Translate this page into:
Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro
⁎Corresponding author. asali@ksu.edu.sa (Ashraf Abdel-Fattah Mostafa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
A high incidence of stalk rot disease of maize causes huge economic losses as well as deleterious effects on the environment and human health resulting from fungicide use. Against this background, the current study was established to evaluate the antagonistic efficacy of Trichoderma viride and Trichoderma harzianum strains against Fusarium proliferatum and Fusarium verticillioides strains, the most common causative agents of stalk rot disease of maize.
Methods
Dual culture assay was performed to determine the antagonistic efficacy of Trichoderma strains against some fusarial pathogens of maize. Mycoparasitic relationships of the antagonistic fungal strains against fungal pathogens were investigated using a slide culture technique. Furthermore, a food poisoning technique was performed to detect the antimycotic efficacy of Trichoderma culture filtrates against fusarial pathogens of maize. Antifungal activity of organic solvent extracts of T. harzianum and T. viride was evaluated using the disc diffusion method. GC–MS analysis was used to detect the active components of these extracts.
Results and conclusion
Trichoderma viride showed antagonistic activity against F. proliferatum and F. verticillioides with mycelial inhibition rates of 80.17% and 70.46%, while T. harzianum exhibited rates of 68.38% and 60.64%, respectively. The culture filtrates of T. viride and T. harzianum strains exhibited antifungal activity against F. verticillioides strain with suppressive rates of 56.7% and 32.2%, while the mycelial inhibition rates against F. proliferatum strain were 44.09% and 23.50%, respectively. Mycoparasitic action of T. harzianum strain against fusarial strains was detected, while no mycoparasitism was observed between T. viride and fungal pathogens. The fungicidal concentration of carbendazim fungicide against F. proliferatum was 2.00 ppm, while F. verticillioides strain exhibited resistance to carbendazim fungicide. Moreover, the acetonic extracts of T. viride and T. harzianum strains exerted the highest antifungal potency against F. proliferatum strain, recording minimum inhibitory concentrations of 0.25 and 0.50 mg/ml, respectively. The main bioactive constituents of the acetonic extracts of T. viride and T. harzianum strains were palmitic acid (22.87%) and acetic acid (21.36%), respectively. In conclusion, the antagonistic strains could be a potential source of novel biological fungicides, especially against carbendazim-resistant F. verticillioides strain, avoiding side effects of chemical fungicides.
Keywords
Stalk rot disease
Trichoderma
Dual culture assay
Mycoparasitism
Secondary metabolite
GC–MS
1 Introduction
Maize is considered to be a key crop for food security, which is cultivated globally across an area of 160 million hectares. It has high nutritional value, which is due to its levels of carbohydrates, lipids, vitamins, proteins, and minerals (Da Silva et al., 2017). Fusarial stalk rot disease reduces the yield of maize crops in severely affected areas by 30%–50%. This disease appears as white or light pink mold on the kernels of maize crops (Oldenburg et al., 2017). Fusarium verticillioides is considered one of the major causative agents of fusarial rot disease of maize (Zea mays L.). This fungus was reported to produce a group of mycotoxins called fumonisins, which are toxic to humans and animals (van Rensburg et al., 2016). Recently, fumonisins were reported to exert deleterious effects on crop yield, resulting in huge economic losses (Silva et al., 2017). Furthermore, Pfordt et al. (2020) reported that F. verticillioides and F. proliferatum were the most common fungal pathogens causing stalk rot disease of maize. The application of fungicides to manage fungal diseases is potentially associated with health hazards due to the deleterious environmental impacts of these fungicides on terrestrial and aquatic ecosystems. Several harmful effects of fungicides threaten human health have been reported, including neurological, gastrointestinal, dermatological, and carcinogenic effects (Thakur et al., 2014). Recently, Trichoderma spp. have been reported to be eco-friendly biological control agents for managing plant diseases, which enable the use of chemical fungicides to be minimized (Puyam, 2016). Trichoderma species are abundant in all types of soil and are considered as potential antagonistic agents against parasitic soil-borne microorganisms (Shahid et al., 2014). Biological control agents were reported to play a key role in the successful management of fungal plant pathogens (Zhang et al., 2013). The antagonistic activity of Trichoderma sp. against different plant pathogens occurs through different mechanisms of action, including antibiosis, mycoparasitism, and competition for nutrients and space (Ghazanfar et al., 2018). Trichoderma harzianum and Trichoderma viride strain have been reported to possess antagonistic activity against Fusarium oxysporum strain, demonstrating mycelial growth inhibition rates of 75.7% and 67.7%, respectively (Singh et al., 2018). Given the harmful effects of chemical fungicides on human and animal health, in addition to the high economic losses due to the high incidence of stalk rot disease of maize, alternative biological control agents are needed. Against this background, the current study was established to evaluate the antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against Fusarium proliferatum and Fusarium verticillioides, the most common fusarial pathogens causing stalk rot disease of maize.
