Translate this page into:
An investigation of the efficacy of hygienic behavior of various honey bee (Apis mellifera) races toward Varroa destructor (Acari: Varroidae) mite infestation
⁎Corresponding author at: Unit of Bee Research and Honey Production, Biology Department, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, saudi Arabia. khalidtalpur@hotmail.com (Khalid Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hygienic behavior in honey bees reflects the social immunity against parasites and diseases and is considered one of the main factors of genetic resistance of the bee breeding program. Hygienic behavior refers to the uncapping and removal of diseased and dead larvae and pupae from sealed brood cells by the worker bees. Here, the hygienic behavior of Apis mellifera races was investigated in response to pin-killed assay and artificial Varroa mite-infested brood cells. Moreover, the reproduction behavior of Varroa mites was determined in various sizes of honey bee race comb cells. The results revealed that the percentage of uncapping and removal of dead broods were significantly higher in the Italian as compared to the Carniolan bee colonies (p < 0.05). Similarly, a significant difference was present in response to artificially infested brood cells with Varroa mites in Italian and Carniolan colonies over the day of inspection (p < 0.05). Overall, the width of cells of the two types of combs was significantly different. The smaller width of cells size combs reduces the Varroa mite reproduction behavior compared to larger cell size combs. More infestation of Varroa mite occurred in drone brood cells as compared to worker brood cells in both old and new comb types. This study contributed to understand the hygienic behavior of A. mellifera races in preparation for selecting and developing hygienic lines of bees given the important contribution of hygienic behavior to honey bee health.
Keywords
Pin-killed assay
Uncapping and removal
Ectoparasitic mite
Reproduction behavior
Cell size
Drone brood cell
1 Introduction
The ectoparasitic mite Varroa destructor Anderson and Trueman (Acari: Varroidae) causes severe damage to honey bee health all around the world. It is the primary pest of Asian honey bee (Apis cerana), but they are currently considered as serious threat to the western honey bee (A. mellifera) (Nazzi and Le Conte, 2016; Eliash and Mikheyev, 2020). Over the past few decades, Varroa mite infestation causes the huge amount of honey bee colony losses in numerous countries (Le Conte et al., 2010; Eliash and Mikheyev, 2020). These mites cause serious damage to honey bees, directly by feeding on fat body tissue / cellular components of immature and mature bees (Ramsey et al., 2019) and indirectly through transmitting various deadly honey bee viruses (Bowen-Walker et al., 1999; Chen et al., 2004; Tantillo et al., 2015), and bacteria (Hubert et al., 2015). The mite infestation leads to lifespan shortening, malformation, weight loss, and weakening of host colony (De Jong et al., 1982; Duay et al., 2003; Garedew et al., 2004; Dainat et al., 2012). From the beginning of the nineteenth century, various techniques such as mechanical, chemical, and natural procedures have been developed to fight against Varroa mite to prevent colony losses (Khan et al., 2020; Masry et al., 2020; Spivak and Danka, 2020). Among them, chemical methods efficiently used to manage mite infestation but it create mite resistance to most of the acaricides and even induce contamination in the hive products (Wallner, 1999; Rosenkranz et al., 2010; González-Cabrera et al., 2016; Beaurepaire et al., 2017; Jamal et al., 2020; Kumar et al., 2020; Naeem-Ullah et al., 2020). Different alternative control strategies, such as physical mite removal and natural materials like essential oils and botanical extracts were used to minimize the mite infestation (Loucif-Ayad et al., 2010; Mahmood et al., 2011; Ansari et al., 2017; Cimmino et al., 2019; Al-Ghamdi et al., 2020a; Masry et al., 2020; Sajid et al., 2020). One of the most promising ways to reduce Varroa mite infestation is by multiple selective breeding mite resistance-honey bees such as Varroa Sensitive Hygienic (VSH) and Minnesota Hygienic (HYG) stocks. Both VSH and HYG colonies showed a higher level of hygienic behavior (Leclercq et al., 2017) and have been considered to show more resistance to Varroa mites as compared to unselected colonies (Spivak and Gilliam, 1998; Harris et al., 2012; Danka et al., 2013).
Naturally, social insects such as honey bees, ants, and termites have developed the different behavioral adoption to prevent pathogens and disease spread inside the colonies (Cremer et al., 2007; Leclercq et al., 2017; Spivak and Danka, 2020; Jamal et al., 2021). Specifically, hygienic behavior in honey bees has been studied for nearly 80 years for understanding the mechanism to decrease the infestation of pathogens and Varroa mites resistance and improve the health of their colonies (Spivak and Danka, 2020). The term “hygienic behavior” was introduced by Rothenbuhler (1964) to describe the ability of worker bees to detect, uncap, and remove the diseased and dead brood from the cells to minimize the infestation (Peng et al., 1987; Boecking and Spivak, 1999; Spivak and Reuter, 2001). Honey bees hygienic behavior was measured by two methods; pin-killed brood (PKB) assay (Kefuss et al., 1996; Palacio et al., 2000) and freeze-killed (FKB) assay (Spivak and Reuter, 1998). This is a successful defense mechanism against bee diseases like, American foulbrood (Spivak and Gilliam, 1998; Spivak and Reuter, 2001; Al-Ghamdi et al., 2018, 2020b), chalkbrood (Palacio et al., 2010), and the destructive Varroa mite (Spivak, 1996; Rinderer et al., 2010; Spivak and Danka, 2020).
