Translate this page into:
Anti-biofilm compound of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane from marine Nocardiopsis sp. DMS 2 (MH900226) against biofilm forming K. pneumoniae
⁎Corresponding author at: School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the 16S rRNA of the marine endophytic actinomycete was characterized and identified as Nocardiopsis sp. strain DMS 2 (MH900226). Partial purification and spectroscopies data were interpreted and confirmed as 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane. Anti-bacterial activity of the crude extract and partially purified compound were shown highest inhibition activity against biofilm forming K. pneumoniae. Minimum biofilm inhibition concentration of identified compound against K. pneumoniae was exhibited at the concentration of 300 µg/mL. Decreased metabolic activity and modified exopolysaccharide production of biofilm forming cells were observed at 350 µg/mL and 300 µg/mL concentration. At the same biofilm inhibition concentration, the intracellular architecture of the biofilm cells and extracellular shape modifications were clearly viewed by confocal laser scanning electron microscope and scanning electron microscope respectively. All the in-vitro inhibition results and microscopic images were strongly suggested that the identified compound was a potential anti-biofilm agent against tested K. pneumoniae.
Keywords
Marine environment
Endophytes
Nocardiopsis
Minimum biofilm inhibition concentration
Metabolic activity
Intracellular damages
1 Introduction
Marine nature is presenting with 70% of water with more complex system, typically Earth was emerged from the Sea (Ismail et al., 2016). The presence of unidentified microbes from marine environments estimates highly in their potential biological diversity compared with terrestrial microbes (Leetanasaksakul and Thamchaipenet, 2018; Valli et al., 2012). Among the microbes, actinomycetes are the important Gram positive, filamentous bacteria holding 80–90% of GC rich content (Ballav et al., 2015). Recent years, the non-Streptomyces are considered as potential sources for new antibiotic synthesis and important hydrolytic enzymes production with promising biomedical and pharmaceutical properties (Zheng et al., 2000). Some of the non-Streptomyces are frequently discovered from various sources; they are Nocardiopsis, Actinomadura, Pseudonocardia, Saccharomonospora, Sreptosporangium and Micromonospora (Rajivgandhi et al., 2018). Most of the non-Streptomyces strains are discovered from marine sources. It is more favor in alkaline and organic matter contains soli, which reproduce the spore formation (Davies-Bolorunduro et al., 2019).
Recent years, the discovery of unpredicted antibiotic derivatives from marine endophytic Nocardiopsis is considered as a prime target. Marine is a vast nature of source for producing new types of antibiotics. The marine environmental behaviors of pH, temperature, NaCl, carbon, nitrogen and some other sources are involved in the growth and also productivity in actinomycetes. The microbes that harboring the inside of host complex system without any harmful is considered as endophytes (Tanvir et al., 2016). Likewise, endophytic actinomycetes are rich and reliable sources for new undefined biomolecule producer. Till-date, endophytic actinomycetes form marine seaweed is rarely explored; the reported result was mainly focused on fungi. Recently, some researcher are reported that the endophytic actinomycetes from marine seaweed is an essential study and it produced enormous biomedical compounds including anti-microbial, anti-viral, anti-oxidants and larvicidal effect (Ganesan et al., 2017). Also, the seaweed derived actinomycetes have the ability to produce different variety of novel compounds that helped against various bacterial infections. Based on the above facts, the present study is highly concentrated on the interesting sources of marine endophytic actinomycetes for inhibit the multi-drug resistant K. pneumoniae.
2 Materials and methods
2.1 Isolation and identification of actinomycetes
The marine seaweed sample of Caulerpa racemosa was collected in Gulf of Mannar Region, Southeast coast of Tamil Nadu, Rameshwaram, India. Small pieces of inner seaweed portions were made by cutting and initially performed surface sterilization including double distilled water, 70% ethanol, sodium hypochlorite. All the sterilizations were made between the time intervals of 5 min. After, dried pieces were gently inserted on the starch casein agar surface and maintained at 28 ℃ for 96 h. Next, the actinomycetes colonies arisen around the seaweed inserted places of starch casein agar surfaces were detected based on the visibly observation of phenotypic characterization.
