Translate this page into:
Anti-cancer ability of chitosan nanoparticles loaded plant essential oil evaluated against A549 human lung cancer cells through invitro approaches
⁎Corresponding author at: School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
⁎⁎Co-Corresponding author: Department of Botany and Microbiology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia. sshine@ksu.edu.sa (Shine Kadaikunnan),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Based on the recent research, the natural polysaccharide rich biopolymer of chitosan is an important carrier molecule for drug loading. In addition, the plant essential oils were another important material that frequently used to treat various infectious diseases. In addition, the chitosan loaded essential oils were heightened recent years due to the excellent biomedical properties. In this regard, the current study result was concentrated with encapsulation of plant essential oils into the chitosan nanoparticles for inhibit against A549 human lung cancer cells. In result, the unique and uniform size morphology of chitosan was clearly shown in scanning electron microscope (SEM) and transmission electron microscope (TEM). Further, the IC50 dose of the chitosan loaded essential oils at 1000 µg/mL concentration against A549 lung cancer cells was confirmed using cytotoxicity assay and this concentration was very effective than chitosan and essential oils alone. At 1000 µg/mL concentration, the high proliferation efficiency with unclear morphological evidence of A549 lung cancer cells was shown in phase contrast microscope. In addition, the AO/EB stain result was indicated more death cells with belbing formation of the treated cells were observed. At 1000 µg/mL of IC50 concentration of chitosan loaded essential oils was damaged the A549 lung cancer cells with high necrotic cells, chromatin condensation, and decreased cell division. Therefore, all the invitro experiments results were clearly stated that the chitosan loaded essential oils as more efficient alternative option to inhibit the cancer cells.
Keywords
Chitosan nanoparticle
Plant essential oils
Cancer cells
In vitro inhibition assay
Cytotoxicity assay
Fluorescence microscopy analysis
1 Introduction
Recent years, applications of nanoscience or nanomaterials in the field of biomedical approaches are called a nanomedicine (Matshetshe et al., 2018). In addition, the variety of forms including nanoparticles, nanoshell, nanobiosensor, nanovaccines, nanocarriers, nanorobotics and various forms are used in the field of biomedical applications (Rajkumar et al., 2020). In biomedical field, it act as a drug delivery system and used as a prolonged time duration and natural affinity. In drug delivery process, various format of nanomaterial was used to treat pathogens and their infections such as micelles, nanoemulsion, nanocolloids, dendrimers, nanocarriers and liposomes. The carrier of nanomaterial used to enhance the drug activity, solubility of hydrocompounds and other natural products. Also, it is increased the bioactivity, bioavailability, decreasing the more amount of drug utilization and decrease the toxicity effect (Esmaeili et al., 2015; Cai et al., 2021).
Among the various anti-pathogenic molecules, the plant essential oils are the most important one due to the excessive inhibition effect than plant extract. In this advantage, the current research is highly focused on plant essential oils are loaded on chitosan molecules for inhibit the cancer cells. As same as the current research is highly concentrated with plant essential oils and increased attention in chitosan loading process (Prasathkumar et al., 2021). In addition, it is an excellent complementary and alternative drug for most of the infectious diseases. Essential oils are the factory of compound producer it arranged in the form of complex chemicals (Taskin et al., 2020). Most of the volatile compounds, hydrocarbons, enzymes, and other related bioactive compound synthesized chemical derivatives and their moieties are present in the complex essential oils (Das et al., 2021; Li et al., 2020).