In honey bees, hygienic behavior is a heritable genetic trait and is controlled by two to seven loci (Moritz, 1988; Kefuss et al., 1996; Lapidge et al., 2002). Moreover, detection of the dead and diseased capped brood mainly relies on volatile odorant signals that penetrate with the wax cell cup (Spivak et al., 2003). Some strains of honey bees have the capability to minimize the mite infestation level, obviously by dropping the reproductive success of mites in worker brood cells (Harris et al., 2012; Danka et al., 2013; Leclercq et al., 2017; Spivak and Danka, 2020).
Therefore, the honey bee colonies that have a higher degree of hygienic behavior are recommended as a natural tactic to manage the infestation of pathogens, pests, and diseases. Generally, both environment and genotype are determinative for the hygienic behavior of honey bees. In present study, various experiments were performed with the following aims: (1) to evaluate the hygienic behavior of Italian and Carniolan bee races toward the uncapped and removal of pin-killed brood in capped brood cells, (2) to assess the hygienic behavior of both bee stocks toward artificially Varroa mite-infested brood cells, (3) to determine the reproduction behavior of Varroa mites in different cell size on foundation sheets (4) to compare the Varroa mite infestation rate in new and old combs. The study developed a new approach to be practiced by beekeepers to manage Varroa mite that may result to improve honey yield and economical condition of beekeepers.
2 Materials and methods
2.1 Hygienic behavior of honey bees by pin-killed brood assay
Eight bee colonies were used and separated into two groups on the base of their queens i.e., one group contained the queen of Italian bees (A. mellifera ligustica), and the second group of Carniolan bees (A. mellifera carnica). Both full-sized bee colonies were kept in Langstroth hives. All the colonies had the fertile queens, workers, broods, plenty of honey, and pollen. To determine the hygienic behavior, perforation of brood cell (pin-killed assay) was applied using the minor modification of the method described by Büchler et al. (2013), and Newton and Ostasiewski Jr (1986). Briefly, the area having the most sealed brood cells was selected, and 100 brood worker larvae were killed with a sterile metal pin, and treated frames were returned to their respective hives. Hygienic behavior was quantified as the removal of killed larvae from brood cells. The percentage of uncapped brood cells and dead brood removal by both honey bee stock was recorded after 12 h, 24 h, and 48 h.
2.2 Hygienic behavior of honey bees toward the Varroa mite-infested brood cells
In order to investigate the hygienic behavior, two groups of equal population honey bee colonies reared in Langstroth hives. Among them, four honey bee colonies were selected to compare the hygienic behavior between two queen races (Italian and Carniolan) against artificial infested brood cells with Varroa mites. All mites were collected from local A. mellifera colonies, and these were obtained from newly-capped broods (pre-pupal or white-eyed stages). We selected the 50 worker brood cell capped one day before and opened these cells at one edge using solvent-washed forceps. One female phoretic Varroa mite was introduced with the help of a fine camel hair brush in each treated cell. After inserting mite, the cells were again capped by using the drop of warm wax, and their positions were marked on transparency sheets. In case of control, the worker cells were perforated with help of a small sterilized pin needle and again closed without inserting the Varroa mites, this served to check whether bees removed the brood due to the presence of the mite or to manipulation of cells with a pin needle. After handling, the combs were returned into their respective colonies. The number of brood cell emptied by the bees in treated and control treatment were inspected after three, five, and seven days intervals. Three replicates were run per test series.
2.3 Comparison of reproduction behavior of Varroa mites in different cell size on foundation sheets
The reproduction behavior of Varroa mites was tested on the basis of the difference in cell size on the foundation sheet made by different races of A. mellifera (Carniolan and Italian). For this purpose, two colonies of A. mellifera were taken; one colony with Carniolan queen and the other Italian queen. Both honey bee stocks received supplemental feeding with sucrose syrup. The drone broods were absent in each colony. The foundation sheet was horizontally cut in two sects; 2 and 3 in. of three combs in each colony respectively and these combs were reintroduced in each respective colony. The cell size was observed on the foundation sheet which was built by each of colony of A. mellifera and the width of cell was measured by the Vernier caliper. The reproduction behavior of Varroa mites was observed in cells of different sizes. The data were recorded after an interval of one-week.
2.4 To compare the Varroa mite infestation rate in drone and worker brood cells in old and new combs
Six colonies of European honey bees were selected to compare the Varroa mite infestation rate in drone and worker brood cells. These colonies were maintained in Langstroth hives. Each colony had a healthy queen with new and old combs. The queen laid eggs in both combs in the same period. After that the Varroa mite infestation was examined in 20 workers and 20 drone brood cells in both new and old combs. The percentage of Varroa mite infestation was calculated. The data were recorded after two days interval.
2.5 Statistical analysis
Quantitative data were presented as mean ± standard error (S. Error) and all statistical data were analyzed through statistical package SPSS (version 26). Student’s t-test was used to test the significant difference between two groups, one-way ANOVA followed by Tukey post-hoc was used to determine the difference between three or more groups. The data regarding hygienic behavior mean and other mean were compared at the 0.05 level.