2.2 Proof of endophytic actinomycetes
The interesting method of proof validation method is used to detect the originality of isolated strains followed by Rajivgandhi et al. (2018). Parallel experiment of last wash surface sterilized water and unsterilized algal samples were inoculated separately on the International Strptomyces Project Medium (ISP-2) agar plate and maintained one to two days at ordinary atmosphere. Then, the plates were observed visibly and noted the result and interpreted the originality of sterilization. In result, without contamination of plate containing no growth and more fungal contamination around the unsterilized seaweed samples were suggested that the sterilization was pure.
2.3 Primary anti-biotic activity of crude extract
Detection of anti-bacterial ability of selected strains was performed agar well diffusion method and the method was followed by Ismail et al. (2016). A full loop of old culture inoculated liquid sample was taken and spread on muller hinton agar surface, followed by made different wells. Then, all the wells were filled by different dose of crude extract of actinomycetes strains and incubated at one day for room atmosphere. Then, the plates were visibly viewed and measured the zone around the wells using measuring scale and anti-bacterial effect was determined. Whereas, the antibiotic disc ceftazidime was used for detect the multi-drug resistant nature of K. pneumoniae.
2.4 Molecular identification of active strain
Based on the anti-bacterial activity, the genomic DNA of DMS 2 strain was used to detect 16S rRNA based molecular identification using previously reported evidence of Shin et al. (2010). In this study, thermal cycler PCR was used to amplify the DNA of DMS 4 using universal primers of forward −5′-CTTATTGTATACCCTTAGCAT-3′ and reverse primer-5′-TACCCTAGCTCACCTTGAACCTGCAATT-3′. The set up was fixed for 35 cycles followed by initial denaturation of 94℃ for 5 min and extended to 2 and 1 min at 54℃ and 72 ℃ respectively. The final extension temperature was continued for 15 min at 72℃. The purity of DNA was checked by using NanoDrop and purified amplicons were received. Consecutively, the amplicons were sent to sequences in Rajiv Gandhi Centre for Biotechnology (RGCB), Thiruvananthapuram, Kerala using automated DNA sequencer. The noise was removed from complete sequences and made contigs from pure sequences. The pure sequences were compared to pair wise identities of similar sequences that received from www.ncbi.nlm.nih.gov/blast. Then, the confirmed sequences were submitted in GenBank. After submission, the accession number was received from NCBI and multiple sequences were aligned by CLUSTAL W program. Maximum 1000 replicates of the resulted data was made phylogenetic tree using neighbour-joining method.
2.5 Partial purification of anti-bacterial compound
The dried crude extract of DMS 2 Based on the movement of DMSO mixed crude extract spot was partially purified by TLC using different solvent combination as mobile phases including toluene:acetic acid:chloroform (1:10:2), chloroform:alcohol (1:10), ethyl acetate:water:chloroform (5:10:5). After movement from mobile pahases, the complete movements of the spots were scrapped and performed anti-bacterial activity to confirm the anti-bacterial ability.
2.6 LC-MS detection of possible anti-bacterial compounds
LC-MS analysis of purified HPLC was performed for detection of available chemical compounds from DMS extract and followed by previous reported evidences of. The LC-MS was fitted through pass of the electro spray ionization mass spectrum (ESIMS). Finnegan surveyor auto sampler was used to pass the 100 μL extract into the ESI and 100–1000 nm range of mass spectra was used to mass analysis. 1 mL/min of gradient elated program was set for the process and 1 h of injection time was used with 4kv voltage. The ion spray voltage was 40kv and capillary filter was used three times. Continuously 1 h run the machine of MS for compound detection and it performed by 2.0 SRI of Xcalibur. The mobile phases of acetonitrile:methanol:alcohol combination.