2 Materials and methods
2.1 Fungal strains
Fusarial pathogens, Fusarium verticillioides ATTC 204499 and Fusarium proliferatum ATTC 76097, were used in the present study. Two antagonistic strains, Trichoderma harzianum ATTC 52443 and Trichoderma viride 26802, were tested for their antagonistic potency against fusarial pathogens. Fungal strains were cultured on freshly prepared potato dextrose agar (PDA) medium and incubated for 5 days at 28 °C. The fresh cultures were subcultured on PDA slants and kept in a refrigerator until use.
2.2 Dual culture assay
Evaluation of the antagonistic potency of T. harzianum and T. viride strains against fusarial strains was performed using a dual culture technique. Mycelial discs (8 mm in diameter) were cut from the margins of a 3-day-old culture of each strain using a cork borer. Mycelial discs of both antagonistic strains and fungal pathogens (F. verticillioides, F. proliferatum) were inoculated over freshly prepared PDA medium and placed 3 cm away from both edges of the plates. In the control group, PDA plates were inoculated only with mycelial discs of fungal pathogens. Both treated and control plates were incubated at 25 °C for 7 days. The mycelial inhibition rate was calculated as follows: Percentage of mycelial inhibition = (A – B)/A × 100, where A refers to the mycelial growth of fungal pathogens in control plates and B refers to the mycelial growth of fungal pathogens in dual culture plates (Awad et al., 2018).
2.3 Mycoparasitic relationships of Trichoderma sp. against fungal pathogens
A slide culture technique was used to evaluate the mycoparasitic behavior of Trichoderma strains against fusarial pathogens. A cube of PDA medium was cut using a sterile blade and placed over a sterile glass slide. The cube was inoculated with fusarial pathogens from one side and with antagonistic strains from the other. The glass slide was inoculated at 25 °C for 5 days. After incubation, the agar cube was removed and a sterile cover slip was placed over the mycelium. Finally, the mycelial interactions between antagonistic strains and fusarial pathogens were inspected using a light microscope (40×) (Naglot et al., 2015).
2.4 Antifungal potency of the culture filtrates of Trichoderma sp. against fungal pathogens
A food poisoning technique was performed to detect the antimicrobial potency of culture filtrates of T. harzianum and T. viride strains against different fungal pathogens. Mycelial agar discs (8 mm in diameter) were inoculated into freshly prepared potato dextrose broth medium and the inoculated flasks were incubated over a rotatory shaker (150 rpm) at 28 °C for 7 days. The cell-free filtrates were attained through filtration using double layers of muslin followed by centrifugation at 9000 rpm for 10 min to discard fungal spores that could potentially disrupt the membrane sterilization. The Trichoderma filtrates were further sterilized using Millipore filters (22 μm) and then the filtrates were added to PDA medium to obtain a final concentration of 25% v/v. Mycelial discs (8 mm in diameter) of fusarial pathogens were inoculated into PDA plates supplemented with Trichoderma sp. filtrates. Control PDA plates were inoculated with 8 mm mycelial discs and then both treated and control groups were incubated at 28 ± 1 °C for 7 days. The mycelial diameters of fungal pathogens in control and treated plates were measured using Vernier calipers and the mycelial inhibition rate was calculated as follows: here, A refers to the radial growth of the fusarial pathogens in the control group and B refers to the mycelial growth diameter of the fungal pathogens in the treated group (Sreedevi et al., 2011).
2.5 Antifungal efficacy of standard fungicide (carbendazim)
The antifungal activity of carbendazim fungicide against the tested fusarial pathogens was detected using a food poisoning technique. Different concentrations of carbendazim fungicide (5.0, 1.00, 1.50, 2.00, 2.50, and 3.00 ppm) were added to the freshly prepared PDA medium. Eight millimeter mycelial discs of fungal pathogens were inoculated into the center of the treated plates. Control PDA plates were also inoculated with 8 mm mycelial discs of fungal strains. Both control and treated groups were incubated at 28 ± 1 °C for 7 days. The radial growth diameter of fungal mycelium was measured using Vernier calipers and the rate of mycelial growth inhibition was calculated as follows: here, A is the mycelial growth diameter of fusarial pathogens in the control group and B is the radial growth diameter of mycelial phytopathogens in PDA plates treated with carbendazim fungicide (Anand et al., 2010).
2.6 Preparation of Trichoderma crude extracts
Mycelial agar discs (8 mm in diameter) of the Trichoderma strains were cultured on 250 ml of potato dextrose broth medium and incubated over a rotatory shaker (150 ppm) at 28 °C for 7 days. Filtration of the fungal cultures was performed using Whatman filter paper no. 1 for the removal of Trichoderma spores and mycelia. The culture filtrates were centrifuged at 9000 rpm for the complete separation of cell pellets. The fungal metabolites were extracted from the cell pellets using methanol solvent. Furthermore, the antifungal metabolites were extracted from the culture filtrates using hexane, n-butanol, and acetone with different polarities of 0.1, 3.9, and 5.1, respectively, to ensure the extraction of all active constituents. The extracts were concentrated using rotatory evaporator to eliminate the solvents (Jantarach and Thanaboripat, 2010).
