Translate this page into:
Analysis of tumour markers in esophageal carcinoma with different age groups
⁎Corresponding authors. venzymes@gmail.com (P. Vijayaraghavan), vkgopalakrishnan@gmail.com (V.K. Gopalakrishnan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Esophageal cancer has been reported in various countries, including Saudi Arabia, India and United States. Many biomarkers have been used to detect cancers at an early stage to improve the survival rate of esophageal cancer.
Methods
The present study investigated the biomarkers, biglycan, myeloperoxidase, cytokine (TNF-α, IFN-γ, IL-10 and IL-12) and carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC) and carbohydrate antigen L1 cell adhesion molecule from the tumour and non-tumour samples.
Results
The increased amount of circulatory biglycan level was detected using the ELISA method in tumour cases compared to control samples (p < 0.001). The expression of biglycan was high in certain tumour cases and was statistically significant (p < 0.01). The variation of myeloperoxidase markers in control and experimental subjects were statistically significant (p < 0.01). Among cancer patients, elevated levels of carcinoembryonic antigen (28.3 ± 1.2 ng/ml and 30.3 ± 1.1 ng/ml), squamous cell carcinoma antigen (5.3 ± 1.1 ng/ml and 4.9 ± 0.5) and carbohydrate antigen 19–9 (18.3 ± 1.1 ng/ml and 15.1 ± 2.6 ng/ml) were detected in male and female cases (p < 0.001). In the normal tissues, the expression of L1 cell adhesion molecule was high and was expressed in low amount in tumour tissues in both male and female individuals (<0.001).
Conclusion
These biomarkers are useful for the early diagnosis of esophageal cancer.
Keywords
Esophageal cancer
Biglycan
Myeloperoxidase
Therapeutic target
Cytokines
L1 cell adhesion molecule
1 Introduction
Esophageal cancer accounts for about 1 % of the total number of cancer patients in various developed countries. Its prevalence in other countries varies, and maximum incidences are reported in Africa, Asia, and Iran. Moreover, the total incidence of esophageal cancer increases continuously and is mainly associated with the increasing incidence of adenocarcinoma (AC) (Ishibashi et al., 2020). In the United States, the incidence of AC and squamous cell carcinoma (SCC) are equal. Furthermore, increased SCC incidence was registered in the 1960’s. The types of carcinoma varied widely based on demographics. SCC and AC types are almost common in men; moreover, SCC has increase prevalence in urban cases in African-Americans, whereas AC is a major disease in Caucasians. Despite the development of new treatment strategies, the change in survival rate is very small. The five-year survival for both SCC and AC was less than 29 % (Qin et al., 2022). The incidence of AC increased considerably over the past three decades, and this sudden rise showed much interest in studying various risk factors associated with esophageal cancer. Smoking is one of the important risk factors associated with AC and SCC, and increased consumption of alcohol is mainly associated with SCC. Dietary factors such as consumption of betel nuts, N-nitroso compounds, beverages, and hot foods are associated with the development of SCC. Obesity is another reported factor and a body mass index (BMI) > 30 was associated with esophageal AC (Zhao and Lim, 2020).
Many esophageal cancers are diagnosed at later stages. Early stage carcinomas are frequently treated with multimodality therapy to improve the survival of individuals. Despite continuous effort, >50 % of recurrent cases have been reported. Abdominal, thoracic, and cervical tumours can be treated with multimodality strategies (Crosby et al., 2009). Treatment output from neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy is not effective in improving the survival rate of patients (Li et al., 2016).There is an urgent need to develop a non-invasive, low-cost, convenient method for the early detection of esophageal cancer. The determination of biomarkers in serum samples is one of the simple and convenient methods for some tumours and in monitoring metastasis or recurrence. The serum biomarkers are defined as the molecules in the serum whole levels can be applied to determine certain cancers, prognosis, diagnosis and clinical management of malignant diseases (Li et al., 2022). In recent years, various biomarkers have been proposed in the diagnosis and treatment of esophageal cancer, including, squamous cell cancer antigen (SCC-Ag), carcinoembryonic antigen (CEA), etc. (Zhang et al., 2015a,b).
