Translate this page into:
Anti-arrhythmogenic effects of quercetin postconditioning in myocardial ischemia/reperfusion injury in a rat model
⁎Corresponding author. zrky1983@sina.com (Ran Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The prevention of myocardial damage occurring from ischemia/reperfusion (IR) is a major issue in ischemic heart disease in civilized worldwide. The object of recent study was based on investigating the anti-arrhythmogenic effect of quercetin postconditioning (QPC) in IR injury in rats, aiming at the involvement of nitric oxide (NO) system and mitochondrial K-ATP (mitoKATP) channels activities. Upon separation of the hearts of 64 male Wistar rats, they were randomly assigned to eight groups (n = 8/group). Except for the sham group, ischemia has exerted by inducing interruption of the aortic supply for 30 min ischemic condition, then 55 min reperfusion. Each experiment lasted 100 min in total with 15 min as stabilization period. The levels of LDH in coronary effluent were estimated colorimetrically. Overall the experiment lambeth convention-based used to assess arrhythmias, which were classified as number and severity of premature ventricular complexes (PVC), number and duration of ventricular fibrillation (VF), and ventricular tachycardia (VT). The arrhythmias induced by IR, LDH and number and duration VF significantly enhanced in control group (P < 0.05 vs. control). The Que group could reduce the LDH level, number of PVC, number and duration of VT, VF and severity of arrhythmias (P < 0.05 compared with control). The effects of Que were abrogated by the concurrent administration of L-NAME (L-nitro-arginine methyl ester) or 5-HD (5-hydroxydecanoate) as selective antagonists of NO system and mitoKATP channel, respectively. Accumulating evidence indicates that the cardioprotective effect of QPC highlights the importance of in anti-arrhythmogenic during IR, thereby opening a new direction for treatment in patients with myocardial infarction (MI).
Keywords
Arrhythmias
Ischemia/reperfusion
Quercetin
Nitric oxide
Mitochondrial K-ATP channel
1 Introduction
Myocardium ischemic damage is most of the causing of disability and deaths in civilized worldwide. The pathological manifestation of coronary artery disease is a myocardial injury, which is produced upon ischemia/reperfusion (IR) insult. Three main changes that are correlated with ischemic heart disease (IHD) include (a) decrease of intracellular pH and [K+], and accumulation of metabolites; (b) [Ca2+]i overload and irreversible cellular damage; and (c) enhancement of oxidative stress, increase the generation of reactive oxygen species (ROS), and eventually mitochondrial impairment of function (Lee et al., 2011; Darband et al., 2018). In fact, the imbalance between the formation of ROS and the endogenous antioxidants resulted in IR. Although optimal therapy for ischemia includes reperfusion is undoubtedly beneficial, it has adverse aspects that can diminish the beneficial effects of myocardial reperfusion, including left ventricular extracellular matrix remodeling, myocardial stunning, ventricular arrhythmias, progressive cell death, microvascular dysfunction, and finally death (Piot et al., 2008; Matejikova et al., 2009; Yousefi et al., 2015; Ibarrola et al., 2019).
Ventricular arrhythmias divided into three major phases during ischemia; phase 1a arrhythmias can occur early first 10 min, phase 1b arrhythmias ensue between 15 and 60 min upon the onset of ischemia, and phase 2 arrhythmias ensue even later after ∼90 min (Foster and Coetzee, 2016). Myocardial arrhythmias are a common problem of IR injuries. Indeed, during the generation of ROS, which may create extrudes H+ gradient, contributing to an influx of Na+ and enhance in [Ca2+]i via the 2Na+/Ca2+ exchanger resulting in the accumulation of [Ca2+]i, which leads to further elevates ATP depletion. As a result, leading cause of enhancing [Ca2+]i has been introduced as a potential accused of reperfusion-arrhythmogenesis (Hausenloy and Yellon, 2013).
In the adult heart, cardioprotection via pre/postconditioning (IPC/IPostC) can be induced by activation of “endogenous cardiac protective pathways” that probably include mitochondrial K-ATP (mitoKATP), mitochondrial permeability transition pore (mPTP), nitric oxide (NO), ROS, and diverse cardioprotective kinases. The inhibition of either of these pathways could abolish their cardioprotective effects in adult (Jin et al., 2012; Montazami et al., 2015; Doul et al., 2019).
