Translate this page into:
Analysis of the chemical composition and in vitro cytotoxic activities of the essential oil of the aerial parts of Lavandula atriplicifolia Benth
⁎Corresponding author. hharrath@ksu.edu.sa (Abdel Halim Harrath)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plants of the Lavandula genus have been widely used in folk and traditional medicine. The aim of this study was to evaluate the qualitative and quantitative chemical composition of the essential oil of the aerial parts of Lavandula atriplicifolia (EOAPLA) grown in Saudi Arabia and its potential cytotoxicity to cancer cell lines. The essential oil was characterized and quantified by GC–MS and GC-FID. The potential cytotoxicity of the essential oil was evaluated against colorectal (LoVo) and hepatocellular (HepG2) carcinoma cell lines by using the MTT and lactate dehydrogenase (LDH) cytotoxicity assays. A total of 36 compounds were identified that constituted 92.3% of the total oil. The chemotype of the species was dominated by C-10 massoia lactone (46.65%) as the major compound followed by oxygenated monoterpenes (28.43%). This is the first report to describe the presence of the rare compound massoia lactone in the essential oils from Lavandula species. The data revealed that EOAPLA inhibited the growth of LoVo and HepG2 cells in a dose-dependent manner. Treatment with 10 μg/mL EOAPLA for 2 days resulted in significant damage to LoVo cells in comparison with the control. In contrast to the control cells, the LoVo cells showed morphological alterations such as cytoplasmic condensation, shrinkage, and the formation of debris. Moreover, EOAPLA treatment induced apoptosis in LoVo cells since it increased the expression of caspase 3/7. The cytotoxic and anti-proliferative properties of EOAPLA make it a good candidate for treatment of various cancers.
Keywords
Chemical composition
Cytotoxic
Essential oil
Lavandula atriplicifolia
Massoia lactone
1 Introduction
It is well known that aromatic plants offer considerable benefits to human nutrition and health owing to their high content of bioactive compounds (Djenane et al., 2012; Costa et al., 2015). In particular, plants of the Lavandula genus, which include 39 species and belong to the family Lamiaceae, have been widely used in folk and traditional medicine (Cavanagh and Wilkinson, 2002; Denner, 2009). Several studies have shown that the species of this genus contain a considerable number of bioactive constituents, such as monoterpenes (Ulubelen et al., 1988), sesquiterpenes, diterpenes (Politi et al., 2002), triterpenes (Topcu et al., 2001), polyphenols (Areias et al., 2000; Upson et al., 2000) and coumarins (Shimizu et al., 1990). These components of lavender essential oil have been shown to possess useful biological properties and pharmacological activities, such as cytotoxicity (Nikolic et al., 2014) antiseptic, anti-inflammatory, and analgesic properties, antioxidant activity (Chrysargyris et al., 2016), antimicrobial and antifungal activities (Özcan et al., 2018; Nikolic et al., 2014; Chrysargyris et al., 2016), and have been used in the food, cosmetics, and perfume industries (Wells et al., 2018; Wilkinson et al., 2003; Ciobanu et al., 2012).
Owing to the extensive and important pharmaceutical and cosmetic applications of the Lavandula species cited above, the global production of lavender oil has reached 200 tons/year. Three particular Lavandula species are principally cultivated to produce essential oils: L. angustifolia (fine lavender), L. latifolia (spike lavender), and the sterile hybrid L. intermedia (lavandin). However, to the best of our knowledge, very limited information on the essential oil of L. atriplicifolia and its biological properties is available (Upson et al., 2000). This species is a woody, perennial, bushy, weak-stemmed, aromatic shrub of approximately 30–100 cm. It is native to Saudi Arabia and the Republic of Yemen and grows on dry rocky areas above 2500 m. Thus, in order to extend our contribution to the study of the aromatic flora of Saudi Arabia and their biological activities, this work focuses on: 1) the determination of the qualitative and quantitative chemical composition of the essential oil of the aerial parts of L. atriplicifolia (EOAPLA) and 2) the evaluation of its cytotoxic activity against colorectal (LoVo) and hepatocellular (HepG2) carcinoma cell lines by using the MTT and lactate dehydrogenase (LDH) assays.