Genetic markers are frequently used for the determination of somatic tumours. However, it has certain limitations due to decreased detection limit in the case of low tumour load in early stages of cancer (Shain et al., 2015). Hence, it is very clear that the genetic markers are considered to lack of sensitivity and specificity for cancer types. Epigenetic markers, mainly DNA methylation are considered one of the important markers for early determination of cancer. This method is useful to study the occurrence, cancer-specific methylation patterns, technical repeatability, and biological stability (Koch et al., 2018). Methylation-based biomarkers are helpful for the diagnosis and prediction of survival of solid tumours. DNA methylation markers are widely used to differentiate tumour tissues and normal tissues in various types of cancers, including, liver, lung, colon, and breast cancer (Hao et al., 2017). Moreover, type-specific epigenomic markers for prognosis and diagnosis of esophageal cancer were not completely elucidated. Aberrant methylation has been frequently reported in various types of cancers, including esophageal cancer, which greatly contributes to carcinogenesis (Kou et al., 2019).In this study, cancer biomarkers were used for the early determination of esophageal tumours.
2 Materials and methods
2.1 Blood samples
The blood samples were collected from cancer patients with written informed consent. The sample details, such as sex, age, type of specimen, and non-tumour specimens, were collected from the individuals. Control samples were collected from healthy individuals matched with a pre-determined age.
2.2 Tissues
Tissue samples were collected from patient’s tumour specimens and control non-tumour specimens from the adjacent places. These tissue samples were collected from patients who underwent treatment with written consent. Ethical approval was obtained from the Institute Ethical Committee (Approval number: SDB-152/2022).
2.3 Determination of biglycan from the sample
An enzyme-linked immunosorbent assay (ELISA) was used to determine biglycan from the blood samples using an ELISA kit as suggested by the manufacturer. The concentration of biglycan was determined and the intensity of the reaction was measured at 450 nm against appropriate controls (Zaidi et al., 2014).
2.4 Isolation of RNA from the tissues and real time reverse transcription polymerase chain reaction (RT-PCR)
The collected frozen tissues were used for the isolation of RNA using a RNA extraction kit as suggested by the manufactures instructions. The extracted RNA was quantified using a Nanodrop spectrophotometer (Thermo Scientific™, USA). A total of 2 mg of total RNA from the tissue sample was used to convert cDNA. The quantitative real-time reverse transcription polymerase analysis was performed by a polymerase chain reaction machine using the forward and reverse primers. The primers used were, 5′AGGTGCCCAAGGGAGTGTTC 3′ (forward) and 5′TGGTCTAGGTGGAGTTCATTCAGG 3′ (reverse) primers. PCR reactions were performed and the products were analyzed. Β-actin was used as the positive control (Johnson et al., 2000).
2.5 Western blotting analysis
Tumour (experimental) and non-tumour (adjacent control) tissues were lysed using radioimmunoprecipitation assay lysis buffer (RIPA lysis buffer). RIPA buffer composed of (1 % Triton X-100, 0.15 M NaCl, 0.1 % sodium dodecyl sulphate (SDS), 0.5 % deoxycholate, 0.05 M Tris, pH 7.4) containing appropriate quantities of protease inhibitors and ethylenediaminetetraacetic acid. The total protein content of the lyzed sample was determined by the Bradford method using bovine serum albumin standard. The protein sample from control and experimental tissue was resolved using the sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (11 % separating gel and 5.5 % stacking gel). The separated protein was blotted from the gel onto a nitrocellulose membrane using electro blotting method. The nitrocellulose membrane of the remaining unbound regions was blocked using bovine serum albumin (5 %) and incubated in buffer B (Tris-buffered saline, pH 7.4, 0.1 % Tween 20) for 3 h. Anti-β actin primary and secondary antibodies were used for the determination of β actin. Biglycan primary antibodies were incubated for overnight at 4 ± 1 °C and horse-radish peroxidase conjugated secondary antibody were incubated for 2 h (Sinkala et al., 2017).
2.6 Myeloperoxidase activity in plasma
The amount of myeloperoxidase was analysed using an ELISA assay method as suggested by the manufacturer (Bio Rad). To perform the assay, anti-MPO autoantibodies were inactivated from the plasma samples. Experiments were performed in duplicates and the results were expressed as mean ± SD (Goiffon et al., 2015).