The cardioprotection role of mitochondria as well as cell death undeniable. During ischemia, intracellular pH and ATP levels reduced that lead to anaerobic metabolism and lactate accumulation, besides, exert perturbation in ATPase-dependent ion transport mechanism. mitoKATP channels, which located in mitochondrial inner membrane play a crucial role in the process of protection through opening these channels. Potassium channel openers can induce cardioprotection effects, whereas, blockers can abolish the effect of IPC and IPostC (Meng et al., 2016; Mohammadzadeh et al., 2015). Indeed, protective effects of mitoKATP channels exert through changes in levels of ROS, mitochondrial Ca2+ uptake, and matrix swelling. Oxidative stress during reperfusion alleviates the bioavailability of the intracellular signaling molecule such as NO, thereby removing its cardioprotective effects. Meanwhile, dual-function were predicted to NO in cardiovascular system and IR injury (Ma et al., 2011). The cardioprotective function of NO during IR is owing to its potential as anti-inflammatory and antioxidant agents. Indeed, NO performs as a repressor of mitochondrial respiration and augmentation oxygen radical scavenger thereby alleviates the generation of oxygen-derived free radicals during IR (Ma et al., 2011).
Quercetin (Que; 3,5,7,3′,4′-pentahydroxyflavone) is a natural phenolic compound existent in many fruits and vegetables such as apples, onions, and red wine. Que exerts numerous beneficial effects on variety of biological functions including anti-inflammatory, anti-coagulation, anti-ischaemic, anti-mutagenic, anti-viral and cardiovascular protection processes (Wan et al., 2009; Jing et al., 2016; Dong et al., 2018).
Given takes to protective effects and therapeutic potential of Que, we speculated that Que may have a cardioprotective effect against arrhythmogenic in myocardial IR injury. Therefore, experiments were undertaken to explore our hypothesis on rat models.
2 Materials and methods
2.1 Drugs and chemical compounds
Que was procured from Sigma Aldrich (USA). L-NAME (L-nitro-arginine methyl ester and 5-HD (5-hydroxydecanoate) as selective antagonists of NO and mitoKATP channel, respectively, were obtained from Tocris Bioscience (UK). It is worth mentioning that all materials and reagents in the present study were procured from commercial sources at the highest quality accessible.
2.2 Animals
A total of 64 healthy and clean 12 weeks old male Wistar rats (270 ± 30 g; from the animal research center of Kunming) were fed ad libitum. And were maintained under standard conditions (12:12 h light/dark cycle at ambient temperature of 25 °C). According to the random index method animals were divided into eight groups (n = 8/group): sham, control, Cremophor-EL (EL-C), Que, 5-HD group, 5-HD + Que, L-NAME, and L-NAME + Que group, which were acclimated to their ambient three days before the experimentation. All surgical and experimental procedures were adapted to internationally accepted standards and approved by the committee on animals.
2.3 Langendorff perfusion
To prevent coagulation of the blood, all rats were receiving (500 IU, i.p.) heparin and anesthetized with ketamine/xylazine (60:10 mg/kg, i.p.), then their hearts were surgical via thoracotomy excised and rapidly immersed in ice-cold. Regional myocardial ischemia was performed in isolated hearts perfused in Langendorff apparatus. Using 4/0 silk suture the left anterior descending (LAD) coronary artery was ligated for 30 min. The hearts were perfused at a constant pressure of 75 mmHg throughout the experiment with Krebs–Henseleit (K–H) solution (containing in mM: 1.2 MgSO4, 4.8 KCl, 118 NaCl, 1.0 KH2PO4, 27.2 NaHCO3, 1.35 CaCl2, and 10 Glucose). A mixture of %95 O2 and %5 CO2 in pH 7.4 was bubbled via the perfusate and thermostatically controlled water circulator at 37 °C (Satchwell Sunvic, UK) (Morand et al., 2018).
2.4 Study design
All isolated hearts received K–H solution within 15 min of the adaptation period, and except the Sham, which was perfused steadily for 85 min, the remaining hearts were exposed to IR for 30 min and 55 min, respectively.
64 male Wistar rats were allocated into eight groups equally according to the random index method (n = 8/group):
Sham; in which the isolated hearts of rats were not exposed to ischemia but were perfused constantly for 85 min with a normal K-H buffer.
Cont; in which the isolated hearts of rats were exposed to a 30 min and 55 min IR with a normal K–H buffer, respectively.
EL-C; the condition was similar to the Cont group except that the hearts were perfused with a K-H buffer comprising 0.1% EL-C 10 min at the onset of reperfusion.