2.7 Minimum biofilm inhibition concentration
Turbidity based inhibition of identified compound treated e was visually inspected in 24-well polystyrene plate using microtitre plate (Vinotha et al., 2019). The MIC is suggested that the highest turbidity of identified compound treated wells of the K. pneumoniae in the lowest concentration. Each well was filled by 10 µL of overnight confluent culture and increasing concentration (25–200 µg/mL) of identified compound into the tryptic soy broth. Plate was monitored at room temperature with one day and then observed visibly for turbidity. Based on the visible observation, the minimum biofilm inhibition concentration was calculated after read at 600 nm of microtitre plate reader (Sigma, Mumbai, India). The O.D values were converted to percentages and inhibition percentages were noted by bellowed equation,
2.8 Biofilm metabolic assay
After 24 h treatment of bacteria plus 25–200 µg/mL of actinomycete compound, the non-adherent cells were removed by using 10x PBS and 10 µL of 2,3-bis(2-methoxy-4-nitro-5 sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) solution was treated on the biofilm cells of the 24-well polystyrene plate. The plate was monitored under room temperature for 5 min and followed by addition of 1 µL of menadione acetone solution for 5 min. The reaction mixture was washed with 10x PBS and maintained in dark condition (Maruthupandy et al., 2020). Finally, 1 mL of ethanol was added into the well and read at 600 nm O.D for detection of bacterial survival. The result was done in triplicate and converted to percentage of inhibition using bellowed formula,
2.9 Intracellular damages by CLSM
The architectural structure of K. pneumoniae biofilm arrangement was abolished by BIC of actinomycete compound and detected by CLSM (Tenconi et al., 2018). Shortly, biofilm forming K. pneumoniae culture was inoculated into the TSB along with BIC of actinomycete compound. After, 1x1 cm size cover slip was soaked in inside of the 24-well polystyrene plate samples. The plate was allowed to grow the culture on cover slip at room temperature for one day. Contrary, without treated bacterial culture was used for control. The plate was maintained at room temperature 24 h. Then, cover slip was taken and washed thrice by 10x PBS and followed by D.D·H2O to remove non-adherent cells. Then, the cover slip was treated with 1 mg/mL acridine orange (AO), and then stained cells were viewed under CLSM using 480 nm arogon laser with 640 nm band pass emission filter.
2.10 SEm
Extracellular morphology of BIC treated or untreated biofilm forming K. pneumoniae was analyzed by SEM using following report of Maruthupandy et al. (2018). Briefly, after incubation of overnight culture with BIC of actinomycete compound combination was centrifuged with 2,500 rpm for 30 min, and washed the sample by 10x PBS. Next, the pellet was diluted by PBS and further centrifuged at same procedure. After, the cells were fixed with 4% fresh glutaraldehyde and kept for 4 h. After fixed, the cells were transferred to polycarbonate membrane filter and followed by dehydrated using ethanol graded serious 30–100%. After, the cells were treated by t-butanol and maintained for 2 h. Finally, the cells were coated by aluminum sputter with 15 nm thickness of gold–palladium metal (60:40 alloys). The cover slip was air dried and viewed under 20kv of SEM (Tokyo, stereo scan Japan).
3 Result
3.1 Isolation and identification of actinomycetes
Clear white color, pale yellow like powdery white, spore forming observation with clear round colonies were detected 15 numbers around the Caulerpa racemosa inserted places. All the phonotypical observation result was shown with arial mass, clear reverse side and melanoid pigment (Fig. 1a). Based on the barge’s theory and previously reported evidences were significantly correlated to present result of visible observation (Rajivgandhi et al., 2018). In addition, absence of growth in the last wash water spread starch casein agar plate was also suggested to present result, and the arisen colonies were originated from inside of the algae. Further, the parallel result of unsterilized algae plate was exhibited with fungal contamination (Fig. 1b). It was also clearly confirm, the surface sterilization was excellent and the emerged colonies were pure colonies (Jacintha Jasmine and Agastian, 2013). Identified pure colonies were streaked on starch casein agar for further studies (Fig. 1c). Our validation evidences were agreed by previous reported result of Ramachandran et al. (2019).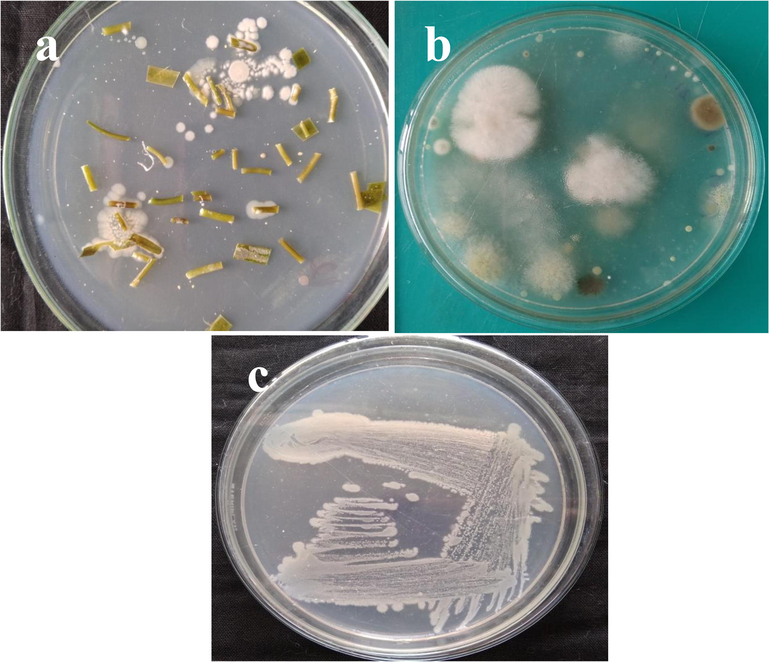
Isolation and identification of endophytic actinomycetes from marine algae (a), parallel experiment of fungal contamination (b) and screening of pure cultured DMS 2 strain in starch casein agar plate.
