Translate this page into:
An overview of antimicrobial and anticancer potential of silver nanoparticles
⁎Corresponding author. sunithasalla@gmail.com (S. Sunitha), djchoi@hongik.ac.kr (Dongjin Choi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nanotechnology has immense potential for the development of novel and necessary products that benefit human health, the environment, and industries. Silver nanoparticles and their nanocomposites have gained tremendous importance in the field of nanotechnology owing to their widespread usage in various realms of science and technology, including electronics, biomedical, environmental protection, textile industry, cosmetics, therapeutics, photonics, agriculture, and so on. Additionally, the market demand for nano silver-based products and high therapeutic usage of silver nanostructures has diverted the focus of researchers towards the exploration and novel use of silver-based nanoparticles for advanced nano based pharmaceuticals, high-tech devices, biosensors and other industrial products. As the characteristic properties of silver nanostructures, like excellent SERS/SPR, surface properties, diversity in shape, surface charge, dissolution rate, controlled silver ion release for mediating the antimicrobial toxicity and cytotoxicity towards cancer cells, and efficient biocompatibility, which render them to be potential antimicrobial, anticancer and diagnostic agents. Here in this review, due attention has been given to highlight the antimicrobial and anticancer properties of silver nanoparticles along with their mechanistic pathways. Finally, some of the miscellaneous applications of silver nanoparticles are briefly mentioned which emphasize the understanding of silver nanoparticles' ubiquitous role in consumer and health care products, biomedical devices, implants, environmental remediation, and so on.
Keywords
Silver nanoparticles
Antimicrobial
Anticancer
Invitro
Mechanism
1 Introduction
Nanotechnology has played an immensely important role in biomedical, diagnostics, therapeutics, industrial sector, scientific research probes, and environmental protection in the last few decades (Farokhzad and Langer, 2009; Guerra et al., 2018). A vast array of nanomaterials of different morphologies (Fig. 1) have been synthesized by employing different synthetic methods and procedures (Iravani et al., 2014; Aygün et al., 2020). Nanomaterials range in size between 1 nm and 100 nm or particles having at least one dimension below the 100 nm size range (Farokhzad and Langer, 2009).
Shapes of AgNPs.
There are various types of metal nanoparticles, which include nanoparticles of iron, gold, silver, titanium, cerium, platinum, thallium, and so on (Piñón-Segundo et al., 2013). Silver nanoparticles are one of the extensively explored metal nanoparticles for diverse scientific purposes because of their characteristic physicochemical and biological properties, viz, large surface area to volume ratio, excellent surface plasmon resonance, ease of functionalization or conjugation with different types of ligands to get desired tailored properties, toxicity against pathogens, efficient cytotoxicity towards cancer cells, catalytic applications and so on (Jain et al., 2008; Akter et al., 2018). Interestingly, matter in the nanometer size range exhibits some unique physical, chemical, and biological properties that are completely different as compared to the matter in its bulk state and are greatly useful in scientific or research scenarios. (Gatoo et al., 2014). Silver nanoparticles are notable among the noble metal nanoparticles and nanocomposites because of their tremendous potential and significant applications (Fig. 2) in the textile and food industries, water purification plants, environmental pollution protection, biomedical/therapeutics (anticancer, antimicrobial, antiangiogenic, contrast agents in imaging techniques for the diagnosis of diseases, biomedical devices, diagnostic probes in biological systems for the detection of several dreadful diseases or complications, conjugation with drugs to overcome the resistance or hindrances facing to their efficient delivery and also to increase the potency and therapeutic index of drugs) (Barkalina et al., 2014; Butola and Mohammad et al., 2016; Yetisen et al., 2016; Deshmukh et al., 2019).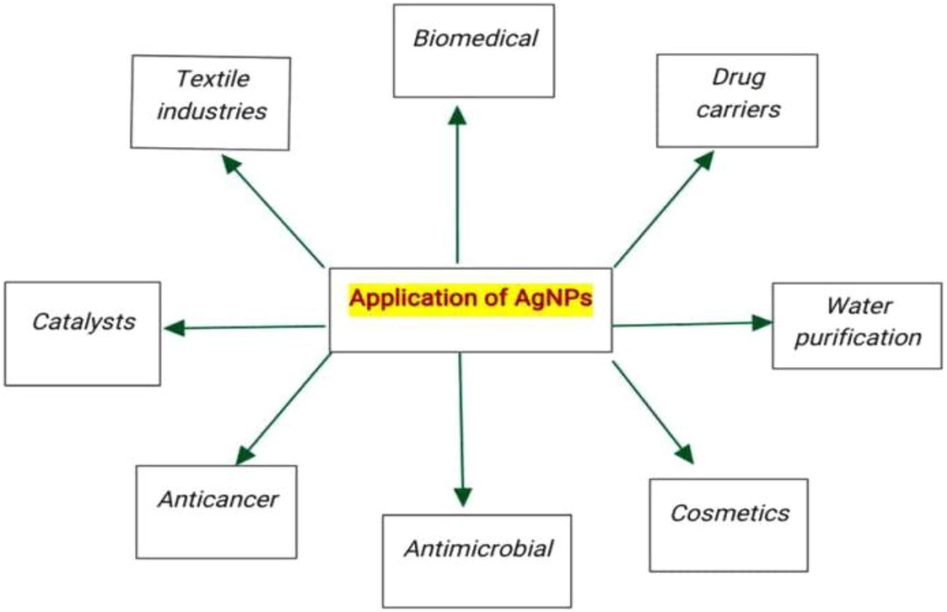
Application of AgNPs.
In this review, an attempt has been made to highlight some of the essential, highly significant in-vitro antimicrobial and anticancer activities of silver nanostructures, and their miscellaneous applications so that the readers can understand the importance and significance of silver nanomaterials and their potential in novel applications of science or nanotechnology. The reading of this review will enhance the fervor for the development of more antimicrobial formulations and also boost the research focus for opening up new strategies in the treatment of cancer, spreading of microbial diseases, and other novel applications in biomedical and other sciences.