Que; the condition was similar to the Cont group except that the hearts were perfused with a K–H buffer comprising 100 nM Que for 10 min at the beginning of reperfusion.
5-HD; the condition was similar to the Cont group except that the hearts were perfused with a K–H solution involving 100 μM 5-HD for 10 min at the beginning of reperfusion.
Que + 5-HD; the condition was similar to the Cont group except that the hearts were perfused with a K–H buffer involving both 100 nM Que and 100 μM 5-HD for 10 min at the beginning of reperfusion.
L-NAME; the condition was similar to the Cont group except that the hearts were perfused with a K-H buffer involving 100 μM L-NAME for 10 min at the beginning of reperfusion.
Que + L-NAME; the condition was similar to the Cont group except that the hearts were perfused with a K–H buffer involving both 100 nM Que and 100 μM 5-HD for 10 min at the beginning of reperfusion (Fig. 1).
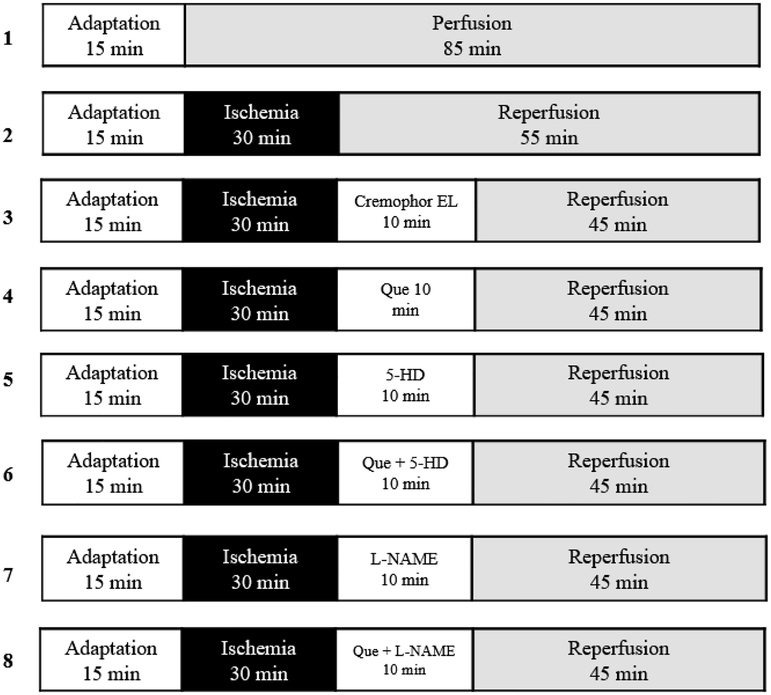
- Experimental protocol. Except the Sham, which was perfused for 85 min, the remaining hearts were exposed for 30 min of ischemia and followed by 55 min of reperfusion, respectively. Administration of 100 nM of Que was conducted for 10 min at the beginning of reperfusion. 5-HD (100 µM) or L-NAME (100 µM) was also administered for 10 min at the beginning of reperfusion with Que presence or lack. L- NAME: L-Nitro-Arginine Methyl Ester; 5-HD: 5-hydroxydecanoate; Que: querctin; 5-HD: mitoKATP channel blocker; L- NAME: NO synthase blocker.
Of Note: There were no significantly differences between EL-C and control groups. Due to this reason we, not considered Cremophor-EL group in the analysis of results.
2.5 Exclusion criteria
In fact, all of the hearts that coronary flow (CF) or left ventricular developed pressure (LVDP) lower than 7.5 ml/min or 70 mmHg, irregular heartbeats, severe arrhythmias, and weak contraction were substituted with the healthy heart.
The exclusion (and replacement) rate of hearts per group were as follows: Sham = 0; control = 1 heart; EL-C receiving group = 1; Que receiving group = 0; 5-HD receiving group = 2; Que plus 5-HD receiving group = 1; L-NAME receiving group = 1; Que plus L-NAME receiving group = 1.
2.6 Electrocardiogram recording and arrhythmias interpretation
Lambeth convention-based used to assess arrhythmias overall the experiment. Throughout the experiment, the impulsive rate and electrical activity of heart were controlled via electrocardiograms (ECGs). ECGs were recorded at baseline, except the sham group, which was perfused continuously for 85 min, the remaining hearts were exposed for 30 min of ischemia followed by 55 min of reperfusion. The arrhythmia were regularly recorded and digitized by a data acquisition system (PowerLab, AD Instruments), and analyzed using Chart v7.7 for Windows Software (AD Instruments). Accordingly, arrhythmias were classified as a ventricular bigeminy (VB), ventricular salvos (VS), ventricular premature beat (VPB), ventricular tachycardia (VT), and ventricular fibrillation (VF). VS, VB, and VPB were defined as the premature ventricular complexes (PVC) and they were not analyzed individually. Indeed, PVC was described as discrete and identifiable premature QRS complexes. ECGs were monitored during the first 30 min of reperfusion and recorded for:
Severity or score of arrhythmias.
