Translate this page into:
Anti-bacterial effect of marine sea grasses mediated endophytic actinomycetes against K. pneumoniae
⁎Corresponding authors. sshine@ksu.edu.sa (Shine Kadaikunnan), rajivgandhimicro@yahoo.com (Govindan Rajivgandhi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the pure, pale white, powdery nature and pigment produced endophytic actinomycetes strains were isolated from marine Sea grasses sample. The isolated strains were endophytes as confirmed by surface sterilization and validation methods. The anti-bacterial efficacy materials of the crude compound were extracted by liquid–liquid extraction using various organic solvents. Anti-bacterial efficacy of the isolated actinomycetes strain RMN 10 was exhibited excellent anti-bacterial activity against K. pneumoniae at increasing concentration. In addition, the minimum inhibition concentration (MIC) of the crude actinomycetes compound has excellent anti-bacterial activity at 250 µg/mL concentration and this concentration was fixed as a MIC. Further, the structural and shape damages of actinomycetes crude compound treated K. pneumoniae cells were clearly identified in SEM images. All the invitro experiments were proved that the actinomycetes compound has excellent anti-bacterial activity against K. pneumoniae.
Keywords
Marine environment
Actinomycetes
Bacterial inactivation
Minimum inhibition concentration
Scanning electron microscope
1 Introduction
Marine environment is very vast environment, and it has interesting and important environment based on the unpredictable sources. Ocean water was spread seventy five percentages of the earth with unpredictable biological materials (Betancur et al., 2020). It covers almost all the sources including living or non-living sources (Seghal Kiran et al., 2018). All these sources are produced beneficial materials except some of them. In unpredictable marine environment, the water sources, plant materials, microbes and algae are very most important than others. All these sources are playing more important biological roles within the marine nature or also outer environments (Du et al., 2019). Recent years, most of the research plate forms are depends on the marine nature because of new materials arrival (Leetanasaksakul and Thamchaipenet, 2018). Compared with terrestrial, oceanic environment based research is very low including physical, chemical and biological studies (Valli et al., 2012). In biological state, the marine is a clean, clear and advantages environment for producing new kind of materials (Ramachandran et al., 2018). Recent years, some researchers are reported that the marine based microbes exhibited new bioactive compounds, and they have enormous biological activities including anti-bacterial, anti-fungal, anti-viral, anti-biofilm, anti-cancer, larvicidal and etc (Rajivgandhi et al., 2019a; Siddharth and Ravishankar Rai, 2019). More amount of unpredictable nutrients such as carbon, nitrogen, diverse chemical metabolites, biodiversity products, enzymes and different levels of pH, temperature, NaCl are present in marine sources only. Apart from other sources, marine microbes are very much interesting factors to synthesize new kind of secondary metabolites for biological activities. It has been reported with various biological activities frequently and it is the most important sources for synthesis novel bioactive compounds.
Mostly, marine microbes are complex structure compared with terrestrial which exposed to extreme environment. This new kind of atmosphere was used to help produce novel secondary metabolite to microbes. It offering potential to understand and treatment for various infections due to the greater physiological structure. Recently, more peptides, proteins, enzymes and unidentified compounds producing microbes are reported by some researchers Laith et al. (2017); Agrawal et al. (2020). Compare with terrestrial, the marine environment is most advisable for researchers to identify new chemical derivative for apply in the field of biological, pharmaceutical and healthcare settings (Rajivgandhi et al., 2016). In marine environment, the microbes can be grown in low, medium, high and extreme temperature. All of them have a different characteristic natures including bioactive metabolites production (Parama Cita et al., 2017). Among the marine microbes, bacteria are the most dominant microbes. It produces novel chemical derivatives frequently (Vijai Sharma and David, 2012). More number of new chemical groups with biological properties is produced by bacteria. Compared with other microbes, bacteria are easy to isolate, identify and characterize. Also, it can be survived in normal atmosphere and also produced new varieties of the compounds. Compared with other sources, marine Sea grasses have excellent bioactivities and chemical properties. In marine environment, the flowering plants are called as a Sea grasses. It comes under the angiosperms and grown in the entire marine environment including tidal and low tidal level of the Sea. Family of Sea grasses are Hydrocharitaceae and Potamogetonaceae. Till-date, 25 genus were reported from various places. The specialty of Sea grasses is holding the large microbial population in their roots, leaves, and rhizomes. They have abundant medicinal and biological properties and chemical derivatives. It has more unique secondary metabolites producer with excellent bioactivities against various infectious pathogens.