2 Synthesis of nanoparticles
There are different synthetic approaches, protocols, or procedures for the preparation of silver nanoparticles. Broadly, the procedures can be categorized into two, viz, top-down and bottom-up approach (Fig. 3). The top-down approach is the mechanical transformation of bulk metal into nanostructures using suitable stabilizing agents. Contrary to this, the bottom-up approach is the transformation or nucleation and subsequent stabilization of matter at the atomic scale into nanostructures (Richards and Bönnemann 2005). Usually and probably all the bottom-up approaches share some common features, i.e., reduction of metal ions obtained from metal precursors by using suitable reducing agents, capping, or stabilizing agents in the preparation and giving the stability to the nanoparticles or nanostructures.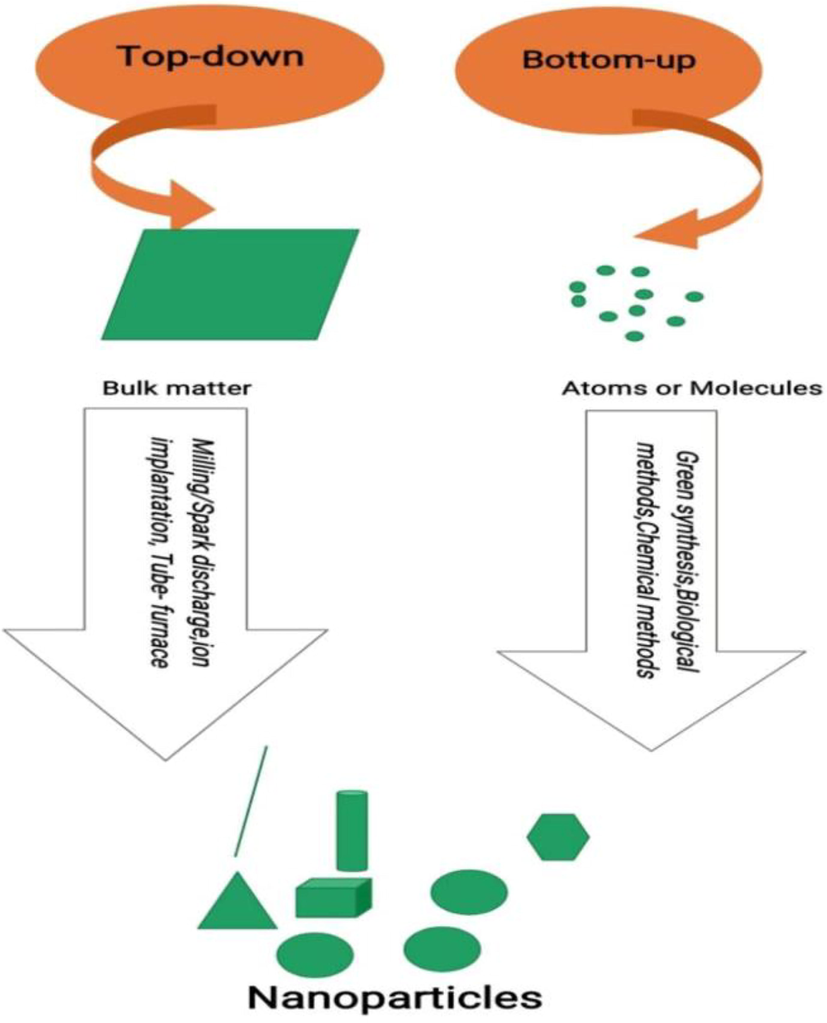
Top-down and Bottom-up approach.
As is mentioned, that silver nanoparticles can be synthesized by three different methods like physical, chemical and biological (Fig. 4) (Iravani et al., 2014). The various physical methods include spark discharging, pyrolysis, tube furnace (evaporation–condensation), laser ablation, gamma-irradiation, microwave processing, and so on. These methods involve certain demerits or downsides, like high energy consumption, low yield, and non-uniform distribution of nanoparticles, but have the positive side of non-use of environmentally hazardous chemicals. Chemical methods for synthesizing silver nanomaterials include Sol-Gel method, Co-precipitation, Pyrolysis, Sono-chemical, Electro-chemical methods, etc. These methods are easy, inexpensive, and efficiently productive as compared to the physical methods. But have disadvantages of the use of harmful and toxic chemicals, which render these methods to be less biocompatible and environmentally toxic. All the drawbacks of physical and chemical methods are being overcome or replaced by the most widely adopted or used method by researchers, that is the biological method, which is the most biocompatible of all the methods. This method of nanoparticle synthesis employs biological systems like plants or plant parts, bacteria, fungi, algae, animal tissues or cells, proteins, nucleic acids, vitamins, and so on, in the synthesis of nanomaterials. This approach of synthesizing nanostructures is eco-friendly, biocompatible, easy, and high-yielding.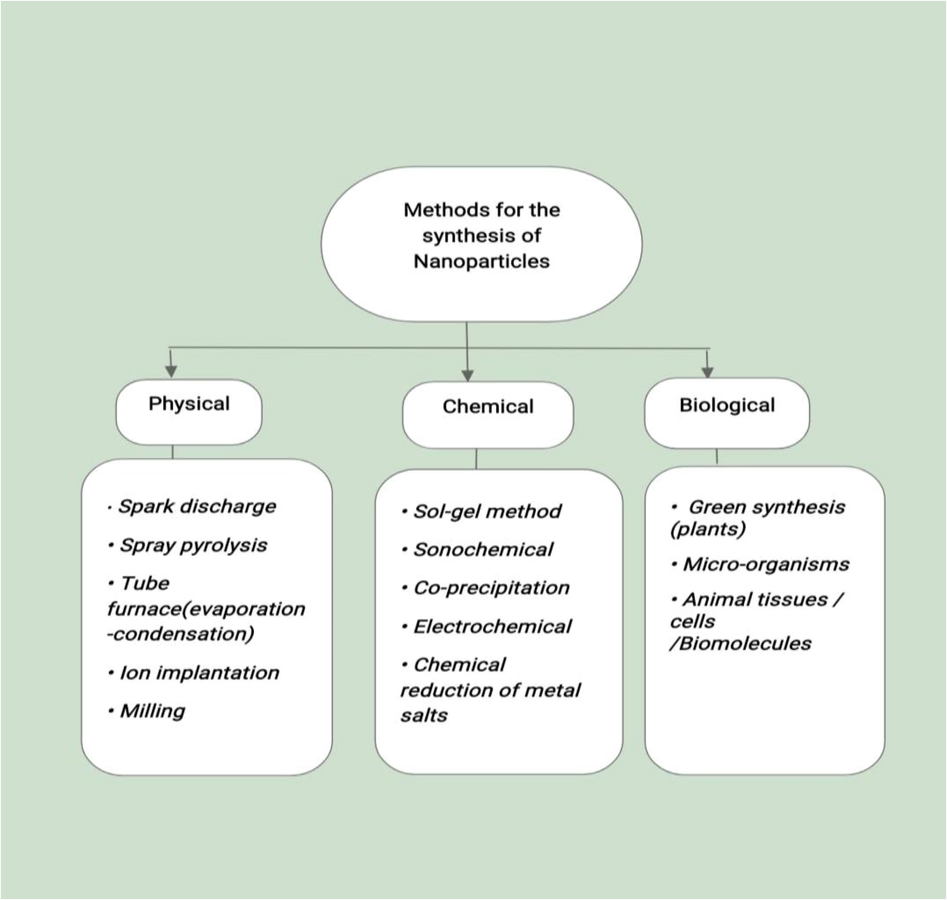
Various methods for synthesizing nanoparticles.
Yet another approach to the biological one is the green synthetic approach, which includes synthesizing nanoparticles solely from plant extracts (Chavan et al., 2021). Nowadays, special emphasis is given to synthesize nanoparticles by adopting a green synthetic approach owing to the eco-friendliness, productivity, economically cheap, also availability of huge diversity of plants, and presence of abundant bioactive phytocompounds in plant extracts (Iravani et al., 2014; Argade et al., 2019). It is important to mention here that plants are huge repositories of naturally bioactive phytocompounds, which not only help in the synthesis of nanoparticles but also offer versatile properties like desired functionalization, long term stability, biodistribution, nanolithography to accomplish the broad spectrum of bioactivities (Shejawal et al., 2020; Ansar et al., 2020). It has been also found that temperature, pressure, pH, type of reducing agent, and precursor agents have a significant impact on controlling the shape, size, surface area to volume ratio, size distribution, morphology, and bioactivity of synthesized silver nanomaterials. (Voronov et al., 2008; Alqadi et al., 2014; Wongpreecha et al., 2018).