Duration and count of VF and VT
Count of PVC
Arrhythmias were characterized using the Lambeth Conventions and arrhythmia severity was depicted based on the criteria of Walker and Curtis (Curtis and Walker, 1988; Curtis et al., 2013). The scoring (severity) of arrhythmias was based on a five grade evaluation system as follows: score 4 for VF, score 3 for VT, score 2 for VB or VS, score 1 for VPB, and score 0 for no arrhythmia (Table 1).
Score
Arrhythmias
0
No arrhythmia
1
VPB
2
VB or VS
3
VT
4
VF
2.7 Lactate dehydrogenase measurement
Lactate dehydrogenase (LDH) is as a glycolytic enzyme that presents in roughly all of the tissues in the body. When the cell membrane harms, LDH releases from cytoplasm quickly. The levels of myocardial LDH in the coronary effluents was collected 10 min after the beginning of reperfusion and were estimated colorimetrically using an auto-analyzer (Abbott, Alcyon 300, USA) and specific detection kit according to the manufacturer’s protocols. The absorbance of the solution was detected at 492 nm by spectrophotometry. Finally, the data were stated in U/L.
2.8 Statistical analysis
In this study data are represented as mean ± SD. The nonparametric Kruskal–Wallis test was applied to analyze the results of arrhythmias between groups. Probabilities of 0.05 or less (P < 0.05) were considered to be significantly.
3 Results
3.1 LDH measurement
To investigate the cardioprotective effect of Que against IR damage, the alterations of LDH release was measured as a myocardial marker. The levels of LDH (in U/l) released into the coronary effluent (CE) were shown in Fig. 2. Which during reperfusion was statically significant enhanced in control compared with the sham P < 0.05, whereas, significantly decreased in Que group administration compared with the control group P < 0.05. Adding the 5-HD, L-NAME, Que + 5-HD, and Que + L-NAME to the reperfusion solution entirely abrogated the LDH decreasing effect of Que (P < 0.05).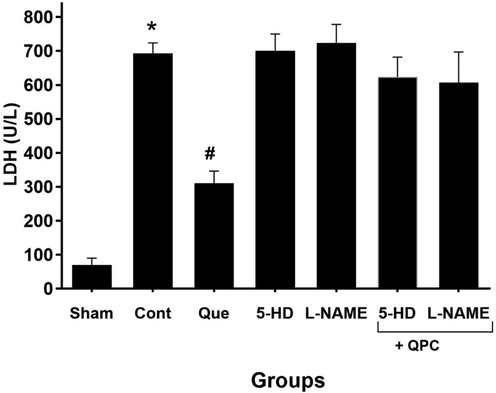
LDH level (U/L) after reperfusion in all experimental groups. * vs. Sham; # vs. control. Data are presented as mean ± SEM. QPC Quercitin postconditioning; 5-HD mitoKATP channel blocker; L-NAME NO synthase blocker.
3.2 Ischemic postconditioning effects of Que on the number of PVC, VT and VF
The ligation of the LAD led to generation of arrhythmias, which the majority occurred as PVC. Our observation reveals that the number of PVCs was decreased in the Que versus control during reperfusion phase; beside, this data was not dramatically significant. Furthermore, compared with Que group, the count of PVC was not dramatically enhanced upon administration of 5-HD, L-NAME, Que + 5-HD, and Que + L-NAME (Fig. 3). Furthermore, in compared with other groups 5-HD more than abolished protective effect of Que during IPostC.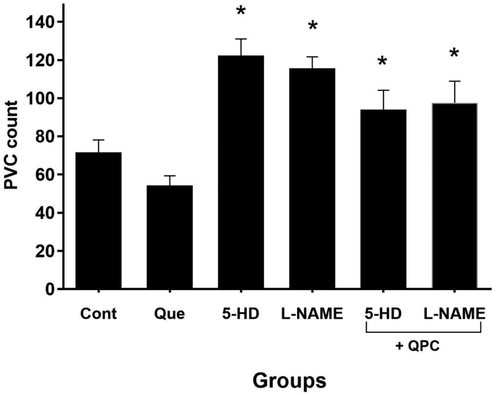
The modifications in premature ventricular complexes (PVC) number. There was no statically significant difference between control and other groups (n = 8 animals/group; P < 0.05 vs. Que).