2.7 Antifungal activity of Trichoderma extracts against fusarial pathogens
A disc diffusion method was used to detect the antifungal efficacy of different Trichoderma extracts against F. proliferatum and F. verticillioides strains. The dried extracts were dissolved in their corresponding organic solvents to attain a final concentration of 10 mg/ml. Ten milliliters of PDA medium was poured into sterile Petri dishes as a basal medium, followed by the addition of 15 ml of seeded medium. The seeded medium was prepared by mixing 1 ml of fungal spore suspension of different Fusarium strains (106 spores/ml) with 100 ml of PDA medium. Sterile filter paper discs (8 mm in diameter) were loaded with the extracts (10 mg/disc) and placed over the seeded plates. Fluconazole antifungal discs (125 µg/disc) were used as positive controls. The plates were incubated at 28 °C for 5 days and the diameters of the inhibition zone were measured using Vernier calipers (Mostafa et al., 2020).
2.8 Determination of minimum inhibitory concentration of the acetonic extracts of T. harzianum and T. viride
The minimum inhibitory concentration (MIC) was determined for the acetonic extracts as these extracts exhibited the highest antifungal activity. Ten milliliters of PDA medium was poured into sterile Petri dishes as a basal medium, followed by the addition of 15 ml of seeded medium. The sterile filter paper discs (8 mm in diameter) were loaded with different concentrations (0.25, 0.50, 1.00, 2.00, 4.00, and 8.00 mg/ml) of the acetonic extracts of T. harzianum and T. viride strains then the discs were dried and placed over the seeded plates. The plates were incubated at 28 °C for 5 days and the diameters of the inhibition zone were measured using Vernier calipers. The lowest concentration of the extract exhibiting antimicrobial activity was recorded as MIC (Yassin et al., 2020).
2.9 GC–MS analysis of the acetonic extracts of T. harzianum and T. viride strains
Chemical analysis of the acetonic extracts of T. viride and T. harzianum strains exhibiting the highest antimicrobial activity was performed using GC–MS using GCMS-QP2010 Plus (Shimadzu, Japan). The analytical conditions were adjusted as described by Yassin et al. (2020). Identification of active ingredients was conducted by comparing the results of GC–MS analysis with reference standards in the NIST database.
2.10 Statistical analysis
The antagonistic activity data were statistically analyzed with GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) using one-way analysis of variance and Tukey’s test. The data were tabulated as mean of triplicates ± standard error and will be considered statistically significant when the (P < 0.05).
3 Results and discussion
3.1 Dual culture assay
Trichoderma viride and T. harzianum strains showed antagonistic efficacy against fusarial pathogens causing stalk rot disease of maize, as shown in Fig. 1. Trichoderma viride strain showed greater inhibition against the Fusarium strains than T. harzianum strain. Trichoderma harzianum strain suppressed the mycelial growth of fusarial pathogens (F. proliferatum and F. verticillioides) by 68.38% and 60.64%, while T. viride strain did so at 80.17% and 70.46%, respectively, as shown in Fig. 2. Our results accord with those of (Nwankiti and Gwa, 2018), who confirmed the antagonistic potency of T. harzianum strain against F. oxysporum, with a mycelial inhibition rate of 45.69%. In addition, (Abhiram and Masih, 2018) reported that T. viride strain suppressed the mycelial growth of F. oxysporum strains demonstrating inhibition rates in the range of 62.50%–71.00%. Furthermore, the antagonistic potential of T. harzianum strain was evaluated by (Gwa and Nwankiti, 2017), who stated that T. harzianum suppressed the mycelial growth of Fusarium moniliforme strain, recording an inhibition rate of 58.70%.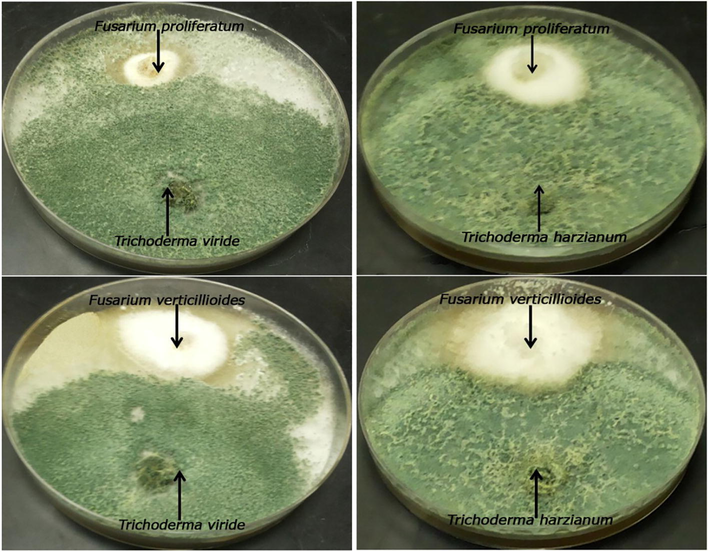
Dual culture assay of antagonistic strains (T. harzianum and T. viride) against fusarial pathogens (F. proliferatum and F. verticillioides).