In this method, sodium tripoly phosphate act as a cross linker for loaded essential oils, drugs, proteins, amino acids, nutrients and enzymes (Damasceno et al., 2018; Rajivgandhi et al., 2021). In the loading process, the ionic interaction between the chitosan molecules containing amino group with positive charges and negatively charged cross linker of sodium try poly phosphate (Fig. 1). After this process, the material has the enhanced diffusion abilities, high dispersion around the target sites and delivered the nano sized drug in inside of the pathogenic cells. Hence, the current study is focused on detect the efficiency of obtained essential oils nature after loaded into the chitosan nanoparticles against A549 human lung cancer cells through various invitro experiment.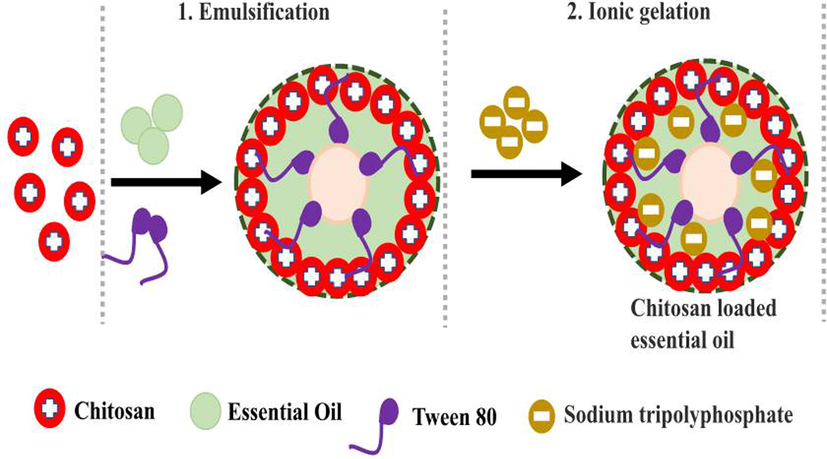
Preparation of chitosan nanoparticles loaded with essential oil through emulsification and ionic gelation methods.
2 Materials and methods
2.1 Culture collection and solution preparation
The chitosan loaded essential oils was obtained from School of Life Sciences, Sun Yat-Sen University, Guangzhou, China. Initially, the characterization and their anti-biofilm efficacy of chitosan loaded essential oils were evaluated and published recently (Rajivgandhi et al., 2021) In addition; the anti-cancer ability of chitosan loaded essential oils was used in this study to detect the anti-cancer ability.
2.2 Reconfirmation of chitosan loaded essential oils by SEM and TEM analysis
In sample preparation, 1 drop of suspended chitosan loaded essential oils was taken in aluminum plate and kept 12 h in desiccator to complete dry. Then, the sample was coated with gold platinum plate, after clear coated the 9 × 10-1 formatted SEM instrument was used (Valizadeh et al., 2021). As same as, all the procedure with copper grid coating sample was taken using syringe and the sample was air dried. Without any contamination of the sample was visualized by TEM (Shimadzhu, Japan). All the same procedure was applied for chitosan nanoparticles and essential oils separately.
2.3 anti-cancer activity
2.3.1 Cell viability assay of chitosan loaded essential oils
The viability based cancer cells detection after treatment with chitosan loaded essential oils was measured under dimethylthiazol-diphenyltetrazolium bromide (Zhang et al., 2020a, 2020b). In procedure, the complete medium was added into the 96-well polystyrene plate with standard procedure and inoculated 2 × 10-4 of 24 h old lung cancer cells, and incubated one day with 95 % humidity and 5 % CO2. Next, 100–1000 µg/mL concentration of chitosan nanoparticle, essential oils, chitosan nanoparticle loaded essential oils were treated properly into the respective wells. Next day, the MTT solution was inoculated on the 24 h incubated side of the all wells and covered by aluminum foil to avoid contamination. Next, formazan crystal formation of the MTT added wells were monitored and noted based on the color formation using 600 nm O.D value by UV-spectrophotometer. After careful reading, the IC50 concentrations of the chitosan nanoparticle, essential oils and chitosan nanoparticle loaded essential oils was detected using bellowed formula,
Percentage of inhibition = Control result O.D – Test result O.D X 100,
2.3.2 Structural differentiation of cancer cells
The complete medium with and without chitosan nanoparticle, and chitosan nanoparticle loaded essential oils treatment samples were taken in 6-well plate. A clean, clear cover slip was pressed slightly into the sample medium and allowed to grown on the cover slip at 37 ℃ for 1 day with standard incubator protocol. Then, formaldehyde (1 %) was fixed on the cover slip and allowed 1 h to complete fixation. Finally, visualized the morphological differentiation between treated and untreated slide samples using phase contrast microscopy (Rajivgandhi et al., 2020).