Moreover, we reported that Que treatment diminished the count of VT and VF vs. control group (Figs. 4 and 5, respectively). These changes were statistically significant in correlation to the number of VT (P < 0.05), but not VF. Also, repressing the mitoKATP and NO dramatically abrogated the effects of Que on both VT and VF counts (Figs. 4 and 5) (P < 0.05).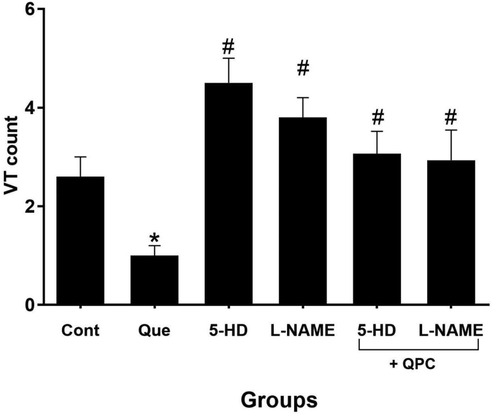
The alterations in ventricular tachycardia (VT) count. n = 8/group. * vs. control, # vs. Que.
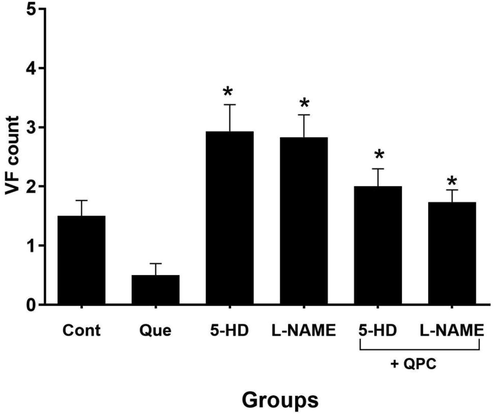
The alterations in ventricular fibrillation (VF) count (n = 8/group). * vs. Que group.
3.3 Ischemic postconditioning effects of Que on the duration episodes of VF and VT
Quercetin postconditioning (QPC) alleviated the duration of VF and VT episodes during reperfusion vs. control. The diminishing effect of Que on the duration of VT was dramatically (P < 0.05), whereas, in VF not statistically significant. After treatment of 5-HD or L-NAME, the antiarrhythmic effects of Que was alleviated, hence, the duration of VT elevated dramatically in the 5-HD or L-NAME groups compared with control (P < 0.05), while not significantly in Que + 5-HD or Que + L-NAME groups and the duration of VF elevated dramatically in the presence of the blockers group vs. corresponding Que group. (P < 0.05) (Figs. 6 and 7, respectively).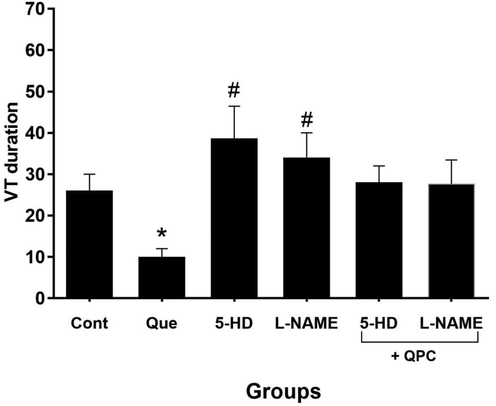
The alterations in ventricular tachycardia (VT) duration (n = 8/group). * vs. control group, and # vs. Que group.
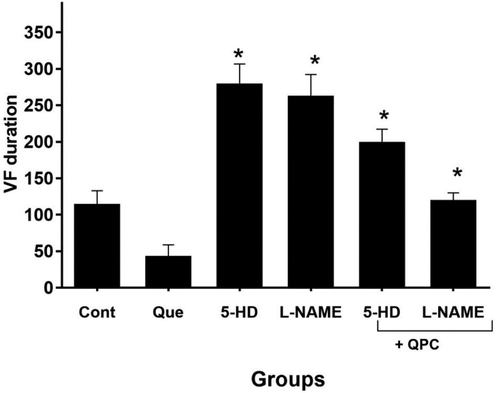
The alterations in ventricular fibrillation (VF) duration (n = 8/group). * vs. Que group.