2.7 Cytokine analysis
Blood samples were used for the determination of cytokine levels. The collected blood was centrifuged at 3000 rpm for 10 min, and the serum sample was used. An ELISA kit was used for the determination of IL-10, IFN-γ, TNF-α and IL-12 (Asselin-Paturel et al., 1998).
2.8 Analysis of serum carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC) and carbohydrate antigen (CA) 19-9
Serum samples were used for the determination of tumour makers such as CEA, SCC and CA 19-9. The serum antigens (ng/ml) were estimated using a commercial immunoassay kit. The amount of CEA above 5 ng/mL and CA 19-9 and SCC above 1.5 ng/mL was considered as CEA positive, CA 19-9 were positive (Mino-Miyagawa et al., 1990).
2.9 L1 cell adhesion molecule (L1CAM) assay
An Enzyme-linked immunosorbent assay was used for the determination of L1 cell adhesion molecule. Briefly, ELISA plate was coated with antibody and incubated at 4 °C. After 1 h of incubation, the microtitre plate was washed and incubated with blocking buffer for 1 h. After clear washing, 100 μl was incubated for 2 h at 28 ± 1 °C. L1CAM was used for the preparation of the standard curve at various concentrations. The colour intensity was read at 450 nm against control as described by the manufacturers protocol (Ichikawa et al., 2019).
2.10 Statistical analysis
The results were expressed as mean ± SD. The statistical significance between non-tumour and tumour was analysed using Student’s t-test. The p value < 0.01 was considered statistically significant.
3 Results
3.1 Biglycan level in blood samples
ELISA test was used for the determination of biglycan from the non-tumour and tumour samples. The circulatory biglycan level was higher in diseased patients than in control samples (p < 0.001). The amount of biglycan level was elevated in tumour tissues (Table 1). The result was expressed as mean ± standard deviation.
Characters
Biglycan (ng/ml)
Cancer patients (n = 60)
Control (n = 12)
Sex
Male (n = 50)
0.873 ± 0.031
0.61 ± 0.04
<0.001
Female (n = 22)
0.764 ± 0.02
0.562 ± 0.03
<0.001
Age group-Male
45–60
0.951 ± 0.05
0.54 ± 0.01
<0.001
61–75
0.892 ± 0.0
0.57 ± 0.04
<0.002
76–90
0.876 ± 0.07
0.62 ± 0.05
<0.003
Age group-Feale
45–60
0.748 ± 0.28
0.538 ± 0.04
<0.005
61–75
0.737 ± 0.16
0.541 ± 0.02
<0.006
76–90
0.738 ± 0.0
0.549 ± 0.05
<0.007
3.2 Western blotting analysis
Western blotting analysis was performed to analyse the expression of biglycan in tumour and non-tumour tissues. Fig. 1 depicted the expressed level and the biglycan and control β-actin. The expression of biglycan was high in certain tumour cases and was significant (p < 0.01). The expressed biglycan was low in non-tumour cells than tumour tissues (Fig. 1).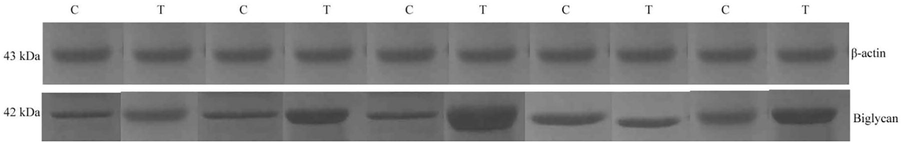
Western blowing analysis of biglycan in oesophageal carcinoma and adjacent normal tissues.
3.3 Myeloperoxidase activity of plasma in tumour and non-tumour cases
The amount of myeloperoxidase was decreased in cancer cases than non-tumour individuals. In the non-tumour group, myeloperoxidase levels ranged from 114.1 ± 1.2 ng/ml to 118.4 ± 10.4 ng/ml. In control subject myeloperoxidase levels were considerably lower. The variation of myeloperoxidase in control and experimental subjects were statistically significant (p < 0.01). The difference in myeloperoxidase level as a function of age was not significant (p > 0.01) (Table 2).