Among the bacteria, actinomycete is the dominant species that used frequently in biological activities. It is gram positive, GC rich microbes and simply termed as fungi like bacteria depends on the spore production (Hameş-Kocabaş and Uzel, 2012). Compared with others, it has been reported 50,000 marine bioactive compounds and all of them having excellent bioactivities. More than 10, 000 compounds were reported against various infections successfully (Babu et al., 2018). In actinomycetes, the Genus Streptomyces is an important microbes, and it only produce 35, 000 bioactive compounds against various infection (Rajivgandhi et al., 2018a, 2018b). In addition, various other genus also present mostly in the actinomycetes group including Nocardiopsis, Micromonaspora, Actinomadura and etc (Khan et al., 2019). Recent years, new kinds of microbes have been emerged worldwide with very excellent bioactivities which are called as endophytes (Zhang et al., 2018; Yara et al., 2020). The microbe that lives inside of the host plant materials is called as endophytes. It has enormous bioactivities compared with others with excellent bioactivities producing abilities.
Endophytic actinomycetes are important microbes, they are emerging recent years with increasing bioactivities against various infections (Rajivgandhi et al., 2019b). Recent reports are more evident for biological activities with increased bioactivities. Also, new, undesired bioactive compounds with excellent anti-microbial, anti-biofilm and anti-cancer activities (Ramachandrana et al., 2019). They are very difficult microbes and produce different bioactivities due to the utilization of plant sources including hormones, nutrients, carbon and nitrogen sources. Based on this nature, the endophytic organisms are produced excellent bioactivities with new classes of antibiotics (Parthasarathy et al., 2020). Altogether, this research is supported by based on the previous reported evidences and compounds. Consequently, we have concentrated on isolation and extraction of endophytic actinomycetes compound from marine sea grasses for inhibit the multi-drug resistant bacterial infections.
2 Materials and methods
2.1 Collection of samples
All the chemicals and glass wares of this study was purchased from ponmani@Co, Tiruchirappalli, India. All the media, PBS, and other solutions of this study were ordered from Ponmani@Co, Tiruchirappalli, India. The sample of the Sea grasses were collected from Gulf of Mannar Region (Latitude 9°15′41.88″N, Longitude 79°04′05.81″E), Rameshwaram, Southeast Coast of India, Tamil nadu, India. The young Sea grasses were collected from 10 m inside from the Sea shores. After collect, the samples were cleaned using Sea water and followed by fresh water. Then, the samples were taken to laboratory and cut in small pieces (6 mm) and processed surface sterilization according to the protocol of Ramachandrana et al. (2019). All the pieces were sterilized in 70% alcohol for 5 min followed by 1.5 M sodium hypochlorite (NaClO) for 5 min. Finally, the samples were washed by double distilled water for discard the more surface sterilants and keep in air dry until the remove of the wet condition. Next, the samples were slightly punched on the actinomycete isolation agar (AIA) plates supplemented with penicillin, nystatin and cycloheximide for suppress the contaminants. Finally, plates were maintained at incubator at room temperature seven days. Finally, the actinomycetes were emerged from inside of the Sea grasses of the samples were identified in AIA plates and confirmed the actinomycetes using phonotypical methods.
2.2 Detection of pure surface sterilization evidence
In endophytic actinomycetes isolation, the surface sterilization method is unavoidable experiment to detect the clear endophytes (Usha Nandhini et al., 2018). Shortly, the last step surface sterilized double distilled water was spread on the starch casein agar plates (SCA) and incubated at 28 ℃ at 48 h. Supportive for surface sterilization, the unsterilized Sea grass samples were also pressed on the AIA plates directly and incubated at 28 ℃ at 48 h. After incubation, no any microbial growths of the last wash water containing AIA plates, and fungal contamination of the unsterilized plates of the reports were noted for differentiation of pure or non-pure endophytes validation.