3.4 Ischemic postconditioning effects of quercetin on the severity of the arrhythmia
Data implied that Que dramatically diminished the severity of arrhythmias vs. control group (P < 0.05). Blocking both mitoKATP channels and NO by specific inhibitors entirely abrogated the cardioprotective effects of Que on the severity of arrhythmias (P < 0.05) (Fig. 8).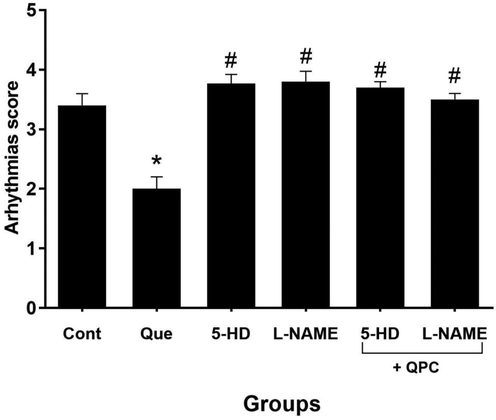
The severity of arrhythmias in compared with control (n = 8 rats/group). * vs. control group, and # vs. Que group.
4 Discussion
The mechanisms involved in the anti-arrhythmogenic effects of Que are still required for the discussion. Our current findings of IPostC implied that Que has anti-arrhythmogenic effects during IR injury. The most important results were revealed that Que could reduce the LDH levels and alleviated the number PVC, and number and duration of VT, VF and score of arrhythmias. Conversely, cardioprotective effects of Que were abrogated by administration of specific inhibitors such as 5-HD and L-NAME, hence, Que may exert its anti-arrhythmogenic effects during reperfusion. Finally, the most important of these findings is that Que could decrease the number and duration of VF as main arrhythmia leading to death in myocardial IR injury.
The pathophysiological mechanisms initiating ventricular arrhythmias show that needed a new approach therapeutic intervention to the management of arrhythmias (Matejikova et al., 2009). Many previous studies have revealed the cardioprotective effect of flavonoids on myocardial IR injury models (Lee et al., 2011). The cardioprotective function of Que investigated in many studies, Liu and colleagues (Liu et al., 2014) revealed that Que pretreatment was protected against myocardium IR injury through decreasing oxidative stress, inhibiting inflammatory and apoptosis pathway and with enhancing PI3K/Akt signaling, which involved in the anti-apoptotic effect. Furthermore, Dong et al. reported that Que through inhibition of the HMGB1/TLR4/NF-κB signaling pathway attenuates myocardial injury during IR (Dong et al., 2018). Collectively, our data of this study suggest that Que exerts anti-arrhythmogenic effects during IR, which coincidence with these studies.
Cell membrane destruction leads in enhanced membrane permeability and the leakage of LDH, and increased levels of LDH allocated as an important biomarker for cardiac injury during reperfusion (Ramezani-Aliakbari et al., 2019). Also, the results of a previous study in this regard reported that Que dramatically alleviated LDH and CK levels compared with the IR group in rat (Dong et al., 2018). In fact, treatment with Que decreased LDH in the coronary effluent in compared with control group. These data revealed that Que may be through this way partly play the crucial role in diminishing reperfusion-induced arrhythmias in rats.
The injury to the heart during myocardial reperfusion leads to arrhythmias, stunning, microvascular injury, and the ‘‘noreflow’’ phenomenon. It is worth mentioning that mitochondria are the principal organelle of ROS generation in the heart. Indeed, in cardiomyocytes, ROS levels were enhanced through the increment activity of complex I and III of the electron transport chain during IR injury (Thummasorn et al., 2016). Increase in mitochondrial ROS level resulted in mitochondrial depolarization, which associated with enhance of cardiac arrhythmia incidence (Thummasorn et al., 2016). Impaired cardiac rhythm is an important issue of the IR that VF progresses into a lethal arrhythmia. Furthermore, the pathophysiologic mechanism is implicated in the development of VT and VF including calcium overload in the early stages of reperfusion and the generation of free oxygen radicals (Ramezani-Aliakbari et al., 2019). Our result showed that myocardial IR led to ventricular arrhythmias, with enhancing in number and duration of PVC, VT, and VF. Which indeed, the number and duration of VT, VF and the scoring of arrhythmia were alleviated in treatment by Que in comparison with control groups. This mechanism of Que may exert through suppress L-type Ca2+, and reduce [Ca2+]i overload during IR by restoring the changes in [Ca2+].