3.2 Initial bacterial inactivation assay
Among the isolated strains, the anti-bacterial effect of the strain against K. pneumoniae was observed and the effective strain was named as DMS 2. Also, the result was shown with excellent zone of inhibition after one day gap with unpurified crude extract against biofilm forming. The 15 mm zone of inhibition was shown at 500 µg/mL concentration (Fig. 2). Whereas, the distilled water loaded well was shown with no zone of inhibition. Therefore, the result was suggested that the crude extract of the DMS 2 extract has anti-bacterial effect. Previously, marine actinomycetes crude extract has better anti-bacterial activity against bacteria and it influences by marine environmental factors including nutrients, salt stress, organic and inorganic nutrient (Valli et al., 2012).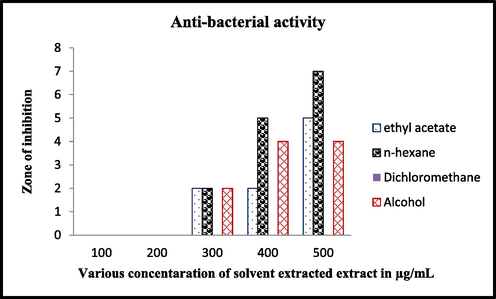
Bacterial inactivation of various solvent extracted crude extract of DMS 2 strain.
3.3 Molecular identification of DMS 2 strain
Excellent anti-bacterial activity of DMS 2 strain was genomically identified based on the obtained sequences. The obtained sequences of DMS 2 strain was compared with more similar NCBI strains sequences and pair wise alignment was made properly. Based on the similarities of related sequences, the DMS 2 strain was closely related to the genus Nocardiopsis. Consecutively, the related sequences similarities were closely adjusted to the genus of Nocardiopsis and indicated as Nocardiopsis DMS 2. Then, NCBI submitted sequences were used to get the accession number. After submission in NCBI, the number of MH900226 was received. Finally, the identified actinomyces strain was named as Nocardiopsis sp. DMS 2 (MH900226) (Fig. 3)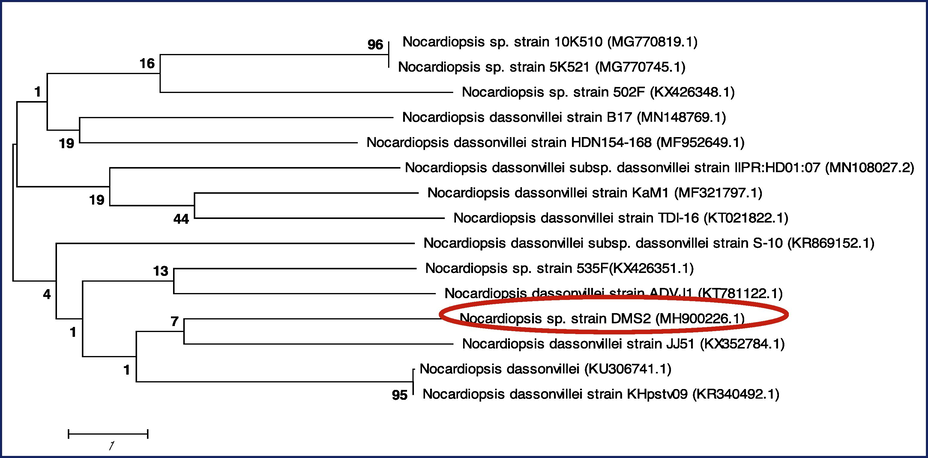
Phylogenetic tree construction of Nocardiopsis sp. DMS 2 (MH900226).