2.3 Extraction of crude compound
For detection of anti-bacterial efficacy, the isolated actinomycete strains were inoculated in actinomycete isolation broth and maintained 17 days to produce potential growth. After 17 days, the fermented broth culture was taken and centrifuged at 10, 000 rpm for 15 min with 4℃. After, the pellets were removed and supernatants were collected and filtered using Whatman NO.1 filter paper and stored at 4℃. Then, the filtered supernatant was extracted using polar and non-polar solutions such as alcohol, dichloromethane, acetic acid, methanol, ethyl acetate and petroleum ether (Lee et al., 2017).
2.4 Inactivation of bacterial cells
Bacterial cells inactivated of extracted crude compound against K. pneumoniae using agar well diffusion method followed by Usha Nandhini et al. (2018). Simplified, first day bacterial inoculated K. pneumoniae culture was spread on the whole nutrient agar containing plate and followed by punch of wells into the agar. Next, different concentration of different solvent extracted actinomycetes crude compound was added into the wells and rotates the plates evenly. All the plates were maintained at room temperature one day. Next day, the plates were seen and calculated the inhibition zones to detect the effect of actinomycetes.
2.5 Minimum inhibition concentration
To identify the minimum inhibition concentration of extracted actinomycetes crude compound against K. pneumonia was performed in 96-well plate, the protocol was chosen from previously evident of Lee et al. (2017). Simplified, evenly add 100 µg/mL of the crude actinomycetes extract into the 96-wells of the plates. Before, 100 µg/mL of the sterile broth was filled and also 10 µg/mL of K. pneumoniae culture. Then, the plates were kept in inside of the incubator with room temperature setup 1 day. After 1 day, the turbidity of the 96-wells was monitored visibly and noted the concentration, followed by taken spectrophotometer result to detect inhibition percentages. To detect the inhibition percentage, the resulted values were compared with untreated pathogen plus sterile media containing well of control. Then, the results were interpreted and identify the minimum inhibition percentage using following formula,
MIC (%) = Control-Test/Control X 100.
2.6 Size and shape damages
Size and shape damages of the K. pneumoniae were detected after treatment with crude actinomycete compound using chemical fixation method. The fixed cells of damaged or undamaged K. pneumoniae cells were confirmed by SEM micrograph. Initially, first day culture was taken and centrifuged at 2,000 rpm for 5 min. Pellet was collected and mixed in 2 mL of PBS and further centrifuged at same procedure. The unwanted cells were removed form PBS and attached cells were again mixed with 2 mL new PBS and fixed on glass slide. Then, the slide was fixed using glutaraldehyde with desired concentration. The fixation was kept at room temperature for 4 h and then washed with PBS initially followed by double distilled water. Then, ethanol serious of 30–90% was used step by step using 10 min time interval. Next, the slide culture was washed by t-butanol and treated by new t-butanol and kept at room temperature 6 h. Finally, the slide was maintained in 4 ℃ in refrigerator. In end, the slide was monitored in 40x SEM instrument, and the treated and untreated cells were differentiated and noted.