Characters
Myeloperoxidase (ng/ml)
Tumour (n = 60)
Non-Tumour (n = 12)
Male (n = 40)
20.3 ± 1.1
118.4 ± 10.4
Female (n = 22)
27.3 ± 2.5
117.1 ± 1.2
Age group-Male
45–60
35.4 ± 2.2
114.1 ± 1.2
61–75
34.8 ± 2.3
117.1 ± 2.7
76–90
33.8 ± 1.1
116.1 ± 2.2
Age group-Female
45–60
37.3 ± 1.3
112.3 ± 8.8
61–75
31.2 ± 2.2
116.2 ± 1.7
76–90
30.4 ± 1.9
115.7 ± 2.9
3.4 The cytokine level in tumour and non-tumour tissues
TNF-α level was elevated over tenfold in tumour group and statistical significance was observed between two groups. The Student’s ‘t’ test revealed a significant difference between the control and the experimental group (p < 0.0001). In serum, the amount of IFN-γ was increased considerably than non-tumour control (p < 0.0001). The mean concentrations of IL-10 and IL-12 elevated about 2-fold in tumour group than control (p < 0.001) (Table 3).
Cytokines (pg/ml)
Tumour group (n = 60)
Non tumour group (n = 12)
TNF-α
64.2 ± 4.8
6.18 ± 0.09
IFN-γ
72.6 ± 1.6
4.02 ± 0.08
IL-10
24.7 ± 0.7
10.3 ± 0.65
IL-12
32.4 ± 0.28
16.2 ± 0.14
3.5 Determination of carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC) and carbohydrate antigen (CA) 19-9 in the serum sample
The positive esophageal cancer was determined based on the amount of CEA, SCC and CA in the serum sample. CEA was 95 ± 2.3 %, 68.9 ± 4.7 %, respectively for the male and female patients. CA 19–9 was considered as one of the important markers and was detected in the blood of 85.4 ± 2.8 % and 80.1 ± 3.1 %, male and female cases. Among cancer patients, elevated levels of CEA (28.3 ± 1.2 ng/ml and 30.3 ± 1.1 ng/ml), SCC (5.3 ± 1.1 ng/ml and 4.9 ± 0.5) and CA 19–9 (18.3 ± 1.1 ng/ml and 15.1 ± 2.6 ng/ml) were detected in male and female cases (p < 0.001). Moreover, the amount of these antigens did not vary significantly based on age group and the results (Table 4).
Patients and age group
CEA
SCC
CA 19–9
%
ng/ml
%
ng/ml
%
ng/ml
Male (n = 50)
95 ± 2.3
28.3 ± 1.2
98.4 ± 2.7
5.3 ± 1.1
85.4 ± 2.8
18.3 ± 1.1
Female (n = 22)
68.9 ± 4.7
30.3 ± 1.1
99.3 ± 1.1
4.9 ± 0.5
80.1 ± 3.1
15.1 ± 2.6
3.6 L1 cell adhesion molecule as a marker for early diagnosis of cancer
Expression of L1CAM protein in the serum sample of cancer tissue and normal tissues were studied. In the normal tissue, the amount of L1CAM protein was high and was expressed in a low amount in the cancerous tissue in both male and female individuals (<0.001). Moreover, the amount of L1CAM was not varied significantly between male and female cases. L1CAM expression changes were observed between cancer and normal tissue in all selected age-groups (Table 5).
Cancerous tissue
Normal tissue
p-value
Patients and age group
Male (n = 50)
31.2 ± 2.4
48.3 ± 2.2
<0.001
Female (n = 22)
30.9 ± 2.7
47.9 ± 1.3
<0.001
Age group
45–60
28.2 ± 1.4
49.1 ± 2.1
<0.001
61–75
31.9 ± 2.2
48.4 ± 1.3
<0.002
76–90
34.2 ± 1.5
49.3 ± 1.2
<0.003
4 Discussion
In cancer patients, inflammation plays a critical role in each stage of the cancer development. Inflammatory cells or tumour cells are able to indirectly or directly stimulate or inhibit tumour growth. In our study, cytokines levels were examined in the control and tumour groups and an elevated level were observed. TNF-α, IFN-γ, IL-10 and IL-12 level increased in the tumour cases. IFN-γ and IL-10 were played critical role in tumour cell growth. IFN-γ induced apoptosis and inhibited tumour cell growth (Wall et al., 2003). IL-10 regulated cell-mediated immunity and it induced tumour growth (Ueda et al., 1994). In this study, elevated level of IL-20 was registered in cancer patients than in non-tumour control and the enhanced level was positively correlated. IL-12 activated antigen-specific and innate adaptive immunity against tumour tissues and inhibited angiogenesis via IFN-γ. In tumour samples, the maximum level of IL-12 indicated the sign of good survival. The amount of MPO was found to be less in tumour cases than non-tumour healthy cases. Myeloperoxidase deficiency has been linked with cancer disease and has been reported previously (Aratani et al., 1999).