3.4 Partial purification of active anti-bacterial compounds by TLC
In the mobile phase of toluene:acetic acid:chloroform (1:10:2), the movement of the spots were observed at 0.30, 0.29 and 0.24 distances. Among the distances, the first spot was shown increased anti-bacterial activity compared to other two spot. Among the all, the spot 1, spot 2 and spot 3 were shown with 15 mm, 0 mm, 6 mm zone of inhibition against K. pneumoniae was observed (Fig. 4). This result was confirmed that the anti-bacterial metabolites of the chemical materials were mostly present in the spot 1 and this spot was taken more amounts by using preparative TLC method for further use. Previously, Ramachandran et al. (2019) reported that the partial purification of TLC method was very suitable method for detection of available compounds. In addition, among the complete chemical groups, the active chemical compounds only moved on TLC plate. It was used to detect the individual compound detection.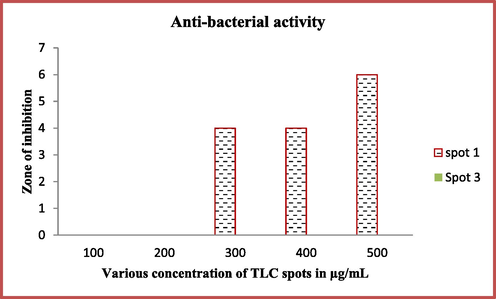
Partial purification of TLC spots mediated active compounds against K. pneumoniae.
3.5 Available chemical components detection by LC-MS
Excellent anti-bacterial activity of TLC spot 1 was effectively monitored by LC-MS for detection of complete chemical compounds analysis. After interpretation with NSIT Wiley library result, the bioactive compounds of 2-hydroxy-3-methyl, phenylethyl alcohol, nicotinic acid, phenol, 2,4-bis(1,1-dimethylethyl), benzene, 1,1′-[oxybis(methylene)]bis, e-14-hexadecenal, 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane, cycloicosane. All these compounds were detected based on the retention time, area, area of percentages. All these chemical compounds may have the biological properties. These compounds have the retention times of 13.25, 14.43, 20.64, 17.45, 30.60, 10.16, and 30.45. The area of 245678, 1237896, 231452, 1786543, 2345897, 1803451, and 4, 45678, and area percentages of 0.86, 1.2, 0.8, 0.9, 1.2, 1. 4 and 1.5 were observed. Based on the literature review, the anti-bacterial activity of the 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane, was highly occupied, and also have more area and more percentages were observed (Fig. 5). Previously, Lin Li et al. (2015), reported that the 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane, compound has excellent anti-bacterial activity. This result was agreed by Ye et al. (2017) and the marine actinomycetes compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane act as an excellent anti-bacterial agents against multi drug resistant bacteria. Previously, Ser et al. (2015) reported that the identified compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane was identified from marine Streptomyces pluripotens and it has more cytotoxicity against various cancer cells. Recently, marine endophytic actinomycete Nocardiopsis sp. was synthesized the 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane compound with excellent anti-microbial activity (Rajivgandhi et al., 2019). The result was agreed by Ramachandran et al. (2019), and partially purified TLC extract was exhibited the excellent anti-bacterial activity compound of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane (Ramachandran et al., 2019).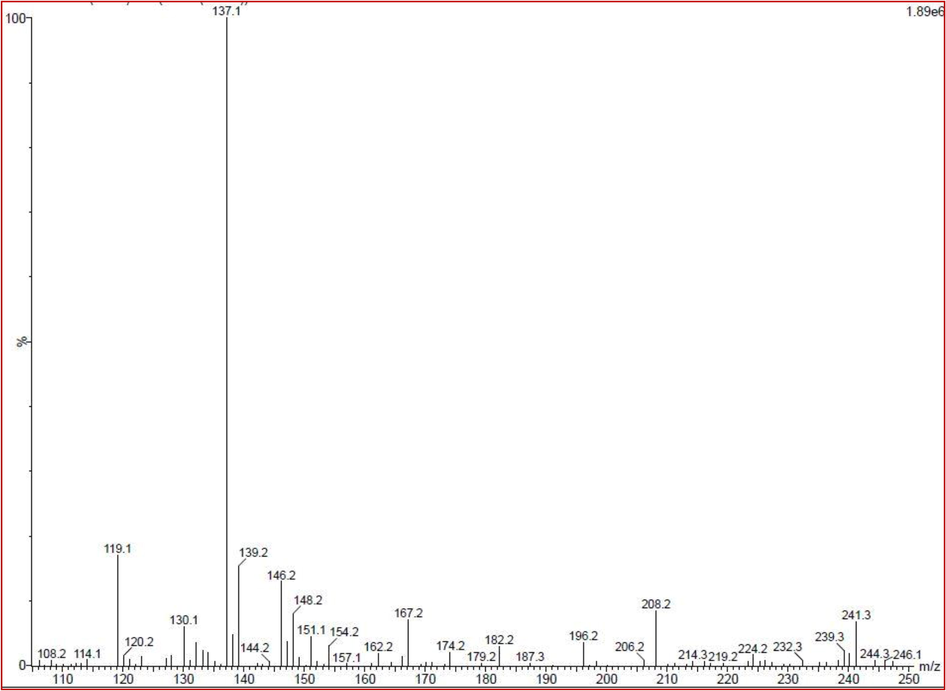
Detection of complete chemical composition of TLC purified actinomycete extract by LC-MS.