3 Results
3.1 Comparison of hygienic behavior between honey bee races
The uncapping and removal percentage of pin killed brood from the cells was used as an indicator of level of hygienic behavior in honey bees. The results (Fig. 1A, B) revealed that the percentage of uncapping and removal of dead brood was significantly higher in the Italian bee as compared to the Carniolan bee colonies. It was observed that after 12 h the uncapping percentage of Italian bees was 91.75%, that was significantly higher than the Carniolan bee with 74.92%. After 24 h, the uncapping percentage of brood cells in Italian bees was significantly higher than the Carniolan bee that was 95.08% and 83.08%, respectively. While after 48 h the Italian bees had removed the 99.25% dead brood significantly higher than the Carniolan bees 89.08%.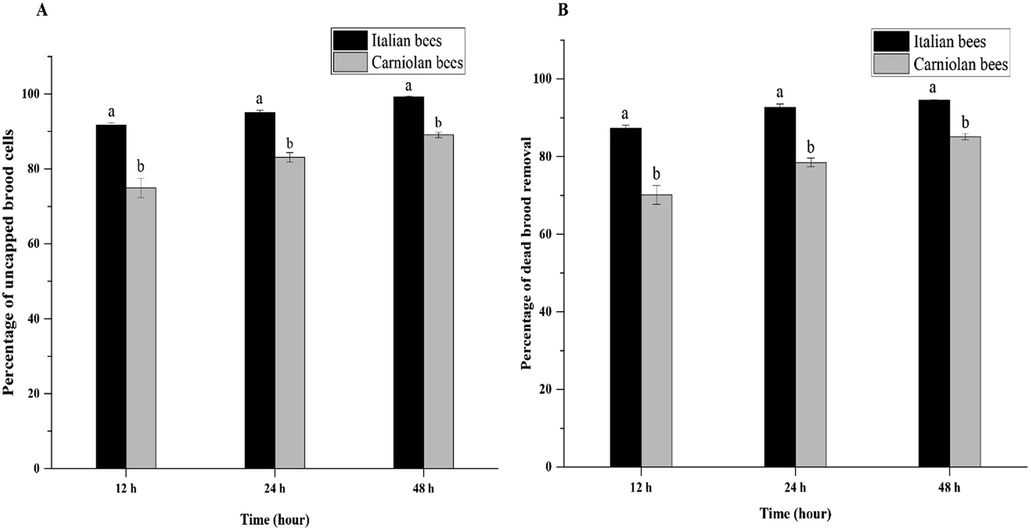
Hygienic behavior (% of mean uncapping and removal of killed brood) over time in colonies of Italian and Carniolan honey bees. Column plots are shown for uncapped brood cells (A), and dead brood removal (B) in Langstroth hives.
Similarly, it was found that after 12 h the removal percentage of dead brood in Italian bees was 87.33%, significantly higher than the Carniolan bee which was 70.17%. After 24 h, the Italian bees had removed the significantly higher dead brood than the Carniolan bees (92.67% and 78.50% respectively). After 48 h, the Italian bees removed 94.50% dead brood which was significantly higher than the Carniolan bee 85.17%. Overall, the uncapping and removal percentages of dead brood within the Italian bee was significantly different over the time while the non-significant difference was observed within the Carniolan bee colonies after 12 h, 24 h, and 48 h.
3.2 Comparison of hygienic behavior of honey bee races towards the removal of artificially Varroa mite infested brood cells
A significant difference was found in response to artificially infested brood cell with Varroa mites in Italian and Carniolan bee colonies over the day of inspection (Fig. 2). After three days, Italian and Carniolan bees emptied 40.17%, 28.33% of the artificially infested cells in treated colonies and removal rates were 24.50% and 19.67% in control colonies. The Italian bee had removed the 69.33% infested brood whereas 48.83% by Carniolan bee after five days. In the control treatment, the removal rate was 37.67% and 27.17%, respectively. Similarly, the removal percentage of infested brood by Italian bee was 78.33% while this was 63.17% in Carniolan bees after seven days interval. In the control, the removal rate of brood from the cells was 57.00% and 42.50% in Italian and Carniolan bee colonies, respectively.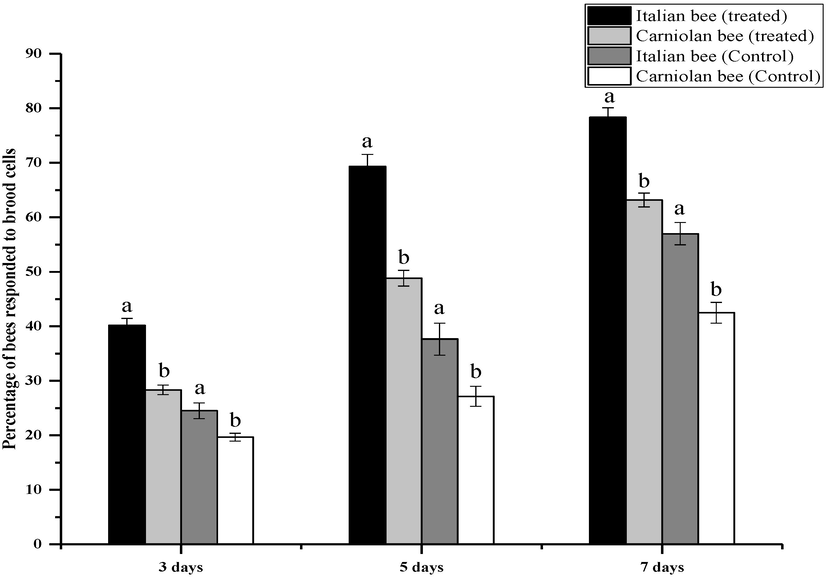
Hygienic behavior of Italian and Carniolan honey bee races after three, five and seven days toward the worker brood cell containing artificially introduced Varroa mite.