Myeloperoxidase catalyses aromatic amines and polycyclic aromatic hydrocarbons and is involved in the biosynthesis of carcinogenic reactive metabolites (Kadlubar et al., 1992). In our study, the inflammatory marker, MPO level decreased and the analysis of this marker may provide a good understanding of oesophagus cancers as tumour markers. MPO is generally present in the neutrophils and diffuses into inflamed tissues.
Carbohydrate antigens are widely used to determine breast, pancreatic, and colon cancers. The amounts of CEA, SCC and CA in the blood sample were quantified. Many studies have conducted to analyse the tumour and prognostic markers in cancer. Niho and Shinkai (2001) and Charalabopoulos et al. (2007) elevated CEA level in BAL fluid in patients have been determined. Moreover, Lazarev et al. (2010) revealed no significant changes of CEA in normal and tumour cases. In diagnosed tumour cases, elevated levels of CA125 and CEA have been reported (Cedrés et al., 2011). The mortality and morbidity of cancer increased in recent times due to delayed diagnosis. In gastric cancer, environmental factors and Helicobacter pylori infection were determined as the main causes of infection (Kusano et al., 2017). In recent years, serum tumour markers were widely used to determine types of cancers. Tumour markers directly reflected cancer and the amount of markers revealed the severity of disease. L1CAM promoted the remodelling of various extracellular matrixes and improved the chemotaxis of tumour cells to the extracellular matrix, promoting tumour invasion and metastasis (Raveh et al., 2009). The expression of L1CAM protein changes has also been studied to analyse the malignancy and prognosis of melanoma, endometrial and ovarian tumours (Geels et al., 2016). In this study, the level of L1CAM protein in oesophageal cancer was analysed in cancer tissues and adjacent normal tissues of patients with oesophageal cancer and the results revealed a moderately elevated level of L1CAM in cancer tissues were compared to normal tissues. The present finding reveals that L1CAM is fairly involved in the development of oesophageal cancer. Recent findings revealed that L1CAM level was elevated in certain tumours than normal tissues, the level of L1CAM was elevated in ovarian, uterine and gastrointestinal stromal tumors, moreover reduced level of expression was observed in esophageal squamous cell carcinoma, which was consistent with the present study (Zhang et al., 2015a,b). In this study, the amount of L1CAM was less in patients diagnosed with esophageal cancer than in healthy controls (p < 0.001). The variation of L1CAM was mainly associated with variations in the expression patterns of L1CAM in various types of cancer (Xu et al., 2018).
5 Conclusions
In this study, we observed expression changes of biglycan, myeloperoxidase, cytokines, carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC) and L1 cell adhesion molecule in esophageal cancer. Analysis of one tumour marker is not recommended to differentiate the cancer cells. Expression of biglycan is one of the classical methods for the determination of esophageal cancer and an elevated level was observed. The amount of myeloperoxidase, cytokine (TNF-α, IFN-γ, IL-10 and IL-12) in blood influenced significant variation in normal tissues and cancer cells. In normal tissue, the amount of expression of L1 cell adhesion molecule protein was high and was expressed in a low amount in cancerous tissues.
Acknowledgment
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR57).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun.. 1999;67(4):1828-1836.
- [Google Scholar]
- Quantitative analysis of Th1, Th2 and TGF-β1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int. J. Cancer. 1998;77(1):7-12.