2.3.3 Dual fluorescence staining assay
The protocol of Salehi et al. (2020) helped to done fluorescence experiment including complete medium with chitosan nanoparticle and chitosan nanoparticle essential oils taken into the 6-well plat. Followed by clean cover slip pressing procedure in inside of the complete medium and permit to grow cancer cells on cover slip. Then, maintained the plate at required standard incubator procedure. Then, the cover slips were taken out from 6-well plate and washed by PBS (1x) and then stained by each 10 µg/mL mixed AO/EB stain combination. Finally, the cover slip was viewed by fluorescence microscope for detection of morphological changes into the cancer cells of treated and untreated cells.
2.3.4 Measurement of damaged parts of mitochondria (ΔΨm)
The experiment was followed the recent report of Rajivgandhi et al., (2020), and protocol was modified below, after successful completion of incubation period with chitosan nanoparticle and chitosan nanoparticle loaded essential oils treatment, the cells were stained by Rhodamine 123 and viewed in fluorescence microscope. In this experiment, the end apoptosis process shown high release of cytochrome c from mitochondrial region to cytosol. Before, the harvested cells was treated by trypsinization and washed by PBS (1 %). Then stained with 10 µg/mL of Rodhamine 123 and waited 30 min and followed by standard incubation. Then, again washed with 1 % PBS and then dry the sample. Finally, the sample was viewed by fluorescence microscopy for detection of mitochondrial variation in treated and untreated cells.
3 Result and discussion
3.1 Morphological evidence of chitosan and chitosan loaded essential oils by SEM and TEM micrographs
Based on the result, the micrograph of Fig. 2b was exhibited the completely chitosan loaded essential oils. Because, the image was entirely varied from native chitosan nanoparticle images of Taskin et al. (2020). Also, the clear differentiated morphology of essential oils was shown within the chitosan nanoparticle outer layer. After seen the microscopic images, the clear rough morphology and tightly packed arrangement of the particles with rough outer morphology were exhibited. Previously, was reported similar statement with current study result, and also helped to suggest the present image of Fig. 2a was native chitosan. In addition, the changed chitosan nanoparticle was shown with single homogeneity structure with flocculent nature. These changes were suggested that the chitosan was changed to chitosan nanoparticle with the help of sodium tri poly phosphate. The Fig. 2b was supported evidence for chitosan nanoparticle, and it proved by recent report of Esmaeili et al., 2015.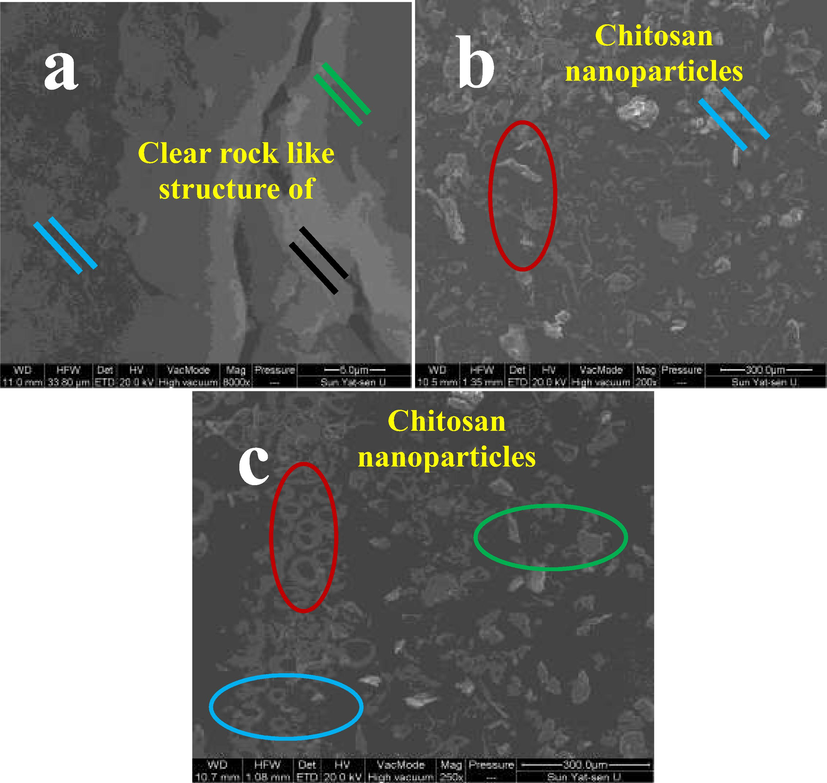
Scanning electron microscope observation for confirmation of obtained chitosan (a), chitosan nanoparticles (b) and chitosan nanoparticles loaded essential oils (c).