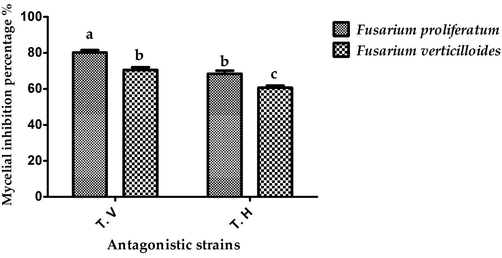
Antagonistic activity of T. harzianum and T. viride strains against fusarial pathogens causing stalk rot disease of maize.
3.2 Mycoparasitic relationships of Trichoderma sp. against fungal pathogens
Trichoderma harzianum strain exhibited mycoparasitic action against F. proliferatum and F. verticillioides strains, while T. viride showed no mycoparasitism. Mycoparasitism of T. harzianum strain was detected in the form of adhesion, coiling, penetration, and lysis of the fusarial mycelium, as shown in Fig. 3. Several mechanisms of action have been reported to enhance the effectiveness of Trichoderma spp. as biological control agents, including mycoparasitism, antibiosis, and competition for nutrients and space (Druzhinina et al., 2011). Lysis of the fusarial mycelium may be attributable to the ability of Trichoderma spp. to produce cell wall-degrading enzymes including β-(1,6)-glucanases, chitinases, and proteases (Sood et al., 2020). Ojha and Chatterjee (2011) confirmed that T. harzianum strain exerted mycoparasitic behavior against F. oxysporum strain through coiling, formation of aspersorium, and lysis of the fungal mycelium.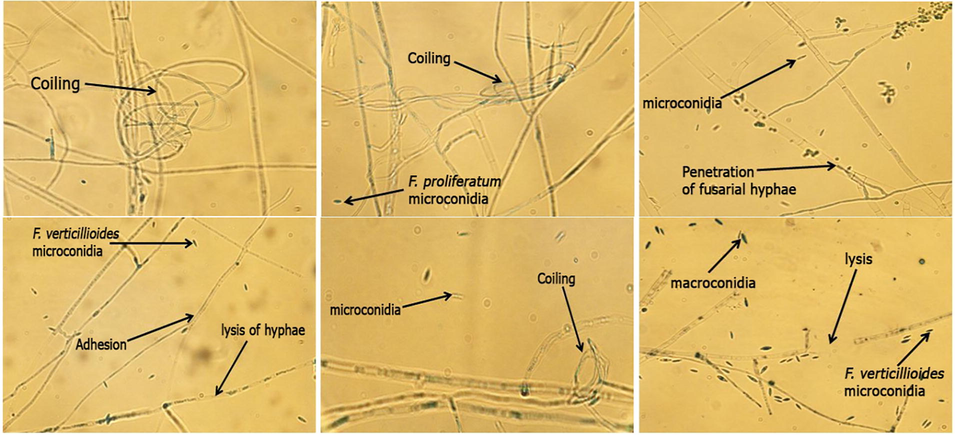
Mycoparasitism of T. harzianum strain against F. proliferatum and F. verticillioides strains.
3.3 Antifungal potency of the culture filtrates of Trichoderma sp. against fungal pathogens
Culture filtrates of T. viride suppressed mycelial growth of F. proliferatum and F. verticillioides, recording inhibition rates of 32.3% and 56.7%, while T. harzianum recorded mycelial inhibition rates of 23.5% and 44.09%, respectively, as seen in Fig. 4. Our findings are consistent with those of Aswini et al. (2016), who reported the antimicrobial efficiency of culture filtrates of T. viride and T. harzianum strains at a concentration of 5% v/v against F. oxysporum strain, recording mycelial inhibition rates of 51.53% and 24.71%, respectively. Marques et al. (2018) confirmed the antifungal potency of culture filtrates of Trichoderma isolates against F. oxysporum strains and attributed the potent activity of these filtrates to the production of active secondary metabolites.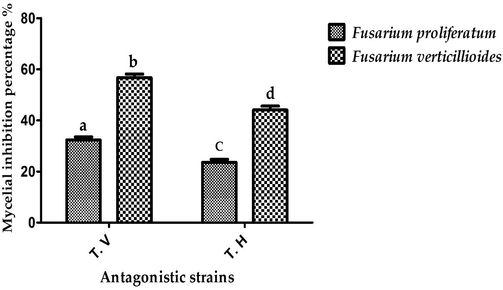
Anti-mycotic potency of culture filtrates of T. harzianum and T. viride strains against fusarial pathogens.