3 Result
3.1 Isolation and identification of actinomycetes
Clear white, pale yellow, slightly yellow mixed powdered colonies, and spore producing ability of 10 different types of actinomycetes strains were identified around the Sea grasses punched places in 7th day. Some strains shown with sparse spore formation producing ability, and also little, half and entire colonies were shown with pigment formation. The areal mass and reverse sides of the colonies looking with clear pigment (Fig. 1a). All the phenotypic evidences are clearly stated that the isolated colonies were actinomycetes. Next, all the clear and differentiated means pigmented colonies were screened on respective AIA plates and allowed to grown till 7 days. After, all the strains were stored separately streaked slant format and stored in 4 ℃.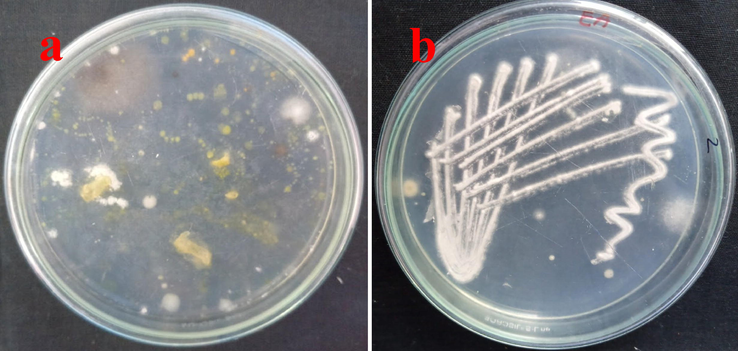
Isolation and identification of endophytic actinomycetes from marine Sea grasses (a) and pure culture of actinomycete strains in actinomycete isolation agar (b).
Further, all the screened strains were endophytic or not was confirmed the surface water validation process was also indicated that the strains were emerged from internal tissue of the Sea grasses. When could seen the last surface sterilized water spread plates, the plates did not contain any growth. Also, there was no any contamination was also observed. This information was clearly stated that the sterilization of Sea grasses samples were clear and uncontaminated samples (Fig. 1b). Finally, all the interpretation of the result was proved that the isolated strains were actinomycetes and also it arisen from inside of the Sea grasses samples. This confirmed evidence was confirmed by surface sterilization validation process. Finally, all the isolated strains were named as endophytic actinomycetes starins 1–10. The result was cross checked with previous result of Javee et al. (2020), and the isolated colonies were actinomycetes due to the phenotypical evidences of color, mass, pigment production (Fig. 2). The followed procedure of Bunbamrung et al. (2020) was supported to current study for confirm the surface sterilization. The result was approved by previous reports of Ramachandrana et al. (2019), the endophytic actinomycetes and surface sterilization was original and also pure.
Validation of endophytic actinomycete by spread plate method using chemical surface sterilization method.
3.2 Extraction of anti-bacterial compounds
After 15 days fermentation, all the endophytic actinomycete strains were extracted using liquid–liquid extraction experiment using various solvent (Fig. 3). After careful consideration, the solvent phase of the RMN 10 strain of ethanol, ethyl acetate, methanol, dichloromethane phases were exhibited some zones against K. pneumoniae containing plate. Surprisingly, ethyl acetate extract of the RMN 10 strain solvent evaporated phase was exhibited increased anti-bacterial activity compare with other strains. In addition, the zones were very high in increasing concentration compared with some lowest concentration. In 250 µg/mL, the RMN 10 extract of actinomycete solvent phase was exhibited with 16 mm zone of inhibition, 10 µg/mL was exhibited 4 mm zone of inhibition against K. pneumoniae (Fig. 4a, b). This anti-bacterial activity result was suggested that the RMN 4 strain of endophytic actinomycetes has anti-bacterial activity against various bacteria. The similar result was reported by Jeeva et al. (2020); marine algae mediated endophytic actinomycete has excellent anti-bacterial activity against various bacteria. This result was more correlated with our previous results (Parama Cita et al., 2017; Khan et al., 2019), and marine algae mediated endophytic actinomycetes has excellent anti-bacterial and anti-cancer activities against multi drug resistant bacteria and cancer cells. Recently, Bunbamrung et al. (2020) reported that the marine sources of endophytic actinomycetes acquired the nutrient sources from algae, plant and respective sources. Sometimes, the marine environmental sources pH, temperature, carbon, nitrogen, minarals and other sources are also influenced the activities of the actinomycetes (de Felício et al., 2015).
Extraction of anti-bacterial compounds from RMN 10 strain of actinomycetes using liquid–liquid extraction.

Anti-bacterial activity of actinomycete extract against K. pneumoniae using various solvent (a), Zones of inhibition against K. pneumoniae using ethyl acetate solvent by agar well diffusion method.