3 Antimicrobial activity of silver nanoparticles
Silver nanoparticles are well-known to have antimicrobial properties (Marambio-Jones and Hoek 2010; Zheng et al., 2018) and luckily seem to be potent and efficient antimicrobial agents as compared to other noble metal nanomaterials, owing to their characteristic properties like large surface to volume ratio (Morones et al., 2005), toxicity (Morones et al., 2005; Navarro et al., 2008; Kruszewski et al., 2011; Beer et al., 2012), possible interaction with the sulfur and phosphorus compounds present in the cells (Morones et al., 2005; Yin et al., 2013), crystallographic characteristics, and so on, which enable them to be useful agents for treating various microbial infectious diseases and also can serve as useful agents to overcome the microbial resistance against the conventional drugs, either used alone or in conjugation with therapeutic antimicrobial formulations (Morones et al., 2005; Khatoon et al., 2019). Silver nanoparticles have also been researched and proved to be efficient antifungal and antiviral agents.
Silver has a long history of being used as an antimicrobial, and its nanoforms are far better and more biocompatible antimicrobial agents. In the context of this section, an ample amount of research work is carried out right from the beginning of the period of silver consumption in nanotechnology. A lot of invivo and invitro research has been done so far with relevance to the antimicrobial potential of nanosilver or its nanoconjugates, which can assist in our understanding about the importance and potential role of silver nanoparticles in the future while tackling microbial diseases by counteracting the loopholes of conventional antimicrobial formulations, combating drug-resistant pathogens and simultaneously setting the foothold of the combinatorial approach in drug action.
Silver nanoparticles are easily synthesized from plant extracts with extreme stability and eco-friendliness and exhibit wide range of antimicrobial properties because of the additive action of nanosilver and broad range of phytoconstituents with inherent antimicrobial properties.
A study conducted by Loo et al., reports that silver nanoparticles (size: 4.06 nm) synthesized from pu-erh tea leaves have been found to have strong antimicrobial activity against gram-ve foodborne pathogens like Salmonella enteritidis, Klebsiella pneumonia, Escherichia coli, and Salmonella typhimurium and MICs reported were 3.9, 3.9, 7.8, and 3.9 μg/ml respectively (Loo et al., 2018). Similarly, Garibo et al., reported synthesis of silver nanoparticles (shape: quasi-spherical and spherical; size: 5 nm) by using an aqueous extract of the perennial tree Lysiloma acapulcensis and found them to be potent antimicrobial agents (Garibo et al. 2020). In an important study, Selim et al. investigated the antimicrobial activity of chemically synthesized silver nanoparticles of an average size of 50 nm on two reference strains (M. tuberculosis and M. bovis), the MDR Mycobacterium tuberculosis strain, and clinical isolates (M. tuberculosis and M. bovis). The synthesized nanoparticles significantly inhibited all the tested samples. Reports of MIC values in case of reference strains M. bovis and M. tuberculosis were found to be 4 μg/ml and 1 μg/ml respectively. In case of the MDR strain of M. tuberculosis, the MIC value obtained was 16 mcg/ml. However, 1–16 μg/ml and 4–32 μg/ml MIC values were reported against clinical isolates of M. tuberculosis and M. bovis respectively. This study suggests that silver nanoformulations can act as potential antitubercular agents (Selim et al., 2018). According to Singh et al., the antibacterial activity of bacteriogenic silver nanoparticles against Acinetobacter baumannii, a harmful nosocomial bacteria associated with hospital-acquired infections with high mortality, that synthesized silver nanoparticles inhibited bacterial growth with a MIC value of 16 μg/ml, which is much lower than ampicillin (4096 μg/ml), amoxicillin (2048 μg/ml), and erythromycin (64 μg/ml) against the selected pathogen (Singh et al., 2018). This study suggests the higher potential and efficacy of AgNPs compared to conventional antibiotics in the treatment of hospital-acquired infections.
Furthermore, silver nanoparticles are conjugated or loaded with antibiotics or antimicrobials for synergistic and enhanced antimicrobial effects, which can lead to the development of efficient, potent, highly biocompatible, broad-spectrum, and least resistant antimicrobial formulations. One of the studies conducted by Bonde et al. has reported strong antimicrobial activity of AgNPs (size 40–80 nm) synthesized from Murraya koenigii against human pathogens like Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The synergistic enhanced antibacterial activity of silver nanoparticles loaded with tetracycline and gentamycin was observed (Bonde et al., 2012).
It is often a practiced method to isolate specific bioactive compounds instead of whole complex crude extracts, to play a major role in nano synthesis and bioactivity with high efficiency, precision, and purity rather than in their crude forms. For example, isolated flucoidan, a phytocompound, from Spatoglossum asperum has been used for the synthesis of AgNPs (size 20–46 nm) showed good results against K. pneumonia (with a zone of inhibition: 18 ± 0.28 mm), comparatively higher than crude fluocidan (zone of inhibition: 12 ± 0.36 mm) (Ravichandran et al., 2018). Similarly, another research work reported that silver nanoparticles synthesized from Euphorbia antiquorum L. have been found to have efficient larvicidal and antimicrobial properties (Rajkuberan et al., 2017). Silver nanoparticles are more toxic and highly bioactive than metallic silver. One such study reported by Ramya and Subapriya revealed that AgNPs are more toxic against commonly found fungi like Aspergillus, Saccharomyces, and Candida than free silver (Ramya and Subapriya, 2012). Additionally, silver nanoparticles are functionalized by coating them with suitable polymers for enhanced therapeutic actions. Elechiguerra et al. found that PVP-coated AgNPs within the 1–10 nm range efficiently inhibit the replication of HIV in a dose-dependent manner (Elechiguerra et al., 2005). Silver nanoparticles have proven in-vitro antiviral activity against different types of deadly viruses, which highlights the potential of silver-based nanomedicines in the treatment of dreadful viral diseases. In such a study, the evaluation of the antiviral activity of AgNPs with or without a polysaccharide coating against the Monkeypox virus showed that AgNPs of around 10 nm in size inhibited the virus effectively (Rogers et al., 2008). A similar type of research work, carried out by Lara et al., revealed that silver nanoparticles attach to gp120 (glycoprotein spikes on the HIV surface) in a way that inhibits the attachment, fusion, and infectivity of CD4-dependent virions, serving as an important antiviral agent against cell-associated and cell-free viruses. Silver nanoparticles also inhibit the post-entry stages of the HIV-1 life cycle (Lara et al. 2010). Even though silver nanoparticles are tremendously exploited for invitro antimicrobial studies, there is still an insufficiency of data regarding invivo research and systemic toxicity assessment in humans.
Surface engineering and functionalization of silver nanoparticles with suitable molecular moieties is the most attractive and fascinating part of silver nanotechnology. It associates or imparts the desired properties, like structural, topographical, binding capacity, synergistic drug efficacy, solubility and distribution of conjugated drugs, and other pharmacokinetic parameters of nanomedicines that enhance the exploration of the novel applications of silver nanoparticles in medicine. Conjugants often used with AgNPs are drugs, bio and inorganic moieties, antibodies, therapeutic proteins, nucleic acids (RNA and DNA), and bioactive therapeutic compounds isolated from plants, animal bodies, bacteria, and fungi.