2 Methods
2.1 Plant material
L. atriplicifolia were harvested in the flourishing season from Elbaha, Saudi Arabia, in May 2015 and then was identified by Faraj Al-Ghamdi. A plant reference specimen (no. 708) was deposited at the Herbarium of the Faculty of Sciences, Northern Border University, Kingdom of Saudi Arabia.
2.2 Extraction of the essential oil
The fresh aerial parts of L. atriplicifolia (500 g) were hand cut into small fragments and hydro-distilled in a Clevenger apparatus for 4 h yielding yellowish oils (0.008% (v/w)). The obtained oils were stored at 4 °C in a colored vial for further testing.
2.3 Oil composition analysis
The separation and identification of the different chemical compounds of the EOAPLA was carried out by gas chromatography (QP2010 Ultra, Shimadzu, Japan), coupled to a mass spectrometer, Ion trap in electronic impact mode (EI) with ionization energy of 70 eV. The column used is non-polar (HP-5MS). The temperature of the column is programmed from 40° to 280 °C. at a rate of 1.5 °C/min. The temperature of the injector is set at 240 °C and the detector (ionization source) is 220 °C. The flow rate of the carrier gas (helium) is set at 1 mL/min. The volume of the sample injected is 1 μL of the oil diluted in hexane. The constituents of the oil essential were identified by their retention indices (RI) calculated from reference to a series of n-alkanes and comparing their mass spectra with those listed in a type library (NIST-MS) and published literature (Adams, 2017; Soskic et al., 2016).
2.4 Cytotoxicity assay
Human epithelial colorectal adenocarcinoma (LoVo cells) and human epithelial hepatocellular carcinoma cells were grown in DMEM (Gibco, USA) supplemented with 10% fetal calf serum (FBS) and incubated at 37 °C and 5% CO2 humidified atmosphere in an incubator (Shel lab CO2 Series, USA). The cytotoxicity was evaluated by using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-terazolium bromide (MTT) reduction inhibition assay [38]. Briefly, the cells were seeded on 24-well plates at a density of 5 × 104 cells/well in 1 mL of fresh medium. After 24 h, the cells were treated for 48 h with different concentrations (10, 25, 50, and 100 μg/mL) of the essential oil. Methanol (0.01%) treated cells were used as controls. At the end of the incubation period, the medium was aspirated, 100 μL MTT solution (0.5 mg/mL) was added to fresh DMEM, and the cells incubated for 2 h. After incubation, 1 mL 0.01% acidified isopropanol was added to dissolve the formed product and the samples were placed in a shaking incubator for 10 mins. Two hundred microliters of the blue dissolved formazan crystals was transferred to a 96-well plate and measured at 570 nm by using a multi-scan plate reader (Thermo, China). The control consistent of treating cells with methanol for the same lengths of time. The cell viability was calculated using the following formula: cell viability (%) = OD treated /OD control ×100.
2.5 Lactate dehydrogenase (LDH) cytotoxicity assay
The cytotoxicity of LoVo cells was measured by using lactate dehydrogenase (LDH) cytotoxicity assay kit (BioVision). This assay measures the release of LDH enzyme from the cytoplasm of damaged cells. The cells were cultured in 24-well plates and treated with the LC50 of the active compound. Methanol (0.01%) treated cells were used as control. After 48 h of treatment, 100 μL of the supernatant was removed and incubated with 100 μL of the reaction mixture. The blue formazan crystals were transferred to 96-well plate and the absorbance was measured at 490 nm by using multi-scan plate reader (Thermo, China).
2.6 Light microscopy
After 2 days of treatment, the structural changes and morphology of LoVo cells were observed with an inverted phase contrast microscope and the images were captured using a MC 170 HD camera (Leica, Germany).
2.7 Caspase-3/7 detection
LoVo cells were treated with the essential oil of L. atriplicifolia for 48 h in 24-well plates. Cells were stained with Cell Event Caspase-3/7 Green Detection Reagent (Life Technologies, Waltham, MA, USA) according to manufacturer procedure for 30 min at 37 °C in the dark and images were captured using fluorescence microscope equipped with a digital camera (EVOS, USA).