3.3 Minimum inhibition concentration
After careful analysis, the more turbidity of the well was visibly detected at 250 µg/mL concentration and it was stated that the concentration was excellent anti-bacterial concentration. When seen in the all wells, the turbidity was shown with lowest to increase at concentration dependent mode. The result was suggested that the RMN 10 extract has anti-bacterial activity against K. pneumoniae at concentration dependent. The concentration dependent inhibition was validated by calculation of O.D values of control and treated results. In the O.D values, we have performed in microtitre plate reader at 640 nm. In microtitre plateanalysis, the inhibition concentration was initially exhibited at 25 µg/mL only. Before, it could not attain the inhibition level. When seen at 100 µg/mL, the inhibition range of 57% was observed. Finally, the complete death cell of 94% was exhibited at 250 µg/mL concentration (Fig. 5). At this concentration was initially shown with more turbidity, and same result was identified after calculate the O.D value. So, finally we have fixed 250 µg/mL concentration was MIC in this study. The similar result of actinomycete extract against bacteria at concentration dependent manner result reported by Ye et al. (2017). Our result was more accordance with previous evidences of Upasana et al. (2020), and marine actinomycete inhibited the bacteria increasing concentration with concentration dependent manner. This stamen also agreed by Soleck et al. (2012) and Rashad et al. (2015) result was also supported to current study. Previously, Law et al. (2019) and Tan et al. (2019) were reported that the marine mangrove environment of pH, temperature, salt stress, carbon, mineral and nitrogen sources have influenced the actinomycetes of bioactivities. Recently, Singh and Dubey (2018) was reported that the Sea grasses mediated endophytic actinobacteria compounds have more anti-microbial activity against various multi drug resistant bacteria. This result was supported by previous reports of Li et al. (2012) and Ravikumar et al. (2010) were reported that the endophytic bacteria were isolated from Sea grasses and they have shown excellent anti-bacterial activity against various pathogenic microorganisms.
Minimum inhibition concentration of RMN 10 strain of actinomycetes extract against K. pneumoniae by microtitre plate method.
3.4 Size and shape damages using SEM
The SEM pictogram of the actinomycete extract treated K. pneumoniae cells were shown with shape damages. The belbing formation was shown internal rod shape, and also rod shape was damaged. The shortened size and shortened shapes were indicated that the K. pneumoniae was affected by actinomycete extract. The original size and shapes were entirely modified and the modified growth was shown with non-rod shape (Fig. 6a, b). The distortion morphology of bacterial cells was shown after retaliated the endophytic actinomycete extract. The inhibited shapes of the rod were shown with endophytic actinomycete extract layers. The layer by layer attachment was shown on the surface of the bacterial shapes were observed. It was suggested that the endophytic actinomycete extract has excellent anti-bacterial activityagainst K. pneumoniae. The supportive evidences were reported by Zheng et al. (2000), the size and shapes were entirely changed due to the influence of endophytic actinomycete extract. Our previous report was closely related to current study, and the endophytic actinomycetes has excellent anti-bacterial activities due to the utilization of Also, the marine endophytic actinomycete compound has excellent anti-bacterial and anti-cancer properties. Recently, Ramachandran et al. (2018) was also reported that the endophytic actinomycete compound has anti-bacterial activity against multi drug resistant bacteria and it has better bioactivities compared with terrestrial due to the influence of marine environments including pH, temperature, salt stress, carbon, nitrogen and organic nutrients.
Detection of size and shape of control (a) and damages of cells after treatment with RMN 10 strain of actinomycetes extract (b) against K. pneumoniae by scanning electron microscope analysis.