3.4 Antifungal efficacy of standard fungicide (carbendazim)
At a concentration of 2.00 ppm, carbendazim showed fungicidal activity against F. proliferatum strain, while F. verticillioides exhibited resistance to this fungicide at all tested concentrations, as demonstrated in Table 1. Carbendazim belongs to a group of broad-spectrum fungicides called the methyl benzimidazole carbamate (MBC) fungicides, which play important roles in combating fungal diseases of many agricultural crops (Karuppaiyan et al., 2015). Resistance of fusarial strains to MBC fungicides was reported in previous studies (Zhang et al., 2016). MBC fungicides bind to β-tubulin, resulting in the inhibition of tubulin biosynthesis and suppression of fungal cell mitosis (Ma and Michailides, 2005). * Different superscript letters in a column indicated that values were significantly different at (P < 0.05).
Carbendazim Concn., ppm
Radial growth diameter (Mycelial inhibition percentage %)
F. proliferatum
F. verticillioides
0.00
75.24 ± 0.15a (0.00%)
57.81 ± 0.32a (0.00%)
0.50
46.27 ± 0.27b (38.5%)
72.14 ± 0.54b (0.00%)
1.00
21.18 ± 0.19c (71.9%)
69.42 ± 0.47b (0.00%)
1.50
12.67 ± 0.34d (83.2%)
65.76 ± 0.26b (0.00%)
2.00
0.00 ± 0.00e (100%)
59.14 ± 0.28c (0.00%)
2.50
0.00 ± 0.00e (100%)
54.17 ± 0.17c (6.3%)
3.00
0.00 ± 0.00e (100%)
49.18 ± 0.58c (14.9%)
3.5 Antifungal activity of Trichoderma extracts against fusarial pathogens
The acetonic extract of T. harzianum strain exhibited the highest antifungal efficacy against F. proliferatum and F. verticillioides strains, with suppressive zones of 27.6 and 22.4 mm, respectively, as seen in Fig. 5. In contrast, the hexanic extract of T. harzianum strain exerted the lowest anti-fusarial activity against F. proliferatum and F. verticillioides, with suppressive zones of 13.3 and 10.5 mm, respectively. These findings accord with those of (Jantarach and Thanaboripat, 2010), who evaluated the antifungal potency of organic solvent extracts of four different Trichoderma isolates. These researchers stated that the hexane extracts showed the lowest antimicrobial efficiency against Aspergillus flavus IMI 242684 strain, with a suppressive zone diameter of 6 mm, while the ethyl acetate extracts exhibited the highest efficacy with inhibition zone diameters ranging from 7.6 to 37 mm. Moreover, the methanolic extract of T. viride showed the lowest antimicrobial efficacy against F. proliferatum and F. verticillioides, with inhibition zone diameters of 14.7 and 10.4 mm, while the acetonic extract exerted the highest activity, with suppressive zones of 33.2 and 27.8 mm, respectively, as shown in Fig. 6.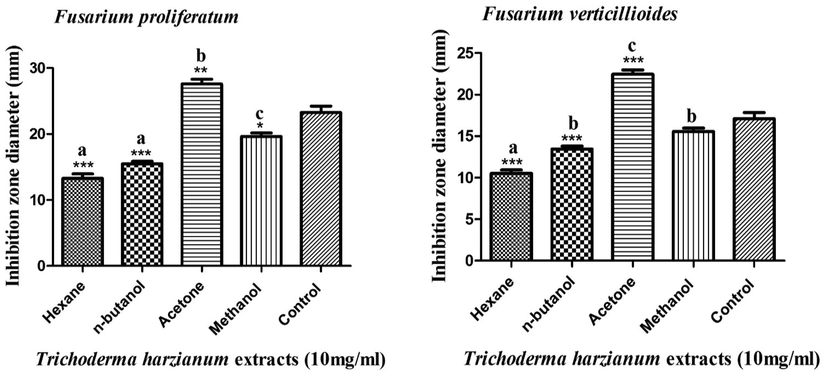
Antifungal activity of different organic extracts of T. harzianum strain against F. proliferatum and F. verticillioides strains.
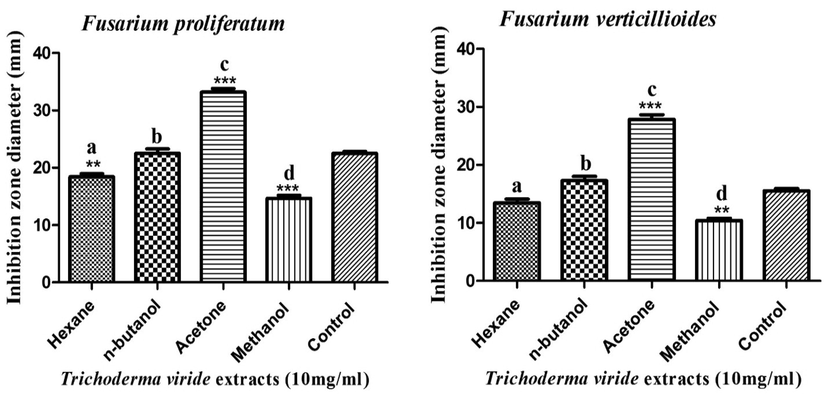
Antifungal activity of different organic extracts of T. viride strain against F. proliferatum and F. verticillioides strains.