2.8 Statistical analysis
We used two-tailed Student’s t-test for comparison (GraphPad). The results were expressed as mean ± standard deviation. p < 0.05 was considered statistically significant when compared to control.
3 Results
3.1 Essential oil composition
In this study, hydro-distillation of L. atriplicifolia aerial parts grown in Saudi Arabia afforded light yellowish oils with a pleasant fragrance. GC–MS analysis of the oils identified 36 different chemical constituents, which represented 92.3% of the total oils from L. atriplicifolia aerial parts. The identified chemical components are reported in Table 1 relating to their order of elution on HP-5MS non-polar column along with the percentage composition of each components and its retention index (RI). Table 2 brings together the various chemical classes in the essential oils of L. atriplicifolia.
Compound
RI
Relative amount (%)
Identification
1
5,5-dimethyl-2(5H)-Furanone
952
1.06
RI, MS
2
2,4-Heptadienal
1009
0.04
RI, MS
3
2-Acetyl-5-methylfuran
1037
0.12
RI, MS
4
5-Ethenyldihydro-5 methyl-2(3H)-Furanone
1041
1.56
RI, MS
5
Artemisia ketone
1064
0.11
RI MS
6
Trans-Linalool oxide (furanoid)
1076
4.56
RI MS
7
Linalool
1100
5.62
RI MS
8
3,7-Dimethyl-1,5,7-Octatrien-3-ol-hotrienol
1106
0.37
RI MS
9
Camphor
1145
5.60
RI MS
10
neroloxide
1151
3.50
RI MS
11
Ethyl benzoate
1170
0.10
RI MS
12
Epoxy linalool
1183
3.83
RIMS
13
Butanoic acid 3-hexenyl ester
1186
0.15
RI MS
14
α-Terpineol
1187
0.42
RI MS
15
Myrtanal
1197
0.23
RI MS
16
Verbenone
1205
2.52
RI MS
17
Pinocarvyl acetate
1287
0.35
RI MS
18
p-mentha-1,8-dien-7-ol
1302
0.37
RI MS
19
4-Acetylanisole
1345
2.15
RI MS
20
β-Damascenone
1388
0.10
RI MS
21
δ-Nonalactone
1404
0.35
RI MS
22
β-Caryophyllene
1417
1.91
RI MS
23
δ-Decalactone
1495
0.67
RIMS
24
C-10 massoia lactone
1501
46.45
RI MS
25
γ-Undecalactone
1573
1.20
RI MS
26
Caryophyllene oxide
1589
3.27
RI MS
27
Humulene epoxide
1606
0.26
RI MS
28
Methyl jasmonate
1644
0.20
RI MS
29
Τ-Cadinol
1679
0.26
RI MS
30
Phytane
1809
0.06
RI MS
31
Hexanoic acid, undec-2-enyl ester
1858
0.41
RI MS
32
Hexadecanoic acid, methyl ester
1928
0.22
RI MS
33
Palmitic acid
1957
0.26
RI MS
34
Oleic acid methyl ester
2103
0.44
RI MS
35
Methyl erucate
2473
0.23
RI MS
36
Squalene
2847
3.50
RI MS
Constituents
Aerial part
Terpenoids
–
Monoterpenes hydrocarbon
–
Sesquiterpenes hydrocarbon
1.42
Oxygenated monoterpenes
28.43
Oxygenated sesquiterpenes
4.03
Lactones
47.47
Furans
2.74
Acids
0.26
Esters
2.10
Hydrocarbons
3.56
Others
2.29
Total
92.30