4 Conclusion
In this result, 10 different strains of actinomycete were isolated from marine Sea grasses. After sterilization validation, the result was confirmed that the isolated strains were emerged from internal tissue of the Sea grasses and the result was suggested that the surface sterilization was original. The excellent anti-bacterial activity of the partially purified actinomycete extract exhibited at 250 µg/mL concentration. Previously, there was no any specific Sea grass mediated actinomycete extract and compounds against any pathogenic microroganisms. In addition, size and shape of the bacterial morphology was also damaged and clearly confirmed by SEM analysis. Altogether, the current study was concluded that the partially purified endophytic actinomycete extract as a promising anti-bacterial agent against K. pneumoniae.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RG-1438- 074.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- L.A. Betancur, A.M. Forero, D.M. Vinchira-Villarraga, J.D. Cárdenas, A. Romero-Otero, F.O. Chagas, M.T. Pupo, L. Castellanos, F.A. Ramos, NMR-based metabolic profiling to follow the production of anti-phytopathogenic compounds in the culture of the marine strain Streptomyces sp. PNM-9, Microbiolog. Res., 239, 2020, 126507.
- An antibiotic agent pyrrolo[1,2-a]pyrazine-1,4- dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv.. 2018;8(32):17837-17846.
- [Google Scholar]
- Pafuranones A and B, two dimeric polyketides from a rare marine algae-derived fungus Paraconiothyrium sp. Chinese Chemical Letters. 2019;30:981-984.
- [Google Scholar]
- Potential anti-biofilm producing marine actinomycetes isolated from sea sediments in Thailand. Agricult. Nat. Resourc.. 2018;52:228-233.
- [Google Scholar]
- S. Valli S.S. Suvathi O.S. Aysha P. Nirmala K.P. Vinoth A. Reena 2 6 2012 469 473
- Isolation and Identification of Antibacterial Compound from Marine Endophytic Actinomycetes against Multi Drug Resistant Bacteria. Ann Microbiol Immunol.. 2018;1(1):1003.
- [Google Scholar]
- Marine sponge alkaloid aaptamine enhances the anti-bacterial and anticancer activity against ESBL producing Gram negative bacteria and HepG 2 human liver carcinoma cells. Biocat. Agricult. Biotechnol.. 2019;17:628-637.
- [Google Scholar]
- Ravishankar Rai, Isolation and characterization of bioactive compounds with antibacterial, antioxidant and enzyme inhibitory activities from marine-derived rare actinobacteria, Nocardiopsis sp. SCA21. Microb. Pathog.. 2019;137:103775.
- [CrossRef] [Google Scholar]
- Metabolomic analysis of marine and mud crabs based on antibacterial Activity. Aquacult. Rep.. 2017;7:7-15.
- [Google Scholar]
- Structural deformation in pathogenic bacteria cells caused by marine fungal metabolites: An in vitro investigation. Microb. Pathog.. 2020;146:104248.
- [CrossRef] [Google Scholar]
- Molecular characterization and antibacterial effect of endophytic actinomycetes Nocardiopsis sp. GRG1 (KT235640) from brown algae against MDR strains of uropathogens. Bioactive Materials. 2016;1(2):140-150.
- [Google Scholar]
- Antibacterial activity of marine bacteria isolated from sponge Xestospongia testudinaria from Sorong, Papua, Asian Pac. J Trop. Biomed. 2017;7(5):450-454.
- [Google Scholar]
- A comparative study on selected marine actinomycetes from Pulicat, Muttukadu, and Ennore estuaries, Asian Pacif. J Trop. Biomed.. 2012;2(3):S1827-S1834.
- [Google Scholar]
- Isolation strategies of marine-derived actinomycetes from sponge and sediment samples. J. Microbiolog. Method.. 2012;88(3):342-347.
- [Google Scholar]
- Marine actinomycetes as bioremediators in Penaeus monodon rearing system. Fish & Shellf. Immunol.. 2018;75:231-242.
- [Google Scholar]
- G. Rajivgandhi, R. Vijayan, M. Maruthupandy, B. Vaseeharan, N. Manoharan, Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs, Microbial Pathogenesis, 118 (2018) 190–198.
- Polyethers isolated from the marine actinobacterium Streptomyces cacaoi inhibit autophagy and induce apoptosis in cancer cells. Chemico-Biological Interact.. 2019;307:167-178.