3.6 Determination of minimum inhibitory concentration of Trichoderma acetonic extracts against F. proliferatum strain
The minimum inhibitory concentration (MIC) was detected for the acetonic extracts of T. viride and T. harzianum strains against F. proliferatum strain, which exhibited the highest susceptibility to these extracts. The acetonic extracts of T. viride and T. harzianum strains demonstrated antifungal efficiency against F. proliferatum strain, recording MIC values of 0.250 and 0.50 mg/ml, respectively, as seen in Fig. 7. The MIC data contradicted those reported in a previous study, which indicated that the alcoholic mycelial extract of T. viride showed anti-fusarial potency against Fusarium solani and Fusarium oxysporum strains with an MIC value of 100 µg/ml. (Jantarach and Thanaboripat, 2010) reported that the MIC value of the ethyl acetate extracts of Trichoderma isolates against A. flavus strain was 1.0 mg/ml, recording suppressive zones ranging from 6 to 13.8 mm in diameter. The difference in MIC values between our findings and the previous studies is attributed to the variation in sensitivity of different fungal strains.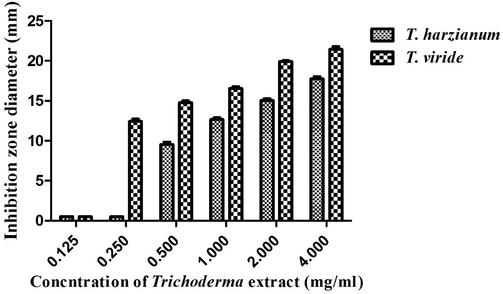
Minimum inhibitory concentration (MIC) of T. viride and T. harzianum acetonic extracts against F. proliferatum strain.
3.7 GC–MS analysis of the acetonic extracts of T. viride and T. harzianum strains
The acetonic extracts of T. viride and T. harzianum strains were analyzed to determine their active chemical constituents. Active constituents of T. viride acetonic extract were palmitic acid (22.87%), propyl benzene (12.75%), oleic acid (10.85%), caryophyllene oxide (9.32%), limonene (8.91%), β-eudesmol (7.14%), propanoic acid (6.78%), 1-pentanol (5.21%), cholic acid (4.93%), α-bisabolol (3.48%), octadecenoic acid (2.98%), ethyl benzene (2.54%), and chavicol (1.87%) as demonstrated in Table 2. GC–MS results were in accordance with those of Ali and El-Ghonemy (2014), demonstrating that the main active ingredient of the n-hexane extract of T. viride was palmitic acid (30.01%). Previous studies indicated that palmitic acid, octadecenoic acid, cholic acid, propanoic acid, β-caryophyllene, limonene, and β-eudesmol compounds inhibited the mycelial growth of different pathogenic fungal strains (Yun and Lee, 2016; Mohy El-Din and Mohyeldin, 2018; Cai et al., 2019). The antifungal activity cannot be attributed to the main constituent (palmitic acid) only but also to the presence of other bioactive constituents which were reported to have antifungal potency. On the other hand, bioctivity may be attributed to the synergistic effect between different bioactive components (Liu et al., 2016). Additionally, the main active component of T. harzianum acetonic extract was acetic acid (21.36%), followed by 2-phenylethyl alcohol (14.61%), hexadecanoic acid (12.98%), diisooctyl phthalate (10.67%), harzianic acid (9.45%), 6-pentyl-alpha-pyrone (7.98%), dihydroxyacetone (5.95%), 1-hexadecanol (4.78%), xylene (3.26%), 2H-pyran-2-one (2.39%), and 9-eicosane (2.14%), as shown in Table 3. The anti-fusarial potency of T. harzianum acetonic extract may be attributable to the presence of many bioactive ingredients as acetic acid, harzianic acid, 6-pentyl-alpha-pyrone and 2H-pyran-2-one, 2-phenylethyl alcohol, dihydroxyacetone, hexadecanoic acid, and 9-eicosane respectively. The antifungal potency of the previous extract may also be referred to the synergism between different bioactive components (Khan et al., 2020). The above conclusion was confirmed by Vinale et al. (2009) who reported that harzianic acid was one of the main components of T. harzianum extract and proved the effectiveness of this acid as an anti-mycotic agent against Pythium irregulare, Sclerotinia sclerotiorum, and Rhizoctonia solani strains. On the other hand, the bioactive alkyl pyrones produced by T. harzianum strain, such as 6-pentyl-alpha-pyrone and 2H-pyran-2-one, were reported to possess antifungal properties (Zeilinger et al., 2016). Moreover, Ismaiel and Ali (2017), showed that 6-pentyl-alpha-pyrone suppressed the mycelial growth of filamentous phytopathogenic fungal strains by 93.5% at a concentration of 250 µg/ml. Furthermore, 2-phenylethyl alcohol, dihydroxyacetone, hexadecanoic acid (Liu et al., 2014), and 9-eicosane compounds were reported to have antimicrobial potency against different fungal strains (Ahsan et al., 2017).