The differentiation of chitosan and chitosan nanoparticles were seen in the Fig. 2c, and the result was proved that the essential oils was completely loaded into the chitosan and morphology was shown very clearly. The oil was precipitate in the rough surface and morphology was shown with follicle nature. The essential oils were seen within the chitosan layers and also shown boll like structure. The highly resembled result of chitosan and chitosan nanoparticle loaded essential oils were observed in TEM images. The clear spherical, unique particles, rubble surface morphology was shown after loaded essential oils. The differentiation of chitosan nanoparticles (Fig. 3a), and chitosan nanoparticles loaded essential oils (Fig. 3b) were clearly available. In TEM, the chitosan, chitosan nanoparticles and chitosan nanoparticles loaded essential oils were exhibited with different morphology. The sticky nature was shown after loaded the essential oils only and the morphological structure exhibited oil covered layer. The oils were shown with dispersed structure into the chitosan molecules and precipitated. Based on the TEM result, the current results were suggested that the plant essential oils were clearly covered by the chitosan layer and it shown ball like structure. In addition, the TEM result was highly similar to SEM and both the evidences were proved that the essential oils were successfully loaded into the chitosan molecules. Kumar et al. (2020) agreed the present result and TEM morphometric was highly similar to essential oil loaded chitosan structure. Recent report of Taskin et al. (2020) was also more evident, the chitosan nanoparticle was the better choice to load the essential oils to perform against various biomedical applications.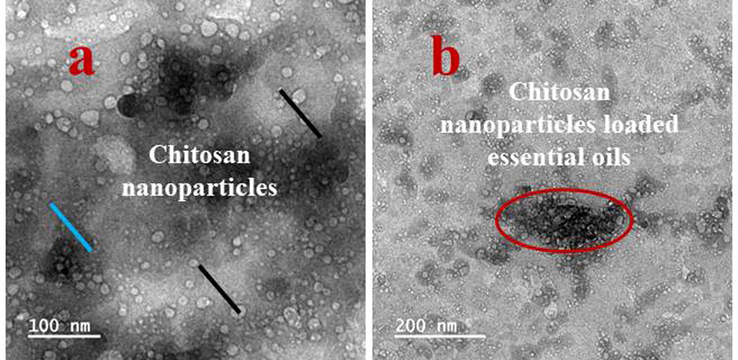
Transmission electron microscope observation of chitosan nanoparticles (a) and chitosan nanoparticles loaded essential oils (b).