3.6 Minimum biofilm inhibition concentration
Based on the O.D values of treated and untreated 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane containing bacterial culture was exhibited in Fig. 6. In our result, the 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated bacterial well was gradually decreased the attachment of bacteria at respective increased concentration. Whereas, the control O.D value result was shown with well grown colonies. It suggested that the 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane has anti-biofilm activity against tested . The comparison of control and test result O.D values was converted to percentages to detect the biofilm inhibition percentages. The highest inhibition rate of 94% was measured at 300 µg/mL and no inhibition was observed only in control well (Fig. 6a). Therefore, it convinced the result of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treatment against biofilm formation. The result was revealed, biofilm inhibition concentration was fixed to 300 µg/mL concentration (Fig. 6a).![Minimum biofilm inhibition concentration (a), biofilm metabolic activity (b) of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane against K. penumoniae.](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.012-fig6.png)
Minimum biofilm inhibition concentration (a), biofilm metabolic activity (b) of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane against K. penumoniae.
3.7 Biofilm metabolic assay
It is a typical experiment for detection of damaged biofilm cells after treatment of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane (Maruthupandy et al., 2002). After addition of XTT solution, the increased death cells were observed at 350 µg/mL concentration due to the decreased production of formazan (Fig. 6b). Due to the decreased production of formazan, the bacteria lost their pathogenicity and cannot be synthesized extracellular materials such as polysaccharides, quorum sensing, ESBLs production and DNA production (Rajivgandhi et al., 2018). The absence of virulence factors of treated cells were shown with minimum viability when compared with control at increasing concentration. The biofilm formed cells were compromised and more sensitive to 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane at 200 µg/mL concentration. The rate of decreased metabolic arrest rate against e was 88%. The inhibition percentage result based on the tested 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane concentration was available in Fig. 7a.![Intracellular modification of of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. pneumonia by CLSM (a, b).](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.012-fig7.png)
Intracellular modification of of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. pneumonia by CLSM (a, b).
3.8 Intracellular damages by CLSM
The demolished architectural arrangement in the BIC of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated cells was observed under CLSM. Based on the observation, the rough surface of bacteria with green color structure was compromised when compared to control. AO is a fluorescence dye, it has the binding ability of bacterial nucleus with green color morphology (Maruthupandy et al., 2020). After addition AO, it permeates through surface layers and bind with damaged and normal nucleus. It differentiated form reduced thickness of biofilm arrangement compared to control and increased death cells. Based on the regulation of AO, the green color emitted tightly clumped biofilm structure of the control (Fig. 7a, c), and sparse with loosely associated separated colonies of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated cells were shown in Fig. 7b, d.
3.9 SEm
Outer membrane morphology of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated biofilm forming cells were shown in Fig. 8. The positive charges of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane was attracted by negative charged bacterial surface and led to form compromised surface. In this process, the chemical groups of the ions were enter into the ion channels of porin and transferred the amino group to remain the anti-bacterial activity (Maruthupandy et al., 2018). After dehydration, the fixed cells were shown with low integration of polysaccharides arrangement and belbing morphology in 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated sample (Fig. 8a). Whereas, the bundle cells aggregation with clear rod shaped morphology of control cells were shown in untreated control sample (Fig. 8b). The stress formation in the bacterial surface morphology had shown damaged rod shape for treated cells. Also, the hank-like polysaccharides containing structure was destructed.![Outer membrane damages of of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. penumoniae by SEM (a, b, c).](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.012-fig8.png)
Outer membrane damages of of actinomycete compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. penumoniae by SEM (a, b, c).