As such silver nanoparticles are functionalized with different groups to form the desired nanostructures like graphene oxide-silver nanocomposite (de Moraes et al., 2015; Ikhsan et al., 2015; Choi et al., 2018), nAg–psf (Zodrow et al., 2009; Verma and Maheshwari 2019), PVP coated silver nanoparticles, etc (El Hotaby et al., 2017), to have enhanced therapeutic efficiencies or other applications. There is a long list of conjugating agents with silver nanoparticles which include drugs: anticancer (Patra et al., 2015; Benyettou et al., 2015), antibacterial (Shanmuganathan et al., 2018; Masri et al., 2018; Kaur and Kumar, 2019); polymers: PEG (Zhao et al., 2019; Cho et al., 2012), PVP (Gélvez et al., 2018; Zhao et al., 2019) PEI (Li et al., 2016), cellulose (Lin et al., 2015), beta cyclodextrins (Xie et al., 2010; Zhai et al., 2017); amino acids (Kumar et al., 2018); proteins (Tai et al., 2014; Joshi and Soni, 2014); fatty acids (Anwar et al., 2019; Rajendran et al., 2019); glucosamine (Veerapandian and Yun, 2010; Veerapandian et al., 2010); 4-aminobenzenethyol (Patel et al., 2015); 4-mercaptopyridine (Li et al., 2012); polyhexamethylene biguanide (PHMB) and so on (Ashraf et al., 2012). These conjugating agents enable silver nanoparticles to have a broad spectrum of applications in therapeutics and biomedical science.
3.1 Mechanism of antimicrobial activity of silver nanoparticles
The mechanism behind the antimicrobial activity of silver nanoparticles is still a topic of deep discussion. Silver nanoparticles are reported to cause structural and physiological changes in microbial cell membranes, such as accumulation and changes in permeability and membrane potential, as well as inhibition of respiratory proteins attached to the membrane, disrupting the cell's homeostasis and ultimately leading to microbial cell death. It is also suggested that a positive charge of Ag ions is essential for antimicrobial properties (Rai et al., 2009; Abbaszadegan et al., 2015). The antimicrobial action of AgNPs is greatly influenced by several parameters, including microbe type, temperature, pH, and AgNO3 concentration (Marambio-Jones and Hoek, 2010). The concentration of Ag+ has an inverse relationship with it. This is due to the fact that smaller particles have a bigger surface area for contact, resulting in greater bactericidal effects than bigger particles having less surface area (Chanda, 2013). It is revealed that the Ag+ ions released from AgNPs mediate the toxicity to the bacterial cells as Ag+ ions penetrate the bacterial cells and get oxidized to Ag2O (Hsueh et al; 2015). Also, Ag+ ions released from internalized AgNPs bind to the sulphydryl groups of proteins and lead to their denaturation. It has been reported that AgNPs attach to the bacterial respiratory chain and disrupt the essential physiological processes that eventually lead to cell death (Menu et al., 2012). Silver nanoparticles have been well-documented to generate reactive oxygen species like hydroxyl and superoxide radicals that induce oxidative stress in the cell, leading to the serious damage or death of the microbial cell (Lok et al., 2006; Kim et al., 2011; Rautela et al., 2019).
4 Anticancer properties of silver nanoparticles
Cancer is a disease of international concern that is a major cause of worldwide human deaths. According to the WHO report, 9.6 million deaths occurred globally in 2018 due to this deadly disease (World Health Organization, 12 Sep 2018; Bray et al., 2018). Any type of tissue or cell in the body is at a risk of abnormal proliferation and that is the reason for the diversity in cancer. The type of cancer varies according to the type of cell, tissue, or organ involved in the disease. Furthermore, the causes of cancer are rapidly increasing, while treatment strategies have simultaneously been expanded and modified into advanced means of combating this dreadful disease. Treatment strategies for various types of cancers include radiation therapy (Balaji et al., 2016; Baskar and Itahana 2017; Deng et al., 2018), surgeries (Rega et al., 2019), chemotherapy (Dickens and Ahmed, 2018), hormonal therapy (Fekete and Győrffy, 2019) immunotherapy (Fang et al., 2017; Thomas et al., 2018) bone marrow transplantation (Gahrton et al., 1991; Giebel et al., 2019), targeted therapy (Gerber 2008), cryoablation (Lee et al., 1994; Bahn et al., 2006), radiofrequency ablation (Brem, 2018; Rajyaguru et al., 2018), and so on. Cancer treatment aided by nanomaterials has emerged with effective therapeutic benefits in the last few decades, capturing the attention and enthusiasm of researchers to develop novel ways of nanotechnology to treat various types of cancers (Barreto et al., 2011; Nazir et al., 2014). Metal oxide nanomaterials (Martinez-Boubeta et al., 2013; Zhou et al., 2014; Vinardell and Mitjans, 2015) and metal nanoparticles (Conde et al., 2012; Sharma et al., 2015) have been particularly effective as anticancer agents. Conjugates of nanomaterials with chemotherapeutic anticancer drugs: doxorubicin (Kievit et al., 2011), fluorouracil (Ashwanikumar et al., 2014), methotrexate, (Wu et al., 2014), etc.; biopolymers (Carvalho et al., 2019); peptides (Yeh et al., 2016); nucleic acids (Guo et al., 2016); folic acid (Boca-Farcau et al., 2014) and so on, have created a synergistic approach to the treatment of cancer. Silver nanoparticles and nanocomposites are highly involved in the list of scientific research and literature to have anti-cancer properties (El-Naggar et al., 2017; Yuan et al., 2017).
In recently reported research work, Shejawal et al. synthesized silver nanoparticles with the help of 1% aqueous extract of the Carotenoid phytopigment “Lycopene”, isolated from tomato, extracted in benzene and observed their anticancer activity. The Lycopene AgNPs were tested on Hella, COLO320DM, H29 cancer cell lines, and it was reported through MTT assay the percent inhibition of 40.9 ± 0.69, 41.41 ± 0.41, and 35.43 ± 0.67 against Hella, COLO320DM and H29 cancer cell lines respectively (Shejawal et al., 2021). In another study conducted by Lin et al., it was found that silver nanoparticles act as anticancer agents by induction of autophagy of cancer cells through activation of the ptdlns3K pathway. Furthermore, they observed that inhibition of autophagy by autophagic inhibitor like wortmannin results in enhanced cancer cell killing efficacy in the mouse melanoma cell model (B16 cell lines) (Lin et al., 2014). Shejawal et al., in their research work, synthesized iron and silver nanoparticles ranging in size from 50 to 100 nm by a green synthesis method from a polyphenolic bioactive phytocompound “proanthocyanidin”, isolated from grape seed and successfully evaluated various biological activities of synthesized nanoparticles. They reported significant anticancer activity against different colon cancer cell lines (COLO320DM and H29) through SRB and MTT assays. According to the SRB assay, proanthocyanidin-AgNPs inhibited the growth of COLO320DM (inhibition: 71.61.97%) and H29 (inhibition: 69.211.86%) cell lines. The MTT assay reveals that proanthocyanidin-AgNPs showed 64.27 ± 1.63 and 63.34 ± 1.64 percent inhibition against COLO320DM and H29 cell lines respectively (Shejawal et al., 2020). In another research work carried out by Mittal et al., biosynthesized silver nanoparticles from plant extracts of Potentilla fulgens caused cytotoxicity in a dose-dependent manner in the U-87 and MCF-7 cell lines. The IC50 value was found to be 8.23 μg/ml and 4.91 μg/mL in U-87 and MCF-7 cell lines respectively (Mittal et al., 2015). Invitro evaluation of the anticancer and antioxidant potential of Morinda pubescens green mediated synthesized silver nanoparticles by Inbathamizh et al., revealed significant cytotoxicity