- [Google Scholar]
- New polycyclic tetramate macrolactams from marine-derived Streptomyces sp. SCSIO 40060. Tetrahedron. 2018;74:6839-6845.
- [Google Scholar]
- Actinomycetes from the Red Sea Sponge Coscinoderma mathewsi: Isolation, Diversity, and Potential for Bioactive Compounds Discovery. Microorganisms. 2020;8:783.
- [Google Scholar]
- G. Rajivgandhi, G. Ramachandran, M. Maruthupandy, B. Vaseeharan, N. Manoharan, Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) and its antibacterial efficacy against isolated ESBL producing bacteria, Microbial Pathogenesis, 126 (2019) 138–148.
- G. Ramachandrana G. Rajivgandhia M. Maruthupandyb N. Manoharana,⁎, Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens, Biocatalysis and Agricultural Biotechnology 17 2019 750 757
- Molecular profiling of marine endophytic fungi from green algae: Assessment of antibacterial and anticancer activities. Process Biochem.. 2020;96:11-20.
- [Google Scholar]
- Isolation, identification and extraction of antimicrobial compounds produced by Streptomyces sps from terrestrial soil. Biocatal. Agricult. Biotechnol.. 2018;15:317-321.
- [Google Scholar]
- Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94-105.
- [Google Scholar]
- A. Javee R. Karuppan N. Subramani Bioactive glycolipid biosurfactant from seaweed Sargassum myriocystum associated bacteria Streptomyces sp. SNJASM6, Biocatal. Agricult Biotechnol. 23 2020 101505
- N. Bunbamrung, C. Intaraudom, A. Dramae, C. Thawai, S. Tadtong, P. Auncharoen, P. Pittayakhajonwut, Antibacterial, antitubercular, antimalarial and cytotoxic substances from the endophytic Streptomyces sp. TBRC7642, Phytochemistry, 172, 2020, 112275.
- Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogens and A549 lung cancer cells. Microbial Pathogenesis. 2018;125:325-335.
- [Google Scholar]
- Antibacterial, antifungal and cytotoxic activities exhibited by endophytic fungi from the Brazilian marine red alga Bostrychia tenella (Ceramiales) Revista Brasileira de Farmacognosia. 2015;25(6):641-650.
- [Google Scholar]
- Antiproliferative cyclodepsipeptides from the marine actinomycete Streptomyces sp. P11–23B downregulating the tumor metabolic enzymes of glycolysis, glutaminolysis, and lipogenesis. Phytochem.. 2017;135:151-159.
- [Google Scholar]
- Staphylococcus xylosus VITURAJ10: Pyrrolo [1,2α] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) (PPDHMP) producing, potential probiotic strain with antibacterial and anticancer activity. Microb. Pathog.. 2020;147:104259
- [Google Scholar]
- N-acetyl-3,4-dihydroxy-L-phenylalanine, a second identified bioactive metabolite produced by Streptomyces sp. 8812, The. J. Antibiot.. 2012;65(4):219-221.
- [Google Scholar]
- Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiolog. Res.. 2015;175:34-47.
- [Google Scholar]
- Streptomyces monashensis sp. nov., a novel mangrove soil actinobacterium from East Malaysia with antioxidative potential. Sci. Rep.. 2019;9(1)
- [CrossRef] [Google Scholar]
- Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents, l. BMC Microbiol.. 2019;19(38):1-16.
- [Google Scholar]
- Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microbiol.. 2018;9:1767.
- [Google Scholar]
- Endophyte-Mediated Effects on the Growth and Physiology of Achnatherum sibiricum Are Conditional on Both N and P Availability. PLoS ONE. 2012;7:48010.
- [Google Scholar]
- S. Ravikumar N. Thajuddin P. Suganthi1, S. Jacob Inbaneson, T. Vinodkumar, Bioactive potential of seagrass bacteria against human bacterial pathogens J. Environment. Biolog. 31 2010 387 389
- Zhonghui Zheng Wei Zeng Yaojian Huang Zhiyuan Yang Jun Li Huirong Cai Wenjin Su Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China 188 1 2000 87 91