- [Google Scholar]
- Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non–small-cell lung cancer (NSCLC) Clin. Lung Cancer. 2011;12(3):172-179.
- [Google Scholar]
- CEA levels in serum and BAL in patients suffering from lung cancer. Med. Oncol.. 2007;24(2):219-225.
- [Google Scholar]
- The management of a patient with an operable carcinoma of the oesophagus. Ann. R. Coll. Surg. Engl.. 2009;91:366-370.
- [Google Scholar]
- L1CAM expression is related to non-endometrioid histology, and prognostic for poor outcome in endometrioid endometrial carcinoma. Pathol. Oncol. Res.. 2016;22(4):863-868.
- [Google Scholar]
- A rapid bioluminescence assay for measuring myeloperoxidase activity in human plasma. Nat. Commun.. 2015;6(1):1-9.
- [Google Scholar]
- DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. U.S.A.. 2017;114(28):7414-7419.
- [Google Scholar]
- Clinical significance and biological role of L1 cell adhesion molecule in gastric cancer. Br. J. Cancer. 2019;121(12):1058-1068.
- [Google Scholar]
- Prognostic significance of systemic inflammatory markers in esophageal cancer: Systematic review and meta-analysis. Ann. Gastroenterol. Surg.. 2020;4(1):56-63.
- [Google Scholar]
- Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal. Biochem.. 2000;278(2):175-184.
- [Google Scholar]
- Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ. Health Perspect.. 1992;98:69-74.
- [Google Scholar]
- Analysis of DNA methylation in cancer: location revisited. Nat. Rev. Clin. Oncol.. 2018;15(7):459-466.
- [Google Scholar]
- Promutagenicity of 8-chloroguanine, a major inflammation-induced halogenated DNA lesion. Molecules. 2019;24(19):3507.
- [Google Scholar]
- The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer. 2017;20(1):16-19.
- [Google Scholar]
- Role of biological tumor markers CEA, Cyfra-21, NSE, TU M2-PK in diagnosis and treatment of lung cancer. VestnikKhirurgiiImeni II Grekova. 2010;169(1):39-43.
- [Google Scholar]
- A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol. Cancer. 2022;21(1):1-13.
- [Google Scholar]
- A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int. J. Biol. Sci.. 2016;12(8):1022.
- [Google Scholar]
- Tumor-antigen 4. Its immunohistochemical distribution and tissue and serum concentrations in squamous cell carcinoma of the lung and esophagus. Cancer. 1990;66(7):1505-1512.
- [Google Scholar]
- Diet and esophageal cancer risk: an umbrella review of systematic reviews and meta-analyses of observational studies. Adv. Nut. 2022
- [CrossRef] [Google Scholar]
- L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett.. 2009;282(2):137-145.
- [Google Scholar]
- The genetic evolution of melanoma from precursor lesions. Eng. J. Med.. 2015;20:1926-1936.
- [Google Scholar]
- Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat. Commun.. 2017;8(1):1-12.
- [Google Scholar]
- Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J. Gastroenterol.. 1994;29(4):423-429.
- [Google Scholar]
- IFN-γ induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin. Canc. Res.. 2003;9:2487-2496.
- [Google Scholar]
- Diagnostic and prognostic value of serum L1-cell adhesion molecule in esophageal squamous cell carcinoma. Clin. Res. Hepato.l Gastroenterol.. 2018;42(6):597-603.
- [Google Scholar]
- Evaluation of a 4-protein serum biomarker panel—biglycan, annexin-A 6, myeloperoxidase, and protein S 100-A 9 (B-AMP)—for the detection of esophageal adenocarcinoma. Cancer. 2014;120(24):3902-3913.
- [Google Scholar]
- Overexpression of L1 cell adhesion molecule correlates with aggressive tumor progression of patients with breast cancer and promotes motility of breast cancer cells. Int. J. Clin. Exp. Pathol.. 2015;8(8):9240-9927.
- [Google Scholar]
- Diagnostic value of multiple tumor markers for patients with esophageal carcinoma. PloS One. 2015;10(2):e0116951.
- [Google Scholar]
- Lifestyle risk factors in esophageal cancer: An integrative review. Crit. Care Nur. Q.. 2020;43(1):86-98.
- [Google Scholar]