3.2 Cell viability assay of chitosan loaded essential oils
In cell viability, the decreased viability was received in increasing concentration of chitosan loaded essential oils, but the complete death cells were observed at very lowest concentration. At 500 µg/mL concentration, the cells were not grown and also fallen the nutrient depletion and leads to more death cells. After entry of chitosan nanoparticle loaded essential oils into the cells, it directly affected the nucleus and promoted the responsible genes for high number of apoptosis production. The continuous production of apoptosis may cause bulk amount of cancer cells death were processed by chitosan nanoparticles (Fig. 4c). In net result, the viability of the cells was decreased and their cell division, growth promoter genes and other virulence genes were inactivated. Previously, the inhibition range was started well in 100 µg/mL only, before we can see the result in 10 µg/mL–100 µg/mL, the death rate was not shown better and viability was remain withstand in the culture medium. It expressed the nature of cancer cells and their effect against human (Kavaz et al., 2019). Apart from this, we could find the inhibition of cancer cells when treated with chitosan nanoparticle also. Compared with chitosan loaded essential oils, the chitosan nanoparticle was shown inhibition with high concentration 100 µg/mL. It was also suggested that the chitosan has anti-cancer ability against tested A549 lung cancer cells at increasing concentration. The IC50 dose of the chitosan was derived from 1000 µg/mL (Fig. 4a). Whereas, the essential oils were also shown anti-cancer properties against tested A549 lung cancer cells at increasing concentration. At 1000 µg/mL concentration, the essential oils were effectively targeted the responsive region and affected the entire nucleus (Fig. 4b). In this reason, the entire cancer cells were lost their effect and conveyed that the plant essential oils has concentration-dependent inhibitor against A549 lung cancer cells. Compared to chitosan loaded nanoparticle, the concentration was double time lower when use chitosan nanoparticles. Previously, Wang et al. (2021); Rajivagndhi et al. (2020) reported that the chitosan nanoparticle has anti-cancer properties against various cancer cells and inhibition was concentration dependent. In addition, recent report of Suresh Kumar et al., (2020), the high level of necrotic formation, increased death cells and chromatin condensation were shown after treatment with chitosan loaded essential oils. Finally, the result was concluded that the chitosan loaded essential oils were shown with more number of death cells and decreased viability against A549 lung cancer cells. These evidences were clearly indicated in Fig. 4, and it constructed its IC50 dose after the O.D values of all the chitosan and chitosan related materials.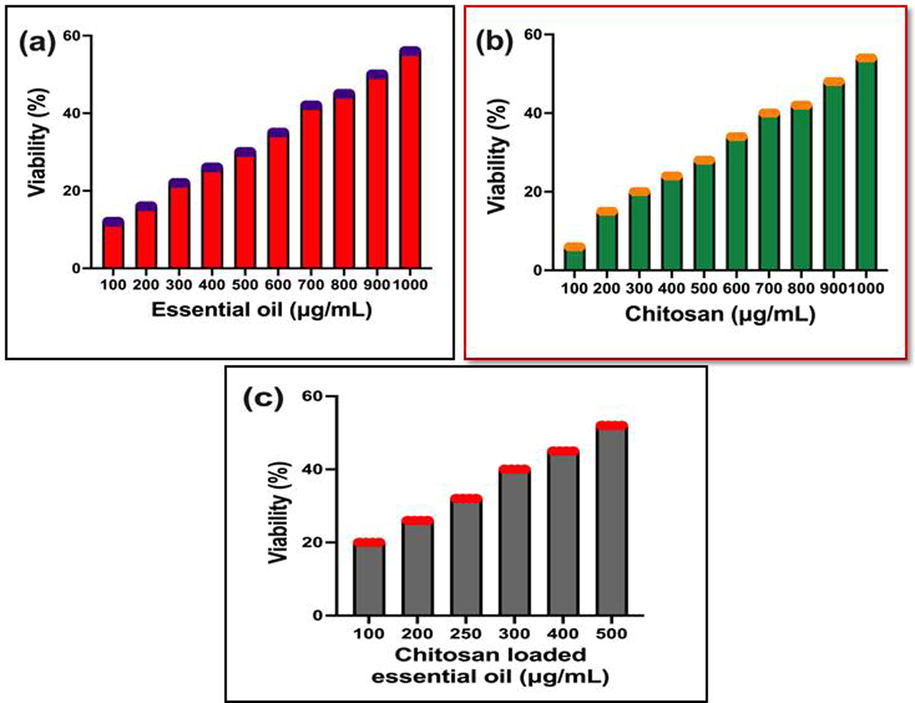
Quantitative measurement of cancer cell viability after treatment with chitosan nanoparticles alone (a), essential oils alone (b) and chitosan nanoparticles loaded essential oils combination (c).