3.3 Comparison of reproduction behavior of Varroa mites based on cell size on foundation sheets
The results indicated that the width of cells of the two types of the combs was significantly different. The data were recorded about the width of cell (inner side) of combs and means were compared by the Student’s t-test at 0.05 shown in Table 1. The mean inner width of Italian comb was significantly different than the inside width of the Carniolan comb that were 5.005 and 5.276 mm, respectively (Table 1). There was no significant difference present within Italian comb cell size ranges from 5.003 to 5.006 mm respectively, while significant difference was in the cell sizes in case of Carniolan 5.144 to 5.457 mm (Table 1). Mean row having the different small letter shows that the significant difference between them at (Student’s t-test, p < 0.05).
Colony
Mean ± S. Error
Colony
Mean ± S. Error
Italian
5.048 ± 0.7919b
Carniolan
5.144 ± 0.1918 ab
Italian
5.034 ± 0.06024b
Carniolan
5.457 ± 0.0363 a
Mean cell size
5.005 ± 0.0464b
Mean cell size
5.276 ± −0.125 a
It was evident that maximum infestation percentage of worker brood with Varroa mite was found in Carniolan comb and less infestation was recorded in case of Italian comb. But the Varroa mite infestation within the Carniolan comb cell and the Italian comb cells was not significantly different (Table 2). The infestation rate of Varroa mite in honey bee colonies may be associated with comb cell width. Table 2 showed that within the same colonies of Carniolan the larger brood cell is more infested as compared to small brood cell. The mean percentage of infested cells in case of Carniolan comb was significantly higher than the Italian comb cells 22.78% and 10.28%, respectively (Table 3). The percentage of the number of Varroa mite in Carniolan comb was significantly higher than the Italian comb which were 30.27% and 12.22%, respectively (Table 3). Row having the different small letter show that the significant difference between them and column having the similar small letter show there is no significant difference between them at (Student’s t-test, p < 0.05). Mean percentage of the infested cell having the small different letter in row were significantly different at p < 0.05 and mean percentage of Varroa mites having the small different letter in row were significantly different at (Student’s t-test, p < 0.05).
Colony
Mean ± S. Error
Colony
Mean ± S. Error
Italian
10.833 ± 0.8333b
Carniolan
20.56 ± 1.368 a
Italian
9.722 ± 1.5278b
Carniolan
25.00 ± 2.32 a
Italian comb
Carniolan comb
Number of brood cells examined
360
360
Mean percentage of infested cells
10.28 ± 0.86b
22.78 ± 1.41 a
Mean percentage of Varroa mites
12.22 ± 1.18b
30.27 ± 1.75 a
In case of Italian honey bees negative correlation was observed between the cell width and the Varroa mite infestation per cell and also there was no significant difference. While the Carniolan bees had both positive and negative correlations, but there was a non-significant difference present between the inner width of brood cells and Varroa mite infestation (Table 4). Almost, similar negative correlation between inner width of brood cells and the number of Varroa mite per cell and the non-significant difference between both honey bee stocks (Table 4). A positive correlation was present between the number of Varroa mite and Varroa mite infestation in all worker cells and highly significant among Italian and Carniolan bee colonies (Table 4). r = Pearson coefficient correlation.
Inner cell width vs Varroa infestation
Inner cell width vs No. of Varroa
No. of Varroa vs Varroa infestation
Colony
r
P value
r
P value
r
P value
Italian
−0.005
0.985
−0.005
0.985
1
0.000***
Italian
−0.297
0.3609
−0.421
0.664
0.718
0.026*
Carniolan
0.232
0.548
−0.471
0.2004
0.498
0.172
Carniolan
−0.207
0.593
−0.266
0.489
0.923
0.0004***
3.4 To compare the rate of infestation of Varroa mite in worker and drone brood cells in old and new combs
Present study indicated that the Varroa mite infestation was higher in drone cell as compared to worker brood cells in both old and new combs (Table 5). In the case of old comb, there was significant difference in the percentage of Varroa mite infestation in drone and worker brood cell in all colonies (Table 5). In drone brood cells, the maximum infestation of Varroa mites was 43.75% and the minimum infestation was 26.25%. While in case of worker brood cells, maximum and minimum rate of infestation of Varroa mite was 31.25% and 15.00%, respectively. In a row, the small different letter shows the significant difference between then, and in column large same letter shows no significant difference between them at (Student’s t-test, p < 0.05).
