pre-treated with dexamethasone at 5 mg/kg b.w. shows significant prevention of inflammation against LPS.
4 Discussion
Acute lung injury or lung inflammation is a fatal disease, which is practically incurable, hence prevention is the best therapeutic option to overcome the problem (Jung et al., 2017). Antioxidant compounds are well-known to possess anti-inflammatory effects in many pathological conditions (Wang et al., 2014; Zhang et al., 2017). LPS is found in gram-negative bacterial outer membrane that induces severe inflammatory response once entered the blood stream, which may eventually cause death (Jing et al., 2015). The lung inflammation response induced by LPS in a mice model closely resembles the pathological changes during acute lung injury in humans (Jing et al., 2015; Jeong et al., 2014). Therefore, D-carvone was chosen to be studied against lung damage instigated by LPS in mice to learn its ability in preventing the progression of lung inflammation. From the results, it was evident that LPS had induced severe lung inflammation in mice, characterized with inflammatory cells accumulation, pulmonary edema, and elevation of pro-inflammatory cytokines similar to past results (San et al., 2014; Ding et al., 2019; Huang et al., 2015).
Lung W/D ratio was increased in LPS-instigated model animals exhibiting edema caused by inflammation. Pre-treatment with D-carvone decreased the W/D ratio by reducing the pulmonary edema caused by inflammation. Increased accumulation of neutrophils, eosinophils, macrophages, and lymphocytes were detected in BALF of LPS-instigated model animals, which relates to the pulmonary edema formation during inflammation. These results were similarly explained by Ran et al. (2014) and Huang et al. (2019). D-carvone treatment efficiently decreased the cell counts in BALF, which explains that D-carvone attenuated the inflammatory reaction induced by LPS. Histopathological findings of this study further evidence the lung inflammation and injury caused by LPS in the model group with edema formation, alveolar walls thickening, and infiltration of inflammatory cells. The pathological alterations caused by LPS were prevented by D-carvone by preserving the alveolar spaces, reduced inflammatory cells infiltration, and preventing the development of edema, suggesting the protective effect of D-carvone against acute lung injury. The histopathological results were similar to other studies using antioxidant compound against LPS-initiated lung damage in mice (Huang et al., 2015; Niu et al., 2017).
LPS triggers inflammatory reaction vigorously in the initial stage of lung damage (Lee et al., 2016). The accumulation of inflammatory cells and edema development is preceded by inflammatory response by pro-inflammatory cytokines and related proteins (Ding et al., 2019). It is learned that inflammatory cytokines are accountable in the pathogenesis of acute lung damage triggered by LPS (Li et al., 2019; Ran et al., 2014). In the results, cytokines were increased in the LPS-instigated model animals, which elucidate the rigorousness of lung damage and inflammation. In contrast, D-carvone treatment significantly downregulated the activities of TNF-α, IL-1β, and IL-6 from LPS-initiated inflammation. The results are similarly reported by Jung et al. (2017) and Fu et al. (2019). Oxidative stress is an important progenitor in inflammatory response (Jing et al., 2015). The levels of GSH, SOD, and CAT were suppressed whereas MDA was elevated in LPS-induced model mice, exhibiting oxidative stress condition due to depletion of defense mechanism. D-carvone pre-treatment prevented lipid peroxidation by means of reduced MDA formations. Amelioration of oxidative stress by D-carvone in LPS-initiated lung inflammation is similar to Chu et al. (2016) report. Overall, the study shows that pre-treatment of D-carvone has successfully prevented LPS from causing lung damage and inflammation in mice as supported by the biochemical and histopathological results.
5 Conclusion
In conclusion, this study proved that D-carvone has anti-inflammatory and preventive effects on LPS-initiated lung damage in mice. D-carvone significantly ameliorated the lung W/D ratio, oxidative stress markers, pro-inflammatory cytokines, and histopathological changes in LPS-challenged mice, compatible with the results of positive control dexamethasone. Together, these results support the use of D-carvone as preventive agent against acute lung injury and inflammation. Further research on the pharmacokinetics, pathway and mechanism of action of D-carvone are necessary prior to conducting clinical trials to promote D-carvone as a commercial anti-inflammatory drug.
Contribution of authors
Dr. Jianhui Du designed the experiment. Dr. Meng Zhao collected data from the experiments. Dr. Jianhui Du and Dr. Meng Zhao analyzed the data and compiled the acquired outcome. Both authors equally prepared and edited the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Eupatorium lindleyanum DC. flavonoids fraction attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol.. 2016;39:23-33.
- [CrossRef] [Google Scholar]
- Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol.. 2019;66:169-176.
- [CrossRef] [Google Scholar]
- Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice. Biosci. Rep.. 2019;39(6) BSR20190789
- [CrossRef] [Google Scholar]
- Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed. Pharmacother.. 2019;111:178-187.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol.. 2015;26(1):265-271.
- [CrossRef] [Google Scholar]
- The therapeutic effects of Jaceosidin on lipopolysaccharide-induced acute lung injury in mice. J. Pharmacol. Sci.. 2019;S1347-8613(19) 35679-8
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem. Biol. Interact.. 2014;212:30-39.
- [CrossRef] [Google Scholar]
- Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol.. 2015;285(2):128-135.
- [CrossRef] [Google Scholar]
- Protective effect of hemopexin on systemic inflammation and acute lung injury in an endotoxemia model. J. Sur. Res.. 2017;212:15-21.
- [CrossRef] [Google Scholar]
- Picrasma quassiodes (D. Don) Benn. attenuates lipopolysaccharide (LPS)-induced acute lung injury. Int. J. Mol. Med.. 2016;38(3):834-844.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. J. Ethnopharmacol.. 2018;210:23-30.
- [CrossRef] [Google Scholar]
- Bee pollen extracts modulate serum metabolism in lipopolysaccharide-induced acute lung injury mice with anti-inflammatory effects. J. Agri. Food Chem.. 2019;67(28):7855-7868.
- [CrossRef] [Google Scholar]
- Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci.. 2018;53(4):e00076
- [CrossRef] [Google Scholar]
- Cavidine ameliorates lipopolysaccharide-induced acute lung injury via NF-κB signaling pathway in vivo and in vitro. Inflammation. 2017;40(4):1111-1122.
- [CrossRef] [Google Scholar]
- Protective effect of veratric acid on lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol.. 2014;740:227-232.
- [CrossRef] [Google Scholar]
- A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18(1):1227-1254.
- [CrossRef] [Google Scholar]
- Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol.. 2014;19(2):342-350.
- [CrossRef] [Google Scholar]
- Modulating effect of D-carvone on 1,2-dimethylhydrazine-induced pre-neoplastic lesions, oxidative stress and biotransforming enzymes, in an experimental model of rat colon carcinogenesis. Cell Prol.. 2013;46(6):705-720.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation. 2014;37(6):2085-2090.
- [CrossRef] [Google Scholar]
- Protective effects of syringin against lipopolysaccharide-induced acute lung injury in mice. J. Sur. Res.. 2017;209:252-257.
- [CrossRef] [Google Scholar]