NO is an active molecule in body that actuates diverse pathophysiological functions in cardiac system, including cardiac vasodilation and contraction, in fact, in the cardiovascular system acts as a ‘‘double-edged sword’’ (Yu et al., 2018). Reperfusion is contributed to the loss of capacity of endothelial cells to release NO. NO, exert its cardioprotective properties by two different signaling pathway; either activation of NO/cG/cGMP, which causes inhibit artery proliferation, inflammatory effects and prevents aggregation that suitable opportunity for the use of drugs administered in circulatory diseases, or can inhibit GSK-3β and mPTP opening via the activation of mito/sarcKATP channels, which are cGMP-independent effects (Zhao et al., 2019). Moreover, anti-arrhythmic effects of NO can form through NO/cGMP, which by regulating calcium homeostasis via the L-type Ca2+ channels cause of inhibiting the influx of Ca2+. Coronary endothelial dysfunction and nitric oxide synthase (NOS) repressors diminish the coronary effluent NO levels and augment the incidence and score of arrhythmias in rat during IR injury (Tamargo et al., 2010). Also, a slight elevate in NO may be exert cardioprotective effects, whereas, a large enhance seems with ONOO− generation to be detrimental to myocardial tissue (Yuan et al., 2015). Neuronal NOS (nNOS) is expressed in cardiomyocytes and regulating cardiac function and Ca2+ homeostasis. In this regard, Burger and colleagues (Burger et al., 2009) data revealed that after MI in nNOS−/− mouse manifest a higher incidence of arrhythmias and VF. In this regard, our result showed that exposed hearts with L-NAME during reperfusion compared control group result in enhance the LDH level as ventricular arrhythmias biomarker, and enhance the number PVC, and number and duration VT, VF and severity of arrhythmias, in fact, revers the anti- arrhythmogenic effects of Que.
Mitochondria is one of the most active and vital organelles in the cardiomyocyte (Yu et al., 2015). mitoKATP channels are a class of potassium channels that couples with electrical and metabolic activities and regulation of cellular function (Meng et al., 2016). The major function of mitoKATP channels is associated with opening in reply to stimuli, such as partial mitigate in cytosolic ATP levels or stress-related signals. Opening of the mitoKATP channel as a final step in cardioprotective signaling mechanisms, which occurs upstream of the mitochondrial ROS generation in the protective pathway (Matejikova et al., 2009). Indeed, mitoKATP channel is involved in the progress of cardiac health and undeniable performance in the face of IR. On the other hand, NO, as a mediator can through NO/cGMP, leads to activation of guanylyl cyclase and, whereby, PKG which activates a mitochondrial PKCε, eventually resulting in the availability of mitoKATP channels. Based on studies result, the cardioprotective effects of flavonoid is undeniable. Meng et al. revealed that naringenin cardioprotective effect against IR injury carried out through activating mito/sarcKATP channels (Meng et al., 2016). It was mentioned that 5-HD, a selective inhibitor of mitoKATP abolished the cardioprotection on heart against IR in rat model (Meng et al., 2016). The opening of mitoKATP channels play a pivotal role in delayed IPC. Indeed, the usage of diazoxide as opener of the mitoKATP channel in mice heart result in dramatically activated eNOS and iNOS proteins via PI3K/Akt pathway, which contributes the expansion of cardiac function and alleviated apoptosis after IR, in fact, diazoxide was totally ineffective in iNOS−/− mice, indicating that mitoKATP channel is an end effector of cardioprotection during delayed IPC (Yu et al., 2018). Beside, Gonca and colleagues (Gonca et al., 2016) revealed that acetamidobenzyl dimethyl-oxazinan (synthetic compound) is a new opener of the mitoKATP channel exert anti-arrhythmic effect via mitoKATP activation during IR-induced ventricular arrhythmias. In this perspective, mitoKATP play key roles in cardioprotection versus ventricular arrhythmias, both as a trigger and an end effector (Gonca et al., 2016). Our results coincidence to these studies revealed that blocking of mitoKATP channels with 5-HD abolished protection against lethal myocardial injury and contractile dysfunction in isolated rat hearts. Therefore, the anti-arrhythmic effect of Que may also be dependent on mitoKATP activation.