% of Total
RT (min)
M.W.
Chemical formula
Compounds
6.78
6.812
74.08
C3H6O2
Propanoic acid
5.21
7.248
88.15
C5H12O
1-Pentanol
2.54
7.921
106.16
C8H10
Ethyl benzene
12.75
9.453
120.19
C9H12
Propyl benzene
1.87
10.342
134.18
C9H10O
Chavicol
8.91
11.708
136.23
C10H16
Limonene
9.32
12.075
220.35
C15H24O
Caryophyllene oxide
3.84
13.734
222.37
C15H26O
α-Bisabolol
7.14
14.281
222.37
C15H26O
Beta-Eudesmol
22.87
16.167
256.42
C16H32O2
Palmitic acid
2.98
21.389
282.47
C18H34O2
Octadecenoic acid
10.85
24.981
282.47
C18H34O2
Oleic acid
4.93
33.076
408.57
C24H40O5
Cholic acid
% of Total
RT (min)
M.W.
Chemical formula
Compounds
21.36
7.365
60.05
C2H4O2
Acetic acid
5.95
8.174
90.08
C3H6O3
Dihydroxyacetone
2.39
9.268
96.08
C5H4O2
2H-pyran-2-one
3.26
10.798
106.16
C8H10
Xylene
14.61
11.243
122.16
C8H10O
2-Phenylethyl alcohol
7.98
13.945
166.22
C10H14O2
6-pentyl-alpha-pyrone
4.78
15.243
242.44
C16H34O
1-Hexadecanol
12.98
18.964
256.42
C16H32O2
Hexadecanoic acid
4.56
21.435
280.45
C18H32O2
Linoleic acid
2.14
23.675
282.50
C20H42
9-Eicosane
9.45
26.783
365.40
C19H27NO6
Harzianic acid
10.67
31.483
390.60
C24H38O4
Diisooctyl phthalate
4 Conclusion
The antagonistic strains T. harzianum and T. viride exhibited potential antimycotic activity against fusarial phytopathogens of maize. The potent antagonistic potency of culture filtrates and organic solvent extracts against fungal pathogens of maize highlights the ability to apply novel and safe biofungicides in order to avoid the harmful impacts of chemical fungicides on the environment and human health. The acetonic extracts exhibited the highest antifungal efficiency, especially against carbendazim-resistant F. verticillioides strain, highlighting the potential for using these bioagents for controlling resistant fungal phytopathogens.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RGP-1438-090).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro Antagonism of Trichoderma viride against Fusarium oxysporum strains. J. Pharmacogn. Phytochem.. 2018;7(2):2816-2819.
- [Google Scholar]
- Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Expr.. 2017;7(1):54.
- [Google Scholar]
- Optimization of culture conditions for the highest lipid production from some oleaginous fungi for biodiesel preparation. Asian J. Appl. Sci.. 2014;2(5)
- [Google Scholar]
- Integrated control of fruit rot and powdery mildew of chilli using the biocontrol agent Pseudomonas fluorescens and a chemical fungicide. Biol. Control. 2010;52(1):1-7.
- [Google Scholar]
- In vitro antifungal activity of Trichoderma strains on pathogenic fungi inciting hot pepper, Capsicum annuum L.) J. Chem. Pharm. Res.. 2016;8(4):425-430.
- [Google Scholar]
- Isolation and characterization of the bioactive metabolites from the soil derived fungus Trichoderma viride. Mycology. 2018;9(1):70-80.
- [Google Scholar]
- Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT. 2019;106:50-56.
- [Google Scholar]
- Da Silva, F.M., Alves, L.S., Botelho Filho, F.B., Silva, I.S., 2017. Liquidez dos contratos futuros de milho negociados na BM&FBOVESPA. Rev. Admin e Negócios da Amazônia, 9(1), 26–44.
- Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol.. 2011;9(10):749-759.
- [Google Scholar]
- Component analysis and antifungal activity of the compounds extracted from four brown seaweeds with different solvents at different seasons. J. Ocean Univ. China. 2018;17(5):1178-1188.