Colony
Rate of Varroa infestation in old combs
Rate of Varroa infestation in new combs
Drone brood infestation (%) Mean ± S. Error
Worker brood infestation (%) Mean ± S. Error
Drone brood infestation (%) Mean ± S. Error
Worker brood infestation (%) Mean ± S. Error
M-101
43.75 ± 2.63 a A
28.75 ± 3.50b AB
33.33 ± 3.33 a A
18.75 ± 3.50b A
4
26.25 ± 3.24 a B
17.50 ± 4.12 a BC
13.75 ± 3.24 a B
10.00 ± 1.18 a A
3
32.50 ± 3.66 a AB
15.00 ± 3.27b C
26.25 ± 3.24 a AB
10.00 ± 2.67b A
18
41.25 ± 4.41 a A
27.50 ± 3.36b AB
20.00 ± 3.78 a B
11.25 ± 3.50 a A
77
43.75 ± 1.83 a A
31.25 ± 2.62b A
35.00 ± 2.67 a A
21.25 ± 2.27b A
73
33.75 ± 3.24 a AB
17.50 ± 2.50b BC
22.50 ± 4.12 a AB
11.25 ± 3.98 a A
In case of new combs, half number of bee colonies showed significantly higher Varroa mite attack in drone brood cells as compared to worker brood which was 33.33%, 26.25%, and 35%, respectively. No significant difference was recorded in colonies (4, 18, and 73) of drone brood cell infested as compared to worker brood cell. On the other hand, there was significant difference within colonies regarded the Varroa mite infestation in worker brood cells (Table 5). While, there was non-significant difference in the percentage of infestation in worker brood cells in all colonies.
4 Discussion
Hygienic behavior is considered as a behavioral defensive response of honey bee workers to remove the dead broods, parasites and inhibit the spreading of infections in the colonies (Rothenbuhler, 1964; Wilson-Rich et al., 2009). In present study, overall data revealed that Italian bees (A. mellifera ligustica) are consistently more efficient in uncapping and removal of dead and infested broods as compared to Carniolan bees (A. mellifera carnica). This is further supported by the higher mite infestation rate in Carniolan bees as compared to Italian bees.
Hygienic behavior is expressed differently among various species, subspecies, honey bee races, and other social insects (Danka et al., 2013; Lazarov et al., 2019; Spivak and Danka, 2020). Rothenbuhler (1964) documented that two genes are responsible for the hygienic behavior in honey bees, one controls the uncapping and the second is responsible for removing the dead brood. Many genetic factors are considered responsible for hygienic behavior in A. mellifera (Moritz, 1988). Recently, the hygienic behavior of A. mellifera ligustica and A. mellifera carnica is compared. These results are in line with (Shakeel et al., 2020) research demonstrating that the uncapping and removal percentage of dead brood is higher after 24 h and 48 h by A. mellifera ligustica. Other previous results compared the hygienic behavior among various honey bee race. For instance, A. mellifera mellifera expressed a higher ability for the removal of dead brood from the comb as compared to A. mellifera carnica (Bąk et al., 2010). Adjlane and Haddad (2014) documented that A. mellifera intermissa removed the 91.56% and 83.55% dead brood, respectively after 24 h. Kamel et al. (2003) reported that the removal percentage of dead brood in A. mellifera jemenitica and A. mellifera carnica were 72.5% and 35.6%, respectively. Balhareth et al. (2012) suggested similar results that the removal percentage of dead broods was significantly higher in A. mellifera jemenitica as compared to A. mellifera carnica over the time of inspection.
The findings (Fig. 2) revealed that the removal percentages of artificially infested brood cells were significantly higher in the Italian bee as compared to Carniolan bee colonies. Similarly, a significant difference was found in the removal of artificially infested brood cells in Italian colonies that have old and newly queen (Saboor et al., 2017). Shrestha et al. (2020) reported that A. mellifera carnica workers uncapped and removed Varroa mite-infested brood significantly faster as compared to sham-manipulated brood (χ2 = 5.5, p = 0.017). In contrast, there was no significant difference reported in response to artificially Varroa mite-infested brood cells in Africanized (χ2 = 0.34, p = 0.56) and Carniolan (χ2 = 0.25, p = 0.63) colonies after the three and seven days of inspection (Aumeier et al., 2000). Aumeier (2001) expressed that the removal rate of infested brood with Varroa mite by Africanized and Carniolan was 30%-40% because the growth of Varroa mite population within the colonies was limited. Vandame (1996) determined that the removal rate in naturally infested brood cells was exceeded more than 40% on average. Those colonies showed slightly higher hygienic behavior and significantly lower Varroa mite infestation on brood and adult honey bees as compared to the un-hygienic colonies (Ibrahim et al., 2007). Bees are more capable to detect and remove the artificially introduced mite from any foreign source because of their different odor (Rosenkranz et al., 1993). Further Bauer et al. (2018) reported that the thermal cues are considered as an important parameters that play a critical role in triggering the hygienic behavior of honey bees toward the Varroa infested brood larvae and pupae.