3.3 External morphological variation using chitosan materials
Based on the IC50 concentration, the changes of outer morphology of the A549 lung cancer cells were exhibited in phase contrast microscope. In result, the original shape was altered and condensed structure was observed in the chitosan loaded essential oils. It clearly confirmed the cell viability assay, the chitosan nanoparticle loaded essential oils have the ability of anti-cancer inhibition. The nucleus was compromised to chitosan loaded nanoparticle and lost their pathogenicity leads to increase the apoptosis. Continuous cell death leads to complete the cancer cells process (Fig. 5b). The viability was altered and complete cell cycle arrest was not allowed the growth generation. In control, the clear, well matured, original A549 lung cancer cells structure without any damages were shown in Fig. 5a. Recently, reported that the outer morphology of the cancer cells were shown differently after treated with chitosan loaded nanoparticles.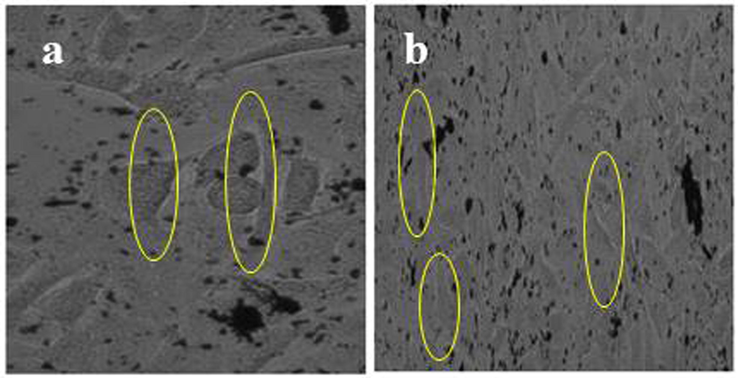
Phase contrast images of outer membrane morphology variations of A549 lung cancer cells of control (a) and chitosan nanoparticles loaded essential oils (b).
3.4 Internal invasion ability of chitosan nanoparticle loaded essential oils
After careful view of dual staining images, the treated cells were shown with irregular size and shape of A549 lung cancer cells. It was clearly stated that the chitosan nanoparticles loaded essential oils very effective in proliferation strategies. In Fig. 6b, the nucleus portion was entirely stained by AO/EB and exhibited internalized cells. The irregular shape and corrosion of size were shown after treat with chitosan loaded essential oils. The internalization process was shown with differential morphology compared with control cells. The control cells were also shown clear, smooth, original size and shapes (Fig. 6a). Recent report also favored to current research and AO/EB is the excellent fluorescence dye for use in the damaged parts of cancer cells. Plant essential oils is an excellent material to eradicate the cancer cells in increasing concentration and after loaded with chitosan, the efficiency against cancer cells were shown with high damaged cells in cancer cells. Recently, Barrera-Martínez et al. (2021) was worked with chitosan nanoparticle loaded chinnamaldehyde against cancer cells very efficiently, and it supported to current result. Similarly, Almanaa et al. (2021); Tamer et al. (2017) were reported that the chitosan loaded essential oils very effective against bacteria and cancer cells. Hence, the current result was proved that the chitosan loaded essential oils were very effective drug molecules for multi drug resistant bacteria and cancer cells.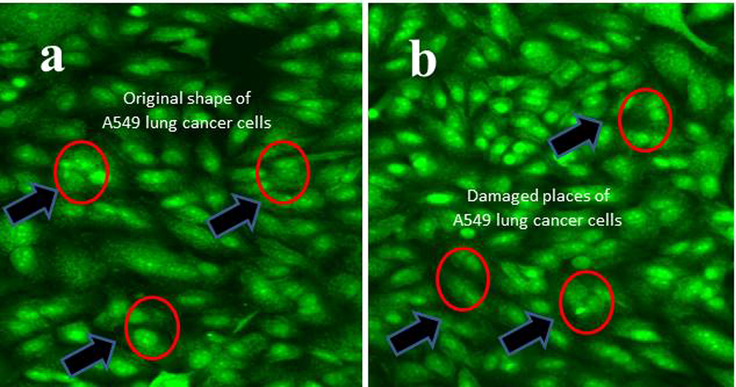
Inner membrane morphological variation using AO/EB florescence dyes viewed by fluorescence microscopy. In result, the original morphology of untreated control (a), and chitosan loaded essential oils (b).