to the HEP.G2 cell lines. The Gi50 value was found to be 93.75 μg/ml (Inbathamizh et al., 2013). Researchers carry out the desired conjugations with AgNPs to have enhanced cytotoxicity towards cancer cells. Preethi and Padma observed the enhanced anticancer activity of silver nano bioconjugates synthesized from the leaf extract of Piper betle and its active polyphenol compound “eugenol”. The cytotoxicity to oral “KB” cancer cell lines was found to be elevated by the application of nanoconjugates in comparison to non-conjugates or crude parts of the plant (Preethi and Padma 2016). Yuan et al., reported synergistic enhanced cytotoxicity and apoptosis of Hela cancer cells with combinatorial therapy of AgNPs and Camptothecin (CPT) (Yuan et al., 2018). Venkatesan et al. biosynthesized porous chitosan-alginate AgNPs and revealed that the nanocomposite has potential anticancer and antimicrobial properties. The nanocomposite was tested in-vitro on MD-MB-231 (breast cancer cells) and the IC50 value is reported to be 4.6 μg/ml (Venkatesan et al., 2017). Benyettou et al., successfully conjugated the anticancer drugs: Doxorubicin and Alendronate with AgNPs (carrier vehicle) and tested the drug-nano conjugate on HeLa cell lines. Interestingly, an enhanced anticancer potential was observed compared to Doxorubicin or Ald used alone. These findings prove that AgNPs could and do act as carriers for efficient transportation and delivery of anticancer chemotherapeutic drugs with increased therapeutic index and potency, thereby sparing the need for a high dose of chemotherapy to lessen the cytotoxic effects on normal healthy body cells (Benyettou et al., 2015). Buttacavoli et al., synthesized silver nanoparticles embedded in polysaccharide (EPS) from the bacteria Klebsiella oxytoca and observed the cytotoxicity to cancer cell lines (SKBR3, 8701-BC, Caco-2, HCT-116 and HT-29). They reported that bio-generated silver nanoparticles under aerobic conditions (AgNPs-EPSaer) are more efficacious in cytotoxicity to breast cancer (SKBR3, 8701-BC) than in colon cancer (Caco-2, HCT-116, HT-29) in terms of their MICs. MIC values for SKBR3, 8701-BC, HT-29, HCT-116, and Caco-2 were found to be 5 ± 0.5, 8.2 ± 0.8, 20 ± 2, 26 ± 2 and 34 ± 4 respectively (Buttacavoli et al., 2018). Gomathi et al., synthesized AgNPs by using the fruit shell of Tamarindus indica, and the resulting biosynthesized nanoparticles proved effective in a dose-dependent manner against the MCF-7 cell line (human breast cancer) (Gomathi et al., 2020). Rajkuberan et al. reported the biosynthesis of AgNPs from the latex of Euphorbia antiquorum L., which was successful in controlling the in-vitro growth of the human cervical carcinoma cell line (Hela). The IC50 value was found to be 28 μg/ml (Rajkuberan et al., 2017).
The above-mentioned research works (invitro analysis) revealed the potential of biosynthesized “especially green synthesis” silver nanoparticles with size-controlled, dose-dependent, and high therapeutic index anticancer activity on different cell lines. Additionally, combinatorial therapy (silver nanoparticles with conventional anticancer chemotherapeutic drugs) delivers a synergistic effect on cancer cells, which is a very essential need in chemotherapy while addressing the toxicity, potency, therapeutic index, biodistribution, dosage, and targeted delivery of anticancer drugs. Further, the green synthesis approach of nanoparticles adds a double benefit owing to the presence of a diversity of medicinal plants with known anticancer properties to be employed in the therapeutics of cancer.
Silver nanoparticles or composites have a dual role, that is, they act as theranostic agents. They absorb and scatter particular wavelengths of visible light, commonly known as surface Plasmon resonance (SPR), and also possess an enhanced SERS. These properties of silver nanomaterials enable them to be useful in imaging different body parts, which assists or facilitates the diagnosis of tumor location, size, stage of cancer, and angiogenesis process. In other words, they act as diagnostic probes and contrast agents for several imaging technologies (Soica et al., 2018).
4.1 Mechanism of anticancer action of silver nanomaterials
There are various mechanisms, as mentioned in the research works through which the anticancer properties of silver nanomaterials can be justified. As is evident, silver nanomaterials efficiently interact with cancer cells because cancer cells show the enhanced permeation and retention effect (EPR) that results in the entry and accumulation of more and more silver nanoparticles, leading to the death of cancer cells or hindering their uncontrolled division. Additionally, AgNPs act by downregulating and upregulating the signaling physiological pathways, resulting in early apoptosis or checking the rapid pace of the division of tumor cells. Further, some research works suggest the activation of p53, caspase-3, and p-Er K1/2 by silver nanomaterials eventually leads to apoptosis and regulates cell division through a series of events taking place in the cells (Gurunathan et al., 2015; Buttacavoli et al., 2018; Murali et al., 2018). Miriam Buttacavoli and coworkers investigated the mechanistic pathway of silver nanoparticles on the breast cancer cell line (SKBR3) by employing sophisticated techniques and procedures. Their findings give a conclusive insight into the mechanism of the anticancer potential of silver nanoparticles on human cancer cell lines (SKBR3). They reported significant inhibition of cell motility and inhibitory action on metalloproteinases (MMPs). Significant morphological changes in the cancer cells were observed by treating them with silver nanoparticles, like shrinkage, irregularity in shape, blebbing of cytoplasm, change in the shape of intracellular vacuoles, and chromatin condensation. In addition to these changes in cancer cells, the production of ROS causes oxidative stress and cell death. Further, they reported the up-regulation of LC3-II, ATG7, beclin-1, ATG5, and downregulation of HSP90, AKT, P62, p-AKT autophagic markers (Buttacavoli et al., 2018)
It is also evident from research work that cancer cells have an enhanced permeation and retention (EPR) effect (Torchilin, 2011), which leads to the intake of more and more nanosilver and generating more cytotoxic silver ions. Oxidative stress, in this way, generated in the tumor cells destroys the vital biomolecules, thereby disrupting the various physiological processes and molecular pathways and destruction of vital cellular organelles, biomembranes, and finally death of the tumor cells (Han et al., 2014; Al-Sheddi et al., 2018).
It is reported that vascular endothelial growth factor (VEGF) promotes angiogenesis, an important process in the progression and spread of tumors by forming new blood vessels (Carmeliet 2005). Reports reveal that silver nanoparticles affect the function of VEGF and hence act as an anti-angiogenic (Kalishwaralal et al., 2009).
Silver nanomaterials act as carrier vehicles for carrying the therapeutic anticancer payload (drugs), resulting in the increased efficiency and potency of anticancer drugs and countering anticancer drug resistance by cancer cells, and also facilitating targeted delivery of anticancer drugs to lessen the cytotoxic effects of chemotherapy on normal healthy tissues (Patra et al., 2015; Karuppaiah et al., 2020).
Lin et al., found that silver nanoparticles act as anticancer agents by induction of autophagy of cancer cells through activation of the ptdlns3K pathway. Furthermore, they observed that inhibition of autophagy by autophagic inhibitor like wortmannin results in enhanced cancer cell killing efficacy in the mouse melanoma cell model (B16 cell lines) (Lin et al., 2014).