The results also revealed that the inner width of the Italian comb was significantly different from the inside width of the Carniolan comb that was 5.005 mm and 5.276 mm, respectively. Similarly, Piccirillo and De Jong (2003) reported that Italian comb cells were smaller than Carniolan comb cells that were 5.16 and 5.27 mm, respectively. The Italian comb had a smaller cell size as compared to the Carniolan comb cell. In the context of reproduction behavior, Varroa mite prefers larger cell-sized comb as compared to smaller cell-sized comb. Gonçalves (1995) determined that the Varroa mite prefers the larger cell-sized comb. Smaller cell size (4.9 mm) comb decreases the Varroa mite infestation while the population grows in larger cell size (5.5 mm) combs (Singer et al., 2019). Oddie et al. (2019) found similar results, the mite reproduction rate was significantly reduced in smaller cell size as compared to larger comb cells. (Piccirillo and De Jong, 2003) reported that the Varroa mite infestation in Carniolan comb was a maximum of 24.4% as compared to Italian comb where the mite infestation was 17.7%. Cell size on the foundation may be an important parameter to manage the Varroa mite infestation. In contrast, Maggi et al. (2010) investigated that a non-significant correlation was found between brood cell width and the number of offspring of Varroa mite. Taylor et al. (2008) found non-significant relation to the influence of cell size on mite reproduction behavior but reported that the rate of infestation increases in smaller cell sizes. Berry et al. (2010) reported similar results, overall mite population was higher in smaller cell size (4.9 mm ± 0.08) as compared to larger comb sizes (5.3 mm ± 0.04). These fluctuating outcomes of various studies provide evidences, need to investigate some other parameters that have not been studied regarding Varroa mite population dynamics to the brood cell sizes. It may be possible that the various methods and environmental factors of each study are responsible for the mixed results. Smaller cell size may potentially useful in minimizing the Varroa mite infestation, but may not be the key factor in achieving Varroa free colonies. This study also suggested that honey bee races largely influences the infestation and growth of Varroa mite population in colonies.
The rate of Varroa infestation is higher in drone brood cells than worker broods in both old and new combs (Table 5). Marcangeli and Damiani (2017) documented that a significant difference in Varroa infestation was observed in old and new combs that were 13.52% ± 3.35 and 6.18% ± 2.12, respectively. Varroa mite shows more preference for older combs because of attractant alien scents of brood cells. Infestation rate increased positively with the width of worker and drone brood cells (Maggi et al., 2010). The Varroa infestation was approximately eight times higher in drone brood cells as compared to worker brood (Fuchs, 1990; Santillán-Galicia et al., 2002). Odemer (2020) reported similar results, Varroa mite infestation significantly higher in drone brood cells as compare to worker brood cells. It is necessary to perform more research trails for a better understanding of the Varroa attraction behavior for old and new combs.
5 Conclusion
Hygienic behavior plays a critical role in the colony’s health of various honey bee races. The results obtained in this study demonstrated that the hygienic behavior of the Italian bee was significantly higher in term of removal of pin-killed brood and artificial Varroa mite-infested cell as compared to Carniolan bee colonies. Smaller cell size combs reduced the Varroa infestation than larger cell size combs. Moreover, the infestation rate of Varroa mite was slightly higher in drone brood cells as compared to worker brood cells in both old and new combs. Investigations of other native bee populations may provide a baseline of hygienic bees to use in the future breeding program. Further studies should focus on how the behavior of various honey bee races affect the survival and efficiency of the whole honey bee colonies against Varroa mite infestation. The author acknowledges Saboor Ahmad for data acquiring and helping in preparing the manuscript.
Acknowledgement
The authors extend their appreciation to the Scientific Research Deanship at King Khalid University and the Ministry of Education in KSA for funding this research work through the project number IFP-KKU-2020/5.
Declaration of Competing Interest
The author declares that there is no known competing financial interests or personal relations that could have appeared to influence the work reported in this paper.
References
- In vitro antagonistic potential of gut bacteria isolated from indigenous honey bee race of Saudi Arabia against Paenibacillus larvae. J. Apic. Res.. 2020;59(5):825-833.
- [Google Scholar]
- Immune investigation of the honeybee Apis mellifera jemenitica broods: a step toward production of a bee-derived antibiotic against the American foulbrood. Saudi J. Biol. Sci. 2020
- [CrossRef] [Google Scholar]
- Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi J. Biol. Sci.. 2018;25(2):383-387.
- [Google Scholar]
- Ansari, M.J., Al-Ghamdi, A., Usmani, S., Khan, K.A., Alqarni, A.S., Kaur, M., et al. (2017). In vitro evaluation of the effects of some plant essential oils on Ascosphaera apis, the causative agent of Chalkbrood disease. 24(5), 1001–1006.
- Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie. 2001;32(1):81-90.
- [Google Scholar]
- A comparison of the hygienic response of Africanized and European (Apis mellifera carnica) honey bees to Varroa-infested brood in tropical Brazil. Genet. Mol. Biol. Evol.. 2000;23(4):787-791.
- [Google Scholar]
- Comparison of hygienic behaviour between five honey bee breeding lines. J. Apicult. Sci.. 2010;54(2):17-24.