Taken together, Que illustrates the anti-arrhythmias effect and improves the survival rate during IR insult. Administering Que during IPostC prevents myocardial damage induced by IR. Accordingly, these data revealed that the anti-arrhythmic effect of Que may be attributed to its role in ameliorating the PVC, VT and VF activities. These results can produce a powerful pharmacological basis for the use of Que in the therapy of acute MI during IR. Nevertheless, the mechanisms underlying the cardioprotective action of Que still in the process of investigation and required more investigations.
Funding
Provincial Basic Research Program (Kunming Medical Special Project) 2019FE001(-068).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345-1354.
- [Google Scholar]
- The Lambeth conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther.. 2013;139:213-248.
- [Google Scholar]
- Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc. Res.. 1988;22:656-665.
- [Google Scholar]
- Quercetin: a functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell Physiol.. 2018;233:6544-6560.
- [Google Scholar]
- Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-κB signaling pathway. Am. J. Transl. Res.. 2018;10:1273-1283.
- [Google Scholar]
- Possible role of mitochondrial K-ATP channel and nitric oxide in protection of the neonatal rat heart. Mol. Cell. Biochem.. 2019;450:35-42.
- [Google Scholar]
- Antiarrhythmic activity of a new spiro-cyclic benzopyran activator of the cardiac mitochondrial ATP dependent potassium channels. Arch. Pharm. Res.. 2016;39:1212-1222.
- [Google Scholar]
- Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest.. 2013;123:92-100.
- [Google Scholar]
- Myocardial injury after ischemia/reperfusion is attenuated by pharmacological galectin-3 inhibition. Sci. Rep.. 2019;9:9607.
- [Google Scholar]
- Mitochondrial K+ channels are involved in ischemic postconditioning in rat hearts. J. Physiol. Sci.. 2012;62:325-332.
- [Google Scholar]
- Protective effect of quercetin on posttraumatic cardiac injury. Sci. Rep.. 2016;6:30812.
- [Google Scholar]
- Wogonin suppresses arrhythmias, inflammatory responses, and apoptosis induced by myocardial ischemia/reperfusion in rats. J. Cardiovasc. Pharmacol.. 2011;58:133-142.
- [Google Scholar]
- Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene. 2014;545:149-155.
- [Google Scholar]
- ATP-dependent potassium channels and mitochondrial permeability transition pores play roles in the cardioprotection of theaflavin in young rat. J. Physiol. Sci.. 2011;61:337-342.
- [Google Scholar]
- Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol. Res.. 2009;58:9-19.
- [Google Scholar]
- The cardioprotective effect of naringenin against ischemia-reperfusion injury through activation of ATP-sensitive potassium channel in rat. Can. J. Physiol. Pharmacol.. 2016;94:973-978.
- [Google Scholar]
- Reduced ABCB1 expression and activity in the presence of acrylic copolymers. Adv. Pharm. Bull.. 2015;4:219-224.
- [Google Scholar]
- Cell. Mol. Biol. (Noisy-le-grand). 2015;61:98-103.
- Chronic intermittent hypoxia promotes myocardial ischemia-related ventricular arrhythmias and sudden cardiac death. Sci. Rep.. 2018;8:2997.
- [Google Scholar]
- Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med.. 2008;359:473-481.
- [Google Scholar]
- Protective effects of gallic acid on cardiac electrophysiology and arrhythmias during reperfusion in diabetes. Iran J. Basic Med. Sci.. 2019;22:515-520.
- [Google Scholar]
- Cardiac electrophysiological effects of nitric oxide. Cardiovasc. Res.. 2010;87:593-600.
- [Google Scholar]
- Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc. Ther.. 2016;34:404-414.
- [Google Scholar]
- Effects of quercetin on gene and protein expression of NOX and NOS after myocardial ischemia and reperfusion in rabbit. Cardiovasc. Ther.. 2009;27:28-33.
- [Google Scholar]
- Anti-proliferative properties of cornus mass fruit in different human cancer cells. Asian Pac. J. Cancer Prev.. 2015;16:5727-5731.
- [Google Scholar]
- Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One. 2015;10:e0134666
- [Google Scholar]
- The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: friend or foe? Oxid. Med. Cell. Longev.. 2018;2018:8364848.
- [Google Scholar]
- Cardioprotective effect of licochalcone D against myocardial ischemia/reperfusion injury in langendorff-perfused rat hearts. PLoS One. 2015;10:e0128375
- [Google Scholar]
- Cardioprotective effect of isosorbide dinitrate postconditioning against rat myocardial ischemia-reperfusion injury in vivo. Med. Sci. Monit.. 2019;25:1629-1636.
- [Google Scholar]