5 Miscellaneous applications
Apart from discussing the antimicrobial and anticancer applications of silver nanoparticles, several other applications are worth to mention here. These include applications or uses in the biomedical field, consumer products, agriculture, cosmetics, air, and water purification, textile industries, automobiles, and so forth (Haider and Kang, 2015; Naidu et al., 2015; Kumar et al., 2020; Shafique and Luo, 2019). These applications define the ubiquitous role of nanosilver in the present era of science and technology.
Silver nanoparticles are being employed as antimicrobial coatings/constituents of various biomedical products like catheters, stents (Yang et al., 2016), artificial heart valves, surgical instruments, contact lenses, cannulas, urinary dilators, intrauterine devices, pacemakers, and disinfecting of hospital bedding. They are used in bandages and dressing of wounds (Naidu et al., 2015; Lem et al., 2012). Silver nanoparticles are used in dentistry as dental alloys, dental resins, prostheses, and dental coatings, thereby protecting the implants from biofilm formation and maintaining long-term oral hygiene (Yin et al., 2020). The usefulness of silver nanoparticles in biomedical products relies on the fact that they are potent and proven antimicrobial agents. The durability, long-term effectiveness, broad-spectrum antimicrobial resistance, and sustained release of silver ions enable them to be used in diverse biomedical and consumer products. Silver nanoparticles are being used in textile industry to create antimicrobial fabrics. AgNPs are blended with fabrics and impart them unique properties like durability, disinfecting, hygienic, and color performance or brightness enhancers (Deshmukh et al., 2019). Silver nanoparticles are increasingly used in consumer products thriving in markets with desired and unique properties. For example, in personal health care products (deodorants, skin creams, body lotions, soaps, toothpaste, antimicrobial socks), kitchen scrubbers, antimicrobial toys, disinfecting sprays, pesticides, keyboard covers, air and water treatment/purification devices, detergents, facial masks, automobile interiors, packaging of food materials and many more (McGillicuddy et al., 2017; Lem et al., 2012; Shafique and Luo, 2019).
Aside from human life, nanoparticles' unique chemical and physical features make them ideal for various high-tech applications, such as the development of new and improved sensing devices, particularly electrochemical sensors and biosensors. AgNPs and other core–shell metal nanoparticles have also been used to mark biomolecules in electroanalysis. An electrochemical DNA biosensor based on the AgNPs label is capable of sensing target oligonucleotides at concentrations as low as 0.5 pM (Luo et al., 2006). In addition, studies have found that AgNPs can be used as an electrical bridge for electron transmission between cytochrome c and the electrode (Liu et al., 2003; Luo et al., 2006). In comparison to many electrically conductive fi llers, the silver particle is a better option for use as adhesives and conductive inks owing to its potential thermal conductivity, electrical conductivity, low cost in comparison to graphene or gold and chemical stability (Ren et al., 2015).
It is a fact that nanosilver based consumer products are increasingly used for their attentive properties and are flooding the markets, but evaluation work regarding the sustained release, accumulation, clearance, and many other toxicity profiles and risk assessments in biological systems and the environment, is unsatisfactory, which is an alarming and wake-up call that needs to be addressed scientifically at the earliest.
6 Conclusion and future perspectives
Nanotechnology represents an emerging and novel approach to develop and validate novel formulations based on metallic nanoparticles. Silver nanoparticles and their nanocomposites have tremendous potential for application in varied fields of science and nanotechnology, especially in biomedical and therapeutics. They represent promising and potential antimicrobial, anticancer, and theranostic agents. Due to the unique properties of silver and its nanomaterials, as already discussed in the above part of article, they are enlisted as valuable and significant nanomaterials with huge potential and applicability in the field of nanotechnology. Despite the tremendous biomedical potential of silver nanoparticles, it is important to note that the possible health and environmental risks posed by silver nanoparticles are still not clear and further analysis with evidence of toxicity is the need of the hour. There needs to be more research and management strategies on systemic toxicity to humans, other animals, aquatic ecosystems, soil, and the atmosphere because of the increasing usage and demand of nano silver-based products in the commercial markets. Moreover, the antiviral activity of silver nanoparticles also needs more clarification. Therefore, the production of nanoparticles with well-controlled morphological and physicochemical characteristics for use in human bodies and other areas still remains an active field of interdisciplinary study.
Acknowledgement
This work was supported by ‘A Project to establishment a regional specialized smart city graduate school’ by Hongik University. Authors also extend their acknowledgement to the International Science & Business Belt support program, through the Korea Innovation Foundation funded by the Ministry of Science and ICT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater.. 2015;2015
- [Google Scholar]
- A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res.. 2018;9:1-16.
- [Google Scholar]
- pH effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater. Sci.-Poland. 2014;32(1):107-111.
- [Google Scholar]
- Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA) Bioinorg. Chem. Appl.. 2018;2018
- [Google Scholar]
- Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep.. 2020;10(1):1-12.
- [Google Scholar]
- Oleic acid–conjugated silver nanoparticles as efficient antiamoebic agent against Acanthamoeba castellanii. Parasitol. Res.. 2019;118(7):2295-2304.
- [Google Scholar]
- Albizzia lebbeck extract mediated synthesis and characterization of Zinc oxide Nanoparticle. Asian J. Pharm. Res.. 2019;9(1):01-06.
- [Google Scholar]
- Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res. Lett.. 2012;7(1):267.
- [Google Scholar]
- 5-Fluorouracil–lipid conjugate: potential candidate for drug delivery through encapsulation in hydrophobic polyester-based nanoparticles. Acta Biomater.. 2014;10(11):4685-4694.
- [Google Scholar]
- Biological synthesis of silver nanoparticles using Rheum ribes and evaluation of their anticarcinogenic and antimicrobial potential: a novel approach in phytonanotechnology. J. Pharm. Biomed. Anal.. 2020;179:113012.
- [Google Scholar]
- Focal prostate cryoablation: initial results show cancer control and potency preservation. J. Endourol.. 2006;20(9):688-692.
- [Google Scholar]
- Radiation therapy for breast cancer: literature review. Med. Dosimetry. 2016;41(3):253-257.
- [Google Scholar]
- Nanomaterials: applications in cancer imaging and therapy. Adv. Mater.. 2011;23(12):H18-H40.
- [Google Scholar]
- Nanotechnology in reproductive medicine: emerging applications of nanomaterials. Nanomed. Nanotechnol. Biol. Med.. 2014;10(5):e921-e938.
- [Google Scholar]
- Radiation therapy and cancer control in developing countries: Can we save more lives? Int. J. Med. Sci.. 2017;14(1):13-17.
- [Google Scholar]
- Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol. Lett.. 2012;208(3):286-292.
- [Google Scholar]
- Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B. 2015;3(36):7237-7245.
- [Google Scholar]
- Folic acid-conjugated, SERS-labeled silver nanotriangles for multimodal detection and targeted photothermal treatment on human ovarian cancer cells. Mol. Pharm.. 2014;11(2):391-399.
- [Google Scholar]
- Murraya koenigii-mediated synthesis of silver nanoparticles and its activity against three human pathogenic bacteria. Nanosci. Methods. 2012;1(1):25-36.
- [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68(6):394-424.
- [Google Scholar]
- Brem, R. F. (2018). Radiofrequency Ablation of Breast Cancer: A Step Forward.
- Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv.. 2016;6(50):44232-44247.
- [Google Scholar]
- Anticancer activity of biogenerated silver nanoparticles: an integrated proteomic investigation. Oncotarget. 2018;9(11):9685-9705.