- [Google Scholar]
- Comparison of hygienic and grooming behaviors of indigenous and exotic honeybee (Apis mellifera) races in Central Saudi Arabia. Int. J. Agricult. Biol. Philos.. 2012;14(6)
- [Google Scholar]
- Recognition of mite-infested brood by honeybee (Apis mellifera) workers may involve thermal sensing. J. Therm. Biol.. 2018;74:311-316.
- [Google Scholar]
- Seasonal cycle of inbreeding and recombination of the parasitic mite Varroa destructor in honeybee colonies and its implications for the selection of acaricide resistance. Infect. Genet. Evol. Dev.. 2017;50:49-54.
- [Google Scholar]
- Small-cell comb foundation does not impede Varroa mite population growth in honey bee colonies. Apidologie. 2010;41(1):40-44.
- [Google Scholar]
- Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie. 1999;30(2-3):141-158.
- [Google Scholar]
- The Transmission of Deformed Wing Virus between Honeybees (Apis melliferaL.) by the Ectoparasitic MiteVarroa jacobsoniOud. J. Invertebr. Pathol.. 1999;73(1):101-106.
- [Google Scholar]
- Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res.. 2013;52(1):1-30.
- [Google Scholar]
- Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie. 2004;35(4):441-448.
- [Google Scholar]
- α-Costic acid, a plant sesquiterpene with acaricidal activity against Varroa destructor parasitizing the honey bee. Nat. Prod. Res. 2019:1-8.
- [CrossRef] [Google Scholar]
- Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol.. 2012;78(4):981-987.
- [Google Scholar]
- Varying congruence of hygienic responses to Varroa destructor and freeze-killed brood among different types of honeybees. Apidologie. 2013;44(4):447-457.
- [Google Scholar]
- Weight loss and other damage to developing worker honeybees from infestation with Varroa jacobsoni. J. Apic. Res.. 1982;21(3):165-167.
- [Google Scholar]
- Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie. 2003;34(1):61-65.
- [Google Scholar]
- Varroa mite evolution: a neglected aspect of worldwide bee collapses? Curr. Opin. Insect Sci.. 2020;39:21-26.
- [Google Scholar]
- Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie. 1990;21(3):193-199.
- [Google Scholar]
- The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie. 2004;35(4):419-430.
- [Google Scholar]
- Effect of the size of worker brood cells of Africanized honey bees on infestation and reproduction of the ectoparasitic mite Varroa jacobsoni Oud. Apidologie. 1995;26(5):381-386.
- [Google Scholar]
- Gonzalez-Cabrera, J., Rodriguez-Vargas, S., Davies, T.E., Field, L.M., Schmehl, D., Ellis, J.D., et al. (2016). Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS One 11(5), e0155332.
- Changes in infestation, cell cap condition, and reproductive status of Varroa destructor (Mesostigmata: Varroidae) in brood exposed to honey bees with Varroa sensitive hygiene. Ann. Entomol. Soc. Am.. 2012;105(3):512-518.
- [Google Scholar]
- Bacteria detected in the honeybee parasitic mite Varroa destructor collected from beehive winter debris. J. Appl. Microbiol.. 2015;119(3):640-654.
- [Google Scholar]
- Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie. 2007;38(1):67-76.
- [Google Scholar]
- Detection of flumethrin acaricide residues from honey and beeswax using high performance liquid chromatography (HPLC) technique. J. King Saud Univ.-Sci.. 2020;32(3):2229-2235.
- [Google Scholar]
- Jamal, Z.A., Abou-Shaara, H.F., Qamer, S., Alotaibi, M.A., Khan, K.A., Khan, M.F., et al. (2021). Future expansion of small hive beetles, Aethina tumida, towards North Africa and South Europe based on temperature factors using maximum entropy algorithm. J. King Saud Univ.-Sci. 33(1), 101242.
- A scientific note on hygienic behavior in Apis mellifera lamarckii and A. m. carnica in Egypt. Apidologie. 2003;34(2):189-190.
- [Google Scholar]
- Insecticidal effect of botanical material for the management of pulse beetle (Caosobruchus chinensis): a step toward ecofriendly control. Fresenius Environ. Bull.. 2020;29(7):5180-5188.
- [Google Scholar]
- Chemical analysis of trace metal contamination in the air of industrial area of Gajraula (UP), India. J. King Saud Univ.-Sci.. 2020;32(1):1106-1110.
- [Google Scholar]
- Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Apidologie. 2002;89(12):565-568.
- [Google Scholar]
- Allozyme genetic characterization of Apis mellifera (Hymenoptera: Apidae) colonies from Bulgaria with different hygienic behaviour. J. Central Eur. Agric.. 2019;20(2):592-597.
- [Google Scholar]
- Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 2010;41(3):353-363.
- [Google Scholar]
- Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review. J. Apic. Res.. 2017;56(4):366-375.
- [Google Scholar]
- Comparative effectiveness of some acaricides used to control Varroa destructor (Mesostigmata: Varroidae) in Algeria. Afr. Entomol. Memoir. 2010;18(2):259-266.
- [Google Scholar]
- Brood cell size of Apis mellifera modifies the reproductive behavior of Varroa destructor. Exp. Appl. Acarol.. 2010;50(3):269-279.