The major compound identified from the aerial part was massoia lactone (46.65%) (Fig. 1). Five additional lactones were also detected at low yields, δ-nonalactone (0.35%), δ-decalactone (0.67%), γ-undecalactone (1.2%), 5-ethenyldihydro-5 methyl-2(3H)-furanone (1.56%), and 5,5-dimethyl-2(5H)-furanone (1.06%). The EOAPLA is moderately rich in oxygenated monoterpenes (28.43%), including linalool (5.62%), camphor (5.60%), trans-linalool oxide (4.56%), epoxy linalool (3.83%), neroloxide (3.5%), and verbenone (2.95%). Additionally, sesquiterpene hydrocarbons and oxygenated sesquiterpenes were identified at low percentages.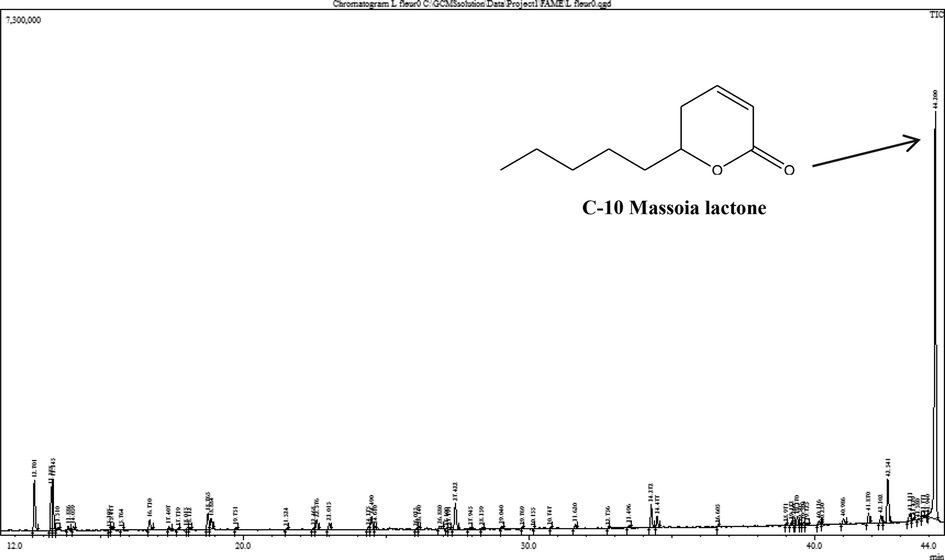
GC–MS chromatogram of essential oil composition of L. atriplicifolia.
3.2 Cytotoxic activity
The MTT assay was used to evaluate the potential cytotoxicity of EOAPLA against two different cancer cell lines. The cytotoxicity was recorded as LC50 (μg/mL), which represents the concentration that kills or inhibits 50% of the cells (Fig. 2). The data revealed that EOAPLA had the potential to inhibit the growth of LoVo and HepG2 cells in a dose-dependent manner. In comparison, the percentage survival of LoVo cells treated with EOAPLA was revealed to be lower than that of HepG2 cells. The LC50 values of the EOAPLA in LoVo and HepG2 cells were 7 μg/mL and 10 μg/mL, respectively. Furthermore, we assessed the cytotoxic effect of EOAPLA on LoVo cells using the LDH release assay. Again, 10 μg/mL EOAPLA resulted in a significant damage to LoVo cells in comparison with the control after 48 h of treatment (Fig. 3). Moreover, after 2 days of treatment, LoVo cancer cells were detached from the surface of the plate and showed morphological alterations under an inverted microscope, in particular cytoplasmic condensation, shrinkage, and debris formation (Fig. 4). However, the control cells maintained their original morphological characteristics and remained attached to the surface of the plate. As shown in Fig. 5, cells incubated with the caspase-3/7 detection reagent for apoptosis detection showed bright fluorescent nuclei in the LoVo cells treated with IC50 of the essential oil of L. atriplicifolia. In contrast, the control group showed very low fluorescence signal as caspase-3/7 was not activated.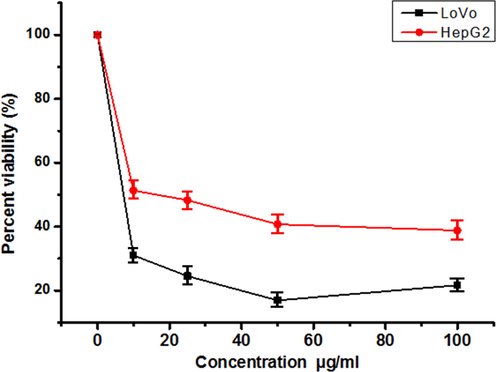
Cytotoxicity of EOAPLA on LoVo and HepG2 cancer cell lines. Values are means of three experiments. Mean ± S.D values of three independent experiments. * < 0.05 indicates significance when compared to the control).