- [Google Scholar]
- VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl. 3):4-10.
- [Google Scholar]
- Bifunctional magnetopolymersomes of iron oxide nanoparticles and carboxymethylcellulose conjugated with doxorubicin for hyperthermo-chemotherapy of brain cancer cells. Biomater. Sci.. 2019;7(5):2102-2122.
- [Google Scholar]
- Silver nanoparticles (medicinal plants mediated): A new generation of antimicrobials to combat microbial pathogens- a review. In: Méndez-Vilas A., ed. Microbial pathogens and strategies for combating them: science, technology and education. Spain: Formatex; 2013. p. :1314-1323.
- [Google Scholar]
- In vivo and in vitro hair growth-promoting effect of silver and iron nanoparticles synthesized via Blumea eriantha DC plant extract. J. Cosmetic Dermatol.. 2021;20(4):1283-1297.
- [Google Scholar]
- Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials. 2012;33(4):1190-1200.
- [Google Scholar]
- Graphene oxide–silver nanocomposite enhances cytotoxic and apoptotic potential of salinomycin in human ovarian cancer stem cells (OvCSCs): a novel approach for cancer therapy. Int. J. Mol. Sci.. 2018;19(3):710.
- [Google Scholar]
- Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed.. 2015;10:6847.
- [Google Scholar]
- Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials. 2018;154:24-33.
- [Google Scholar]
- Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng., C. 2019;97:954-965.
- [Google Scholar]
- Principles of cancer treatment by chemotherapy. Surgery (Oxford). 2018;36(3):134-138.
- [Google Scholar]
- Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol.. 2005;3(1):1-10.
- [Google Scholar]
- Assessment of in situ-prepared polyvinylpyrrolidone-silver nanocomposite for antimicrobial applications. Acta Phys. Pol. A. 2017;131(6)
- [Google Scholar]
- Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep.. 2017;7(1):1-20.
- [Google Scholar]
- NK cell-based immunotherapy for cancer. In: Seminars in immunology. Academic Press; 2017. p. :37-54.
- [Google Scholar]
- ROCplot. org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int. J. Cancer. 2019;145(11):3140-3151.
- [Google Scholar]
- Allogeneic bone marrow transplantation in multiple myeloma. N. Engl. J. Med.. 1991;325(18):1267-1273.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep.. 2020;10(1):1-11.
- [Google Scholar]
- Physicochemical properties of nanomaterials: implication in associated toxic manifestations. BioMed Res. Int.. 2014;2014
- [Google Scholar]
- Biosynthesis, characterization and leishmanicidal activity of a biocomposite containing AgNPs-PVP-glucantime. Nanomedicine. 2018;13(4):373-390.
- [Google Scholar]
- Targeted therapies: a new generation of cancer treatments. Am. Fam. Physician. 2008;77(3):311-319.
- [Google Scholar]
- Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant.. 2019;54(6):798-809.
- [Google Scholar]
- Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Delivery Sci. Technol.. 2020;55:101376.
- [Google Scholar]
- Nanotechnology for environmental remediation: materials and applications. Molecules. 2018;23(7):1760.
- [Google Scholar]
- Bioconjugated gold nanoparticles enhance cellular uptake: a proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm.. 2016;509(1-2):16-27.
- [Google Scholar]
- Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int. J. Nanomed.. 2015;10:4203.
- [Google Scholar]
- Preparation of silver nanoparticles and their industrial and biomedical applications: a comprehensive review. Adv. Mater. Sci. Eng.. 2015;2015
- [Google Scholar]
- Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res. Lett.. 2014;9(1):459.
- [Google Scholar]
- The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions. PLoS ONE. 2015;10(12):e0144306.
- [Google Scholar]
- Facile synthesis of graphene oxide–silver nanocomposite and its modified electrode for enhanced electrochemical detection of nitrite ions. Talanta. 2015;144:908-914.
- [Google Scholar]
- In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J. Pharm. Res.. 2013;6(1):32-38.
- [Google Scholar]
- Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci.. 2014;9(6):385.
- [Google Scholar]
- Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res.. 2008;41(12):1578-1586.
- [Google Scholar]
- Laser-induced synthesis of silver nanoparticles and their conjugation with protein. Appl. Phys. A. 2014;116(2):635-641.
- [Google Scholar]
- Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf., B. 2009;73(1):51-57.
- [Google Scholar]
- Synthesis and characterization of folic acid conjugated gemcitabine tethered silver nanoparticles (FA-GEM-AgNPs) for targeted delivery. Curr. Pharm. Des. 2020
- [Google Scholar]
- Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv.. 2019;9(2):1095-1105.
- [Google Scholar]
- Ampicillin silver nanoformulations against multidrug-resistant bacteria. Sci. Rep.. 2019;9
- [CrossRef] [Google Scholar]
- Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Control. Release. 2011;152(1):76-83.
- [Google Scholar]
- Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011;39(1):77-85.
- [Google Scholar]
- Toxicity of silver nanomaterials in higher eukaryotes. In: Advances in Molecular Toxicology. Elsevier; 2011. p. :179-218.
- [Google Scholar]
- Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep.. 2020;10(1):1-10.
- [Google Scholar]
- Synthesis, characterization, mechanistic studies and antimicrobial efficacy of biomolecule capped and pH modulated silver nanoparticles. J. Mol. Liq.. 2018;249:1145-1150.
- [Google Scholar]
- Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol.. 2010;8(1):1-10.
- [Google Scholar]
- US-guided percutaneous cryoablation of prostate cancer. Radiology. 1994;192(3):769-776.
- [Google Scholar]
- A stable and reproducible nanosilver-aggregation-4-mercaptopyridine surface-enhanced Raman scattering probe for rapid determination of trace Hg2+. Talanta. 2012;99:890-896.
- [Google Scholar]
- Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int. J. Nanomed.. 2016;11:6693.
- [Google Scholar]
- Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. 2014;10(11):2006-2020.
- [Google Scholar]
- Novel antimicrobial chitosan–cellulose composite films bioconjugated with silver nanoparticles. Ind. Crops Prod.. 2015;70:395-403.
- [Google Scholar]
- Wiring electrons of cytochrome c with silver nanoparticles in layered films. Eur. J. Chem. Phys. Phys. Chem.. 2003;4(12):1364-1366.
- [Google Scholar]
- Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res.. 2006;5(4):916-924.
- [Google Scholar]
- In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol.. 2018;9:1555.
- [Google Scholar]
- Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18(4):319-326.
- [Google Scholar]
- A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res.. 2010;12(5):1531-1551.
- [Google Scholar]
- Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci. Rep.. 2013;3(1)
- [CrossRef] [Google Scholar]
- Silver nanoparticle conjugation-enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics. 2018;7(4):100.
- [Google Scholar]
- Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci. Total Environ.. 2017;575:231-246.
- [Google Scholar]
- Menu, P., Mayor, A., Zhou, R., Tardivel, A., Ichijo, H., Mori, K., Tschopp, J. (2012). ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis., 3(1), e261-e261.
- Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng., C. 2015;53:120-127.
- [Google Scholar]
- Murali Satyanarayana Bethu, Vasudeva Reddy Netala, Latha Domdi, Vijaya Tartte, Venkateswara Rao Janapala (2018) Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: an insight into the mechanism, Artificial Cells, Nanomedicine, and Biotechnology, 46:sup1, 104-114.
- Biomedical applications and toxicity of nanosilver: a review. Med. Technol. SA. 2015;29(2):13-19.