3.5 High invasion efficacy of chitosan loaded plant essential oils in mitochondrial membrane
The morphological variations of cancer cells confirmation using phase contrast microscope and dual AO/EB dyes results were proved by mitochondrial membrane alteration result. The result of Fig. 7b was effectively shown the belbing, high necrotic cells, chromatin condensation, and decreased cell division after treatment with IC50 dose of chitosan loaded essential oils. In contrast, clear, original morphology of control cells were shown in full images (Fig. 7a). In addition, the necrotic lesions, nucleus parts deformation, irregular structure was seen in fluorescence microscope using Rodhamine 123. The result was clearly and confidently stated that the chitosan loaded essential oils very efficient against A549 lung cancer cells. Recently Farrag et al. (2021) reported that the anti-tumor effect of chitosan loaded essential oils, and it has more damaged effect than essential oil alone. Also, anti-lung cancer effect of Chen et al. (2018); Yee Kuen et al. (2020) were shown more necrotic lesion with sparse arrangement in cancer cells. It was conveyed to current study result, the chitosan loaded essential oil was more efficient than essential oil alone. In all the invitro experiments confirm, the chitosan nanoparticles has better efficiency, biocompatibility and biodegradation ability in various infections.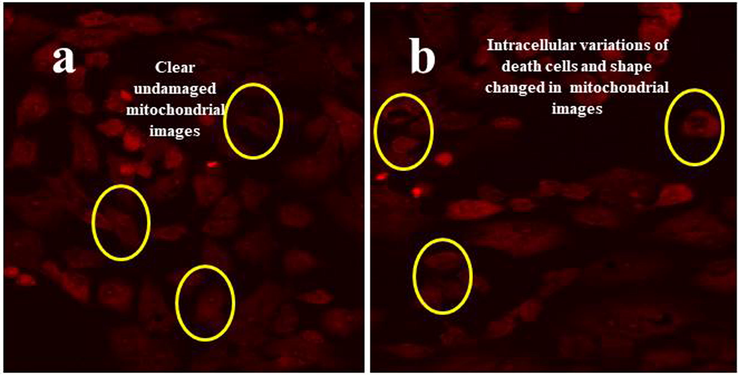
Complete structure of mitochondrial membrane variation using Rhodamine 123 florescence dyes viewed by fluorescence microscopy. In result, the original morphology of untreated control (a) and chitosan loaded essential oils (b).
4 Conclusion
Obviously, chitosan is an excellent biopolymer and derived from nature, which has been used for various biomedical applications. As same as the highly efficient biological properties of the molecules containing plant essential oils were currently dominant in research field. In addition, the plant essential oils were high competitor to antibiotics in the biomedical research field, especially focused in the area of cancer cells inhibition. The current result of chitosan loaded essential oils was very efficient against A549 lung cancer cells in concentration dependent manner. After careful observation of morphological studies, the treated cancer cells were clearly indicated that the chitosan loaded essential oils damaged the intracellular and extracellular parts. Finally, the result was concluded that the chitosan loaded essential oils not only inhibit the bacterial strains, it also very active against cancer cells also.