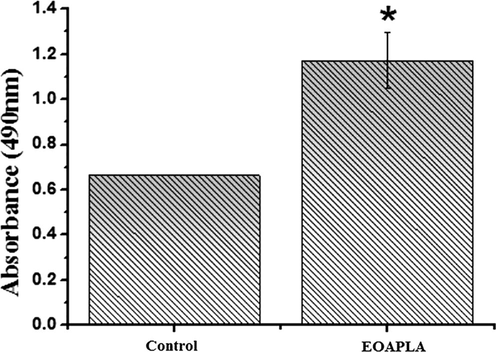
Lactate Dehydrogenase release from LoVo cells treated with EOAPLA after 48 h of treatment. The number of cells used for treatment was 5 × 104. * means significant. Mean ± S.D values of three independent experiments. * < 0.05 indicates significance when compared to the control.
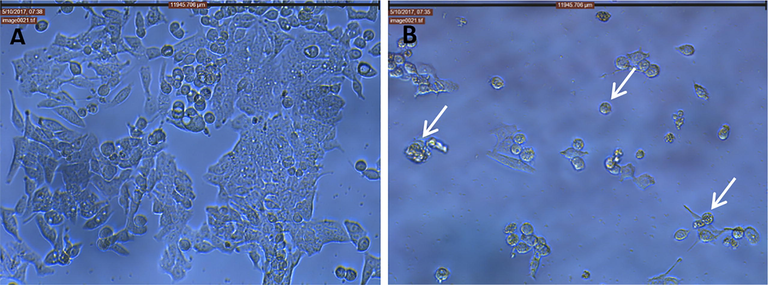
Effect of EOAPLA on LoVo cancer cells when observed at 20× using phase contrast microscope. (A) Control (Methanol) cells; (B) treated cells (10 µg/mL). We can see the rounding and detachment of cells and cytoplasmic shrinkage (white arrows).
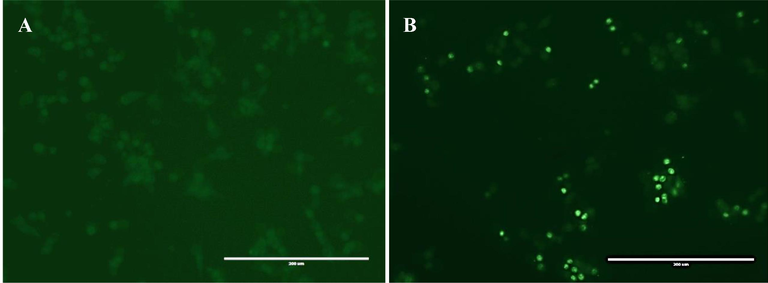
Detection of caspase 3/7 activity by fluorescence microscopy in LoVo cancer cells after treatment with the essential oil of L. atriplicifolia. A: control group B: Treated group.
4 Discussion
It has been noted many studies on the properties and characteristics of essential oils of the economic important lavender species, including L. angustifolia, L. intermedia, and L. latifolia, are highly species-specific (Lesage-Meessen et al., 2015). Indeed, terpenes are the largest class of volatile compounds found in the essential oils from lavender species (Lesage-Meessen et al., 2015; Bakkali et al., 2008) and monoterpenes represent the most abundant component of lavender essential oil (Chrysargyris et al., 2016). The most abundant compound identified in EOAPLA was massoia lactone (46.65%), which reflects the importance of the studied species L. atriplicifolia as an interesting source of lactone production with a relatively good yield in comparison to the other known lavender species. Lactones are essential oil components that are rarely found in the plant kingdom (Knudsen et al., 2006; Barros et al., 2014). In nature, massoia lactone was identified by Abe (1937) for the first time in the essential oil of the bark of Cryptocarya massoy and subsequently found in other plants at low yields, such as Polianthes tuberosa, Achillea fragrantissima, and Cyathea cunninghamii (Cavill et al., 1968; Rali et al., 2007). Lavender lactone is attributable for the floral scents of Lavandula vera (Timmer et al., 1975), whereas δ-decalactone is attributable for the fruity odors of several Prunus species (Tamura et al., 2005).