- [Google Scholar]
- Trichoderma as potential biocontrol agent, its exploitation in agriculture: a review. Plant Protection. 2018;2(3)
- [Google Scholar]
- In vitro antagonistic potential of Trichoderma harzianum for biological control of Fusarium moniliforme isolated from Dioscorea rotundata tubers. Virol. Mycol.. 2017;6(2):2-8.
- [Google Scholar]
- Antimicrobial properties of 6-pentyl-α-pyrone produced by endophytic strains of Trichoderma koningii and its effect on aflatoxin B1 production. Biologia. 2017;72(12):1403-1415.
- [Google Scholar]
- The efficacy of ethyl acetate extract of Trichoderma culture broth on growth inhibition and aflatoxin production by Aspergillus flavus IMI 242684. Curr. Appl. Sci. Technol.. 2010;10(1):19-29.
- [Google Scholar]
- The incidence of pokkah boeng in indigenous and exotic sugarcane (Saccharum officinarum) clones. Indian J. Agric. Sci.. 2015;85(4):596-601.
- [Google Scholar]
- Khan, R. A. A., Najeeb, S., Hussain, S., Xie, B., Li, Y., 2020. Bioactive secondary metabolites from trichoderma spp. against phytopathogenic fungi. Microorganisms, 8(6), 817.
- Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014;14(1):242.
- [Google Scholar]
- Synergistic effects and related bioactive mechanism of Potentilla fruticosa L. leaves combined with Ginkgo biloba extracts studied with microbial test system (MTS) BMC Complement Altern. Med.. 2016;16(1):495.
- [Google Scholar]
- Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot.. 2005;24(10):853-863.
- [Google Scholar]
- Marques, E., Martins, I., Mello, S. C. M. d., 2018. Antifungal potential of crude extracts of Trichoderma spp. Biota Neotropica, 18(1).
- Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J. Biol. Sci.. 2020;27(6):1482-1487.
- [Google Scholar]
- Antagonistic potential of native trichoderma viride strain against potent tea fungal pathogens in North East India. The Plant Pathology Journal. 2015;31(3):278-289.
- [Google Scholar]
- Evaluation of antagonistic effect of Trichoderma harzianum against Fusarium oxysporum causal agent of white yam (Dioscorearotundata poir) tuber rot. Trends Tech. Sci. Res.. 2018;1(1):0012-0018.
- [Google Scholar]
- Ojha, S., Chatterjee, N., 2011. Mycoparasitism of Trichoderma spp. in biocontrol of fusarial wilt of tomato. Arch. Phytopathol. Plant Protect., 44(8), 771–782.
- Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res.. 2017;33(3):167-182.
- [Google Scholar]
- Pfordt, A., Ramos Romero, L., Schiwek, S., Karlovsky, P., von Tiedemann, A., 2020. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear-and stalk rot in maize. Pathogens, 9(3), 236.
- Puyam, A., 2016. Advent of Trichoderma as a bio-control agent-a review. J. Appl. Nat. Sci., 8(2), 1100–1109.
- Comparative study of biological agents, Trichoderma harzianum (Th-Azad) and Trichoderma viride (01PP) for controlling wilt disease in pigeon pea. J. Microbial Biochem. Technol.. 2014;6:110-115.
- [Google Scholar]
- Genetic structure of Fusarium verticillioides populations and occurrence of fumonisins in maize grown in Southern Brazil. Crop Prot.. 2017;99:160-167.
- [Google Scholar]
- In vitro evaluation of trichoderma species against Fusarium oxysporum f. sp. lycopersici causing tomato wilt. Plant Pathol. J.. 2018;17(2):59-64.
- [Google Scholar]
- Sood, M., Kapoor, D., Kumar, V., Sheteiwy, M. S., Ramakrishnan, M., Landi, M., Araniti, F., Sharma, A., 2020. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants, 9(6), 762.
- Isolation and screening of effective Trichoderma spp. against the root rot pathogen Macrophomina phaseolina. J. Agric. Technol.. 2011;7(3):623-635.
- [Google Scholar]
- The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop Prot.. 2016;79:56-63.
- [Google Scholar]
- Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod.. 2009;72(11):2032-2035.
- [Google Scholar]
- A novel fungal killing mechanism of propionic acid. FEMS Yeast Res.. 2016;16(7):fow089.
- [Google Scholar]
- Secondary metabolism in Trichoderma – chemistry meets genomics. Fungal Biol. Rev.. 2016;30(2):74-90.
- [Google Scholar]
- Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil. 2013;368(1-2):433-444.
- [Google Scholar]
- Zhang, H., Brankovics, B., van der Lee, T. A., Waalwijk, C., van Diepeningen, A. A., Xu, J., Chen, W., Feng, J., 2016. A single-nucleotide-polymorphism-based genotyping assay for simultaneous detection of different carbendazim-resistant genotypes in the Fusarium graminearum species complex. PeerJ, 4, e2609.
- In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC Complement Med. Ther.. 2020;20(1):25.
- [Google Scholar]