Acknowledgement
G. Rajivagndhi and F. Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R696), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-biofilm effect of Nerium oleander essential oils against biofilm forming Pseudomonas aeruginosa on urinary tract infections, Journal of King Saud University –. Science. 2021;33:101340
- [Google Scholar]
- Chitosan microparticles as entrapment system for trans-cinnamaldehyde: Synthesis, drug loading, and in vitro cytotoxicity evaluation. Int. J. Biol. Macromol.. 2021;166:322-332.
- [Google Scholar]
- Pleurotus eryngii polysaccharide nanofiber containing pomegranate peel polyphenol/chitosan nanoparticles for control of E. coli O157: H7. Int. J. Biol. Macromol.. 2021;192:939-949.
- [Google Scholar]
- Demethoxycurcumin-Loaded chitosan nanoparticle downregulates DNA repair pathway to improve cisplatin-induced apoptosis in non-small cell lung cancer. Molecules. 2018;23:3217.
- [Google Scholar]
- L.L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Ind. Crop. Prod.. 2018;125:85-94.
- [Google Scholar]
- Eugenol loaded chitosan nanoemulsion for food protection and inhibition of Aflatoxin B1 synthesizing genes based on molecular docking. Carbohydr. Polym.. 2021;255:117339
- [Google Scholar]
- In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol.. 2015;81:283-290.
- [Google Scholar]
- Green tea essential oil encapsulated chitosan nanoparticles-based radiopharmaceutical as a new trend for solid tumor theranosis. Int. J. Biol. Macromol.. 2021;186:811-819.
- [Google Scholar]
- Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol.. 2019;123:837-845.
- [Google Scholar]
- Apoptotic induction and anti-metastatic activity of eugenol encapsulated chitosan nanopolymer on rat glioma C6 cells via alleviating the MMP signaling pathway. J. Photochem. Photobiol. B Biol.. 2020;2013:111773
- [Google Scholar]
- Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol.. 2018;118:676-682.
- [Google Scholar]
- Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol.. 2021;186:656-685.
- [Google Scholar]
- Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol.. 2020;164:4010-4021.
- [Google Scholar]
- Physiochemical characterization and anti-carbapenemase activity of chitosan nanoparticles loaded Aegle marmelos essential oil against K. pneumoniae through DNA fragmentation assay. Surfaces and Interfaces. 2021;23:100932
- [Google Scholar]
- Development of encapsulated peppermint essential oil in chitosan nanoparticles: characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Physiol.. 2020;170:104679
- [Google Scholar]
- Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: A nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biol. Macromol.. 2020;143:382-392.
- [Google Scholar]
- Caspase dependent apoptotic activity of polycyclic cage-like heterocyclic hybrids. Saudi J. Biol. Sci.. 2020;27:3290-3300.
- [Google Scholar]
- Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr. Polym.. 2017;169:441-450.
- [Google Scholar]
- Bioassay-guided isolation and antiproliferative efficacy of extract loaded in chitosan nanoparticles and LC-QTOF-MS/MS analysis of Achillea magnifica. S. Afr. J. Bot.. 2020;133:236-244.
- [Google Scholar]
- Controlled release of turmeric oil from chitosan nanoparticles extends shelf life of Agaricus bisporus and preserves its postharvest quality. Food Biosci.. 2021;44:101401
- [Google Scholar]
- Prospects and challenges of anticancer agents’ delivery via chitosan-based drug carriers to combat breast cancer: a review. Carbohydr. Polym.. 2021;268:118192
- [Google Scholar]
- Increased cytotoxic efficacy of protocatechuic acid in A549 human lung cancer delivered via hydrophobically modified-chitosan nanoparticles as an anticancer modality. Polymers. 2020;12:1951.
- [Google Scholar]
- Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci.. 2020;166:108137
- [Google Scholar]
- Biosynthesized silver nanoparticles using Caulerpa taxifolia against A549 lung cancer cell line through cytotoxicity effect/morphological damage. Saudi J. Biol. Sci.. 2020;27:3421-3427.
- [Google Scholar]