It is interesting to note that the variability in the chemical composition of terpenoid compounds of many essential oils results from many factors, such as environmental conditions (climatic and geographical) and genetics of the plant (Zheljazkov et al., 2013). Šoskic and co-workers compared the chemical composition of lavender aerial part essential oils collected from different areas in Serbia and Montenegro (Budva, Rovinj, and Podgorica) and found that oxygenated monoterpenes were the most abundant compounds in the different groups studied (Soskic et al., 2016). Moreover, they noted a significant difference in oxygenated monoterpene content between the samples from Budva (71.74%), Rovinj (91.79%), and Podgorica (87.63%). However, our chemical analysis of L. atriplicifolia revealed that oxygenated monoterpenes represented the second most abundant compound (28.43%). The natural terpenes found in lavender oil may have protective effects against a number of pathologies (Aquilano et al., 2008), including lipid peroxidation and dementia (Yang et al., 2010; Hancianu et al., 2013), anti-inflammatory activity (Peana et al., 2002), and anti-apoptotic activity (Hancianu et al., 2013).
At the pharmaceutical level, massoia lactone has been demonstrated to play an important role in the regulation of bacterial virulence genes (Smith et al., 2002) and exhibits antimicrobial actions against bacterial species, especially Candida albicans, Pseudomonas aeruginosa, and Staphylococcus aureus (Hertiani et al., 2016). It also induces concentration-dependent inflammatory activity, exerting an anti-inflammatory effect at a low concentration and proinflammatory action at a high concentration (Cooley et al., 2008). In the present study, the leakage of LDH into the culture medium was an indication of cell death that resulted from irreversible damage to the cell membrane or lysis (Korzeniewski and Callewaert, 1983). Moreover, EOAPLA treatment induced apoptosis in LoVo cells since it increased the expression of caspase 3/7 as shown in Fig. 5. Thus, EOAPLA, decreased the viability and growth of HepG2 and LoVo cells in a dose-dependent manner at a low concentration. This result was in agreement with those reported in previous studies in which lactones inhibited cell proliferation and function (Chhabra et al., 2003; Ritchie et al., 2003). Thus, the cytotoxic and anti-proliferative properties of EOAPLA make it a good candidate for treatment of various cancers.
5 Conclusions
The present study demonstrated the cytoplasmic condensation and shrinkage of LoVo cancer cells after 2 days of treatment with EOAPLA, which suggested the induction of apoptotic cell death. We believe that massoia lactone was the major component responsible for the induction of apoptosis in the cancer cells tested.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group RG-164.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification of essential oils components by gas chromatography/ quadrupole mass spectroscopy. Allured Publishing Corporation, Illinois. J. Am. Soc. Mass Spectrom.. 2017;16(11):1902-1903.
- [Google Scholar]
- Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res.. 2008;33:2416-2426.
- [Google Scholar]
- HPLC/DAD analysis of phenolic compounds from lavender and its application to quality control. J. Liq. Chromatogr. Relat. Technol.. 2000;23(16):2563-2572.
- [Google Scholar]
- Biological effects of essential oils: a review. Food Chem. Toxicol.. 2008;46:446-475.
- [Google Scholar]
- Synthesis and evaluation of Massoialactone and analogues as potential anticancer and anti-inflammatory agents. Eur. J. Med. Chem.. 2014;76:291-300.
- [Google Scholar]
- Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol.. 2015;45:336-354.
- [Google Scholar]
- Insect venoms attractants and repellents Massoilactone from 2 species of formicine ants and some observations on constituents of bark oil of Cryptocarya massoia. Aust. J. Chem.. 1968;21:2819-2823.