4 Conclusion
Based on the molecular identification, the marine actinomycetes Nocardiopsis sp. strain DMS 2 (MH900226) was identified. Based on the agar well diffusion, the partially purified TLC extract was exhibited more inhibition against tested bacteria. Further, the minimum biofilm inhibition concentration study result was exhibited the anti-biofilm effect at 300 µg/mL concentration. The decreased metabolic activity of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. pneumoniae result was exhibited at the concentration dependent inhibition. Importantly, the architectural damages of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane treated K. pneumoniae cells were showed with decreased biofilm arrangement. The microscopic results of CLSM and SEM were proved the intracellular and extracellular damages in the treated K. pneumoniae. Therefore, the present study was concluded that the compound 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane was dominant compound and it has promising anti-biofilm effect against K. pneumoniae.
Acknowledgments
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573, 91951205, 31670009 and 31850410475) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Halophilic and halotolerant actinomycetes from a marine saltern of Goa, India producing anti-bacterial metabolites. J. Bioscie. Bioeng.. 2015;119:323-330.
- [Google Scholar]
- Anticancer potential of metabolic compounds from marine actinomycetes isolated from Lagos Lagoon sediment. J. Pharmaceut. Anal.. 2019;9:201-208.
- [Google Scholar]
- Isolation and molecular characterization of actinomycetes with antimicrobial and mosquito larvicidal properties. Beni-Suef Univ. J. Basic Appl. Sci.. 2017;6:209-217.
- [Google Scholar]
- Antimicrobial activities of bacteria associated with the brown alga padina pavonica. Front. Microbiol.. 2016;7:1072.
- [Google Scholar]
- In vitro antioxidant activity and in vivo alpha glucosidase activity of endophytic actinomycetes isolated from Catharanthus roseus (l.) J. Pharm. Res.. 2013;6:674-678.
- [Google Scholar]
- Potential anti-biofilm producing marine actinomycetes isolated from sea sediments in Thailand. Agricult. Nat. Resour.. 2018;52:228-233.
- [Google Scholar]
- Acetylcholinesterase inhibitory dimeric indole derivatives from the marine actinomycetes Rubrobacter radiotolerans. Fitoterapia. 2015;102:203-207.
- [Google Scholar]
- Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog.. 2018;121:224-231.
- [Google Scholar]
- Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa. Carbohydrate Polym.. 2020;230:115646.
- [Google Scholar]
- Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs. Microb. Pathogenesis. 2018;118:190-198.
- [Google Scholar]
- Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) and its antibacterial efficacy against isolated ESBL producing bacteria. Microb. Pathogenesis. 2019;126:138-148.
- [Google Scholar]
- Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens. Biocatal. Agricult. Biotechnol.. 2019;17:750-757.
- [Google Scholar]
- Evaluation of antioxidative and cytotoxic activities of streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front. Microbiol.. 2015;6:1398.
- [Google Scholar]
- An angiogenesis inhibitor isolated from a marine-derived actinomycete, Nocardiopsis sp. 03N67. Phytochem. Lett.. 2010;3:194-197.
- [Google Scholar]
- Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation. Microbiolog. Res.. 2016;185:22-35.
- [Google Scholar]
- Production of prodiginines is part of a programmed cell death process in streptomyces coelicolor. Front. Microbiol.. 2018;9:1742.
- [Google Scholar]
- Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pacif J. Trop. Biomed. 2012:469-473.
- [Google Scholar]
- Synthesis of ZnO nanoparticles using insulin-rich leaf extract: anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B: Biolog.. 2019;197:111541.
- [Google Scholar]
- Antiproliferative cyclodepsipeptides from the marine actinomycete Streptomyces sp. P11–23B downregulating the tumor metabolic enzymes of glycolysis, glutaminolysis, and lipogenesis. Phytochemistry. 2017;135:151-159.
- [Google Scholar]
- Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS Microbiol. Lett.. 2000;188:87-91.
- [Google Scholar]