- [Google Scholar]
- Effect of thymol and formic acid against ectoparasitic brood mite Tropilaelaps clareae in Apis mellifera colonies. Pakistan J. Zool.. 2011;43(1)
- [Google Scholar]
- Infestation levels of the mite Varroa destructor (Acari: Varroidae) in new and old honeybee brood combs of Apis mellifera (Hymenoptera: Apidae) Rev. Soc. Entomol. Argentina. 2017;66(1–2)
- [Google Scholar]
- Evaluating the impact of jatropha oil extract against the Varroa mite, Varroa destructor Anderson & Trueman (Arachnida: Acari: Varroidae), infesting honeybee colonies (Apis mellifera L.). Egypt. J. Biol. Pest Control. 2020;30(1):1-7.
- [Google Scholar]
- A reevaluation of the two-locus model for hygienic behavior in honeybees (Apis mellifera L.) J. Hered.. 1988;79(4):257-262.
- [Google Scholar]
- Naeem-Ullah, U., Ramzan, M., Saeed, S., Iqbal, N., Sarwar, Z.M., Ali, M., et al., 2020. Toxicity of four different insecticides against Trilocha varians (Bombycidae: Lepidoptera). J. King Saud Univ.-Sci. 32(3), 1853-1855.
- Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol.. 2016;61(1):417-432.
- [Google Scholar]
- A simplified bioassay for behavioral resistance to American foulbrood in honey bees. Am. Bee. J. 1986
- [Google Scholar]
- Cell size and Varroa destructor mite infestations in susceptible and naturally-surviving honeybee (Apis mellifera) colonies. Apidologie. 2019;50(1):1-10.
- [Google Scholar]
- Reproductive capacity of varroa destructor in four different honey bee subspecies. Saudi J. Biol. Sci.. 2020;27(1):247-250.
- [Google Scholar]
- Changes in a population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie. 2000;31(4):471-478.
- [Google Scholar]
- Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie. 2010;41(6):602-612.
- [Google Scholar]
- The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol.. 1987;49(1):54-60.
- [Google Scholar]
- The influence of brood comb cell size on the reproductive behavior of the ectoparasitic mite Varroa destructor in Africanized honey bee colonies. Genet. Mol. Res. 2003;2(1):36-42.
- [Google Scholar]
- Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci.. 2019;116(5):1792-1801.
- [Google Scholar]
- Breeding for resistance to Varroa destructor in North America. Apidologie. 2010;41(3):409-424.
- [Google Scholar]
- Differential hygienic behaviour towards Varroa jacobsoni in capped worker brood of Apis cerana depends on alien scent adhering to the mites. J. Apic. Res.. 1993;32(2):89-93.
- [Google Scholar]
- Biology and control of Varroa destructor. J. Invertebr. Pathol.. 2010;103:S96-S119.
- [Google Scholar]
- Behavior genetics of nest cleaning in honey bees. IV. Responses of F 1 and backcross generations to disease-killed brood. Am. Zool.. 1964;4(2):111-123.
- [Google Scholar]
- Effect of queen age on hygienic and grooming behavior of Apis mellifera Ligustica against Varroa destructor (Anderson and Trueman) Asian J. Agric. Biol.. 2017;5:113.
- [Google Scholar]
- Efficacy assessment of soft and hard acaricides against Varroa destructor mite infesting honey bee (Apis mellifera) colonies, through sugar roll method. Saudi J. Biol. Sci.. 2020;27(1):53-59.
- [Google Scholar]
- Varroa destructor (Acari: Varroidae) infestation in queen, worker, and drone brood of Apis mellifera (Hymenoptera: Apidae) Can. Entomol.. 2002;134(3):381-390.
- [Google Scholar]
- Comparison of hygienic behavior of exotic honey bee Apis mellifera L. and indigenous honey bee Apis cerana of Pakistan. Sociobiology. 2020;67(1):74-79.
- [Google Scholar]
- Individual-level comparisons of honey bee (Hymenoptera: Apoidea) hygienic behavior towards brood infested with Varroa destructor (Parasitiformes: Varroidae) or Tropilaelaps mercedesae (Mesostigmata: Laelapidae) Insects. 2020;11(8):510.
- [Google Scholar]
- Interactions between honeybees and Varroa mites influenced by cell sizes and hygienic behaviour. Entomol. Generalis. 2019;38(3):255-273.
- [Google Scholar]
- Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie. 1996;27(4):245-260.
- [Google Scholar]
- Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 2020:1-16.
- [Google Scholar]
- Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa: Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World. 1998;79(4):169-186.
- [Google Scholar]
- Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J. Neurobiol.. 2003;55(3):341-354.
- [Google Scholar]
- Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie. 2001;32(6):555-565.
- [Google Scholar]
- The effect of honey bee worker brood cell size on Varroa destructor infestation and reproduction. J. Apic. Res.. 2008;47(4):239-242.
- [Google Scholar]
- Importance de l'hybridation de l'hôte dans la tolérance à un parasite: cas de l'acarien parasite Varroa jacobsoni chez les races d'abeille Apis mellifera européenne et africanisée, en climat tropical humide du Mexique. Lyon. 1996;1
- [Google Scholar]
- Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol.. 2009;54(1):405-423.
- [Google Scholar]