- [Google Scholar]
- Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr. Polym.. 2012;87:1963-1970.
- [Google Scholar]
- Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.) Ind. Crops Prod.. 2016;83:577-586.
- [Google Scholar]
- Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-L-homoserine lactone as immune modulators. J. Med. Chem.. 2003;46:97-104.
- [Google Scholar]
- N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem. Biol.. 2008;15:1141-1147.
- [Google Scholar]
- Lavandula angustifolia Miller: English Lavender. Holist. Nurs. Pract.. 2009;23(1):57-64.
- [Google Scholar]
- Antioxidant and antibacterial effects of Lavandula and Mentha essential oils in minced beef inoculated with E. coli O157:H7 and S. aureus during storage atabuse refrigeration temperature. Meat Sci.. 2012;2:667-674.
- [Google Scholar]
- Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine. 2013;20:446-452.
- [Google Scholar]
- Potency of massoia bark in combating immunosuppressed-related infection. Pharmacogn. Mag.. 2016;12:363-370.
- [Google Scholar]
- An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods. 1983;64:313-320.
- [Google Scholar]
- Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Appl. Microbiol. Biot.. 2015;99:3375-3385.
- [Google Scholar]
- Chemical composition antimicrobial and cytotoxic properties of five Lamiaceae essential oils. Ind. Crop. Prod.. 2014;61:225-232.
- [Google Scholar]
- Chemical composition and antifungal activity of lavender (Lavandula stoechas) oil. Nat. Prod. Commun.. 2018;13:895-898.
- [Google Scholar]
- Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721-726.
- [Google Scholar]
- Structure and absolute configuration of new diterpenes from Lavandula multifida. J. Nat. Prod.. 2002;65:1742-1745.
- [Google Scholar]
- Comparative chemical analysis of the essential oil constituents in the bark, heatwood and fruits of Cryptocarya massoy (Oken) Kosterm. (Lauraceae) from Papua New Guinea. Moelcules. 2007;12:149-154.
- [Google Scholar]
- Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Infect. Immun.. 2003;71:4421-4431.
- [Google Scholar]
- Anti-inflammatory constituents of topically applied crude drugs. IV. Constituents and anti-inflammatory effect of Paraguayan crude drug “Alhucema” (Lavandula latifolia Vill.) Chem. Pharm. Bull.. 1990;38:2283-2284.
- [Google Scholar]
- The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol.. 2002;184:1132-1139.
- [Google Scholar]
- Comparative Chemical analysis of essential oils from Lavende Lavender of different geographic origin. Stud. U. Babes Bol. Che.. 2016;61:127-136.
- [Google Scholar]
- Authenticity assessment of γ- and δ-decalactone from Prunus fruits by gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (GC-C/P-IRMS) J. Agr. Food Chem.. 2005;53:5397-5401.
- [Google Scholar]
- Analysis of the lactone fraction of lavender oil (Lavandula vera D.C.) J. Agr. Food Chem.. 1975;23:53-56.
- [Google Scholar]
- Triterpenoids of the roots of Lavandula stoechas ssp. Stoechas. Die Pharm.. 2001;56(11):892-895.
- [Google Scholar]
- Longipinene derivatives from Lavandula stoechas subsp. stoechas. Phytochemistry. 1988;27:3966-3967.
- [Google Scholar]
- Leaf flavonoids as systematic characters in the genera Lavandula an Sabaudia. Biochem. Syst. Ecol.. 2000;28(10):991-1007.
- [Google Scholar]
- Lavandula essential oils: a current review of applications in medicinal, food, and cosmetic industries of Lavender. Nat. Prod. Commun.. 2018;13:1403-1417.
- [Google Scholar]
- Bioactivity of Backhousia citriodora: antibacterial and antifungal activity. J. Agric. Food. Chem.. 2003;51:76-81.
- [Google Scholar]
- Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res.. 2010;24:140-151.
- [Google Scholar]
- Distillation time effect on lavender essential oil yield and composition. J. Oleo. Sci.. 2013;62:195-199.
- [Google Scholar]