- [Google Scholar]
- Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol.. 2008;42(23):8959-8964.
- [Google Scholar]
- Nanomaterials in combating cancer: therapeutic applications and developments. Nanomed. Nanotechnol. Biol. Med.. 2014;10(1):19-34.
- [Google Scholar]
- Recognition of carbendazim fungicide in environmental samples by using 4-aminobenzenethiol functionalized silver nanoparticles as a colorimetric sensor. Sens. Actuators, B. 2015;206:684-691.
- [Google Scholar]
- Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng., C. 2015;53:298-309.
- [Google Scholar]
- Nanoparticles as dental drug-delivery systems. In: Nanobiomaterials in Clinical Dentistry. William Andrew Publishing; 2013. p. :475-495.
- [Google Scholar]
- Anticancer activity of silver nanobioconjugates synthesized from Piper betle leaves extract and its active compound eugenol. Int. J. Pharm. Pharm. Sci.. 2016;8(9):201-205.
- [Google Scholar]
- Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv.. 2009;27(1):76-83.
- [Google Scholar]
- Oleic acid coated silver nanoparticles showed better in vitro amoebicidal effects against Naegleria fowleri than Amphotericin B. ACS Chem. Neurosci. 2019
- [Google Scholar]
- Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Soc.. 2017;21(8):911-919.
- [Google Scholar]
- Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J. Clin. Oncol.. 2018;36(6):600-608.
- [Google Scholar]
- Green synthesis of silver nanoparticles. Int. J. Pharm. Med. Biol. Sci.. 2012;1(1):54-61.
- [Google Scholar]
- Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol.. 2019;10(1):1-10.
- [Google Scholar]
- Phyto-mediated synthesis of silver nanoparticles using fucoidan isolated from Spatoglossum asperum and assessment of antibacterial activities. J. Photochem. Photobiol., B. 2018;185:117-125.
- [Google Scholar]
- Treatment of splenic flexure colon cancer: a comparison of three different surgical procedures: experience of a high volume cancer center. Sci. Rep.. 2019;9(1):1-7.
- [Google Scholar]
- One-step preparation of silver hexagonal microsheets as electrically conductive adhesive fillers for printed electronics. ACS Appl. Mater. Interfaces. 2015;7:13685-13692.
- [Google Scholar]
- Synthetic approaches to metallic nanomaterials. Nanofabrication Towards Biomed. Appl. 2005:1-32.
- [Google Scholar]
- A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett.. 2008;3(4):129-133.
- [Google Scholar]
- Antibacterial activity of silver nanoparticles against field and reference strains of Mycobacterium tuberculosis, Mycobacterium bovis and multiple-drug-resistant tuberculosis strains. Rev. Sci. Tech.. 2018;37(3):823-830.
- [Google Scholar]
- Nanotechnology in transportation vehicles: an overview of its applications, environmental, health and safety concerns. Materials. 2019;12(15):2493.
- [Google Scholar]
- An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ. Sci. Pollut. Res.. 2018;25(11):10362-10370.
- [Google Scholar]
- Metal nanoparticles: a theranostic nanotool against cancer. Drug Discovery Today. 2015;20(9):1143-1151.
- [Google Scholar]
- Green synthesis of silver, iron and gold nanoparticles of lycopene extracted from tomato: their characterization and cytotoxicity against COLO320DM, HT29 and Hella cell. J. Mater. Sci. - Mater. Med.. 2021;32(2):1-12.
- [Google Scholar]
- Green synthesis of silver and iron nanoparticles of isolated proanthocyanidin: its characterization, antioxidant, antimicrobial, and cytotoxic activities against COLO320DM and HT29. J. Genet. Eng. Biotechnol.. 2020;18(1):1-11.
- [Google Scholar]
- Antibacterial activities of bacteriagenic silver nanoparticles against nosocomial Acinetobacter baumannii. J. Nanosci. Nanotechnol.. 2018;18(6):3806-3815.
- [Google Scholar]
- Silver-, gold-, and iron-based metallic nanoparticles: Biomedical applications as theranostic agents for cancer. In: Design of nanostructures for theranostics applications. William Andrew Publishing; 2018. p. :161-242.
- [Google Scholar]
- Protein–silver nanoparticle interactions to colloidal stability in acidic environments. Langmuir. 2014;30(43):12755-12764.
- [Google Scholar]
- NY-ESO-1 based immunotherapy of cancer: current perspectives. Front. Immunol.. 2018;9:947.
- [Google Scholar]
- Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev.. 2011;63(3):131-135.
- [Google Scholar]
- Synthesis of silver nanoclusters and functionalization with glucosamine for glyconanoparticles. Synth. React. Inorg., Met.-Org., Nano-Met. Chem.. 2010;40(1):56-64.
- [Google Scholar]
- Glucosamine-functionalized silver glyconanoparticles: characterization and antibacterial activity. Anal. Bioanal. Chem.. 2010;398(2):867-876.
- [Google Scholar]
- Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol.. 2017;98:515-525.
- [Google Scholar]
- Applications of Silver nanoparticles in diverse sectors. Int. J. Nano Dimension. 2019;10(1):18-36.
- [Google Scholar]
- Antitumor activities of metal oxide nanoparticles. Nanomaterials. 2015;5(2):1004-1021.
- [Google Scholar]
- Mechanism of silver ion reduction in concentrated solutions of amphiphilic invertible polyesters in nonpolar solvent at room temperature. Langmuir. 2008;24(21):12587-12594.
- [Google Scholar]
- W Lem, K., Choudhury, A., A Lakhani, A., Kuyate, P., R Haw, J., S Lee, D., et al. (2012). Use of nanosilver in consumer products. Recent Patents Nanotechnol., 6(1), 60-72.
- One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym.. 2018;199:641-648.
- [Google Scholar]
- World Health Organization,12 sep.2018.Latest global cancer data:Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018.
- Methotrexate-conjugated AgInS2/ZnS quantum dots for optical imaging and drug delivery. Mater. Lett.. 2014;128:412-416.
- [Google Scholar]
- Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst. 2010;135(6):1389-1394.
- [Google Scholar]
- A novel biliary stent coated with silver nanoparticles prolongs the unobstructed period and survival via anti-bacterial activity. Sci. Rep.. 2016;6(1):1-11.
- [Google Scholar]
- Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials. 2016;99:1-15.
- [Google Scholar]
- The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed.. 2020;15:2555.
- [Google Scholar]
- Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci.. 2013;441:73-82.
- [Google Scholar]
- Quercetin-mediated synthesis of graphene oxide–silver nanoparticle nanocomposites: a suitable alternative nanotherapy for neuroblastoma. Int. J. Nanomed.. 2017;12:5819.
- [Google Scholar]
- Yuan, Y. G., Zhang, S., Hwang, J. Y., Kong, I. K. (2018). Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxidative Med. Cell. Longevity, 2018.
- Uptake of silver nanoparticles by DHA-treated cancer cells examined by surface-enhanced Raman spectroscopy in a microfluidic chip. Lab Chip. 2017;17(7):1306-1313.
- [Google Scholar]
- Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int. J. Nanomed.. 2019;14:9483.
- [Google Scholar]
- Tungsten oxide nanorods: an efficient nanoplatform for tumor CT imaging and photothermal therapy. Sci. Rep.. 2014;4:3653.
- [Google Scholar]
- Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res.. 2009;43(3):715-723.
- [Google Scholar]







