Translate this page into:
An investigation of physicochemical properties of Nigella sativa L. Seed oil from Al-Qassim by different extraction methods
⁎Corresponding authors at: Laboratory for Biolubricant, Biofuels and Bioenergy Research, Centre for Advanced Materials & Renewable Resources, Faculty of Sciences and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia (M. Alrashidi). monir3090@hotmail.com (Moneer Alrashidi), fir_my@ukm.edu.my (Mohamad Firdaus Yusoff)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An investigation of physicochemical properties of Nigella sativa seed oil from Al-Qassim, KSA was conducted using cold press and Soxhlet extraction methods. The effect of solvent polarity on the oil components and properties were examined using hexane, tetrahydrofuran, ethanol, dichloromethane, methanol and methanol-water binary system. The results demonstrated a high yield of oil with the Soxhlet method using ethanol (40.16%) while the samples extracted with the methanol–water binary system produced the lowest yield (28.28%). Linoleic acid was the major free fatty acid in all samples followed by oleic and palmitic acids. Moreover, the triacylglycerol analysis was carried out using a high-performance liquid chromatography system. The results revealed that the studied oil samples were rich in unsaturated triacylglycerols mostly as 3 Linoleic acid (LLL) but low in saturated triacylglycerols. Thymoquinone, which is known as a powerful antioxidative and antiradical agent was detected in all samples except the sample extracted with the methanol–water binary system. The effect of solvent polarity and the solvent boiling point was observed through the quantity of the main components of each oil sample even though they are from the same source of seed. This study showed that the aforementioned properties were affected by the polarity of the solvent used during the extraction process.
Keywords
Nigella sativa seed oil
Physicochemical properties
Al-Qassim, KSA
1 Introduction
Nigella sativa seed oil has been widely used as a medicinal plant to cure a variety of diseases in many countries. The seed of the plant is identified through various local names according to regions (Liu et al., 2012). The plant has been used as a flavor, a preservative and in folkloric medication for many decades. Likewise, it is considered as a newer source of edible oil (Kiralan et al., 2017). There have been many studies examined the constituents of Nigella sativa seed oil in addition to identifying many important compounds, such as water-soluble vitamins and minerals (Neirgiz and Otles, 1993), phenolic compounds and essential fatty acids (Karacabey, 2016, Kinki, 2020).
Furthermore, substantial amounts of fat-soluble bio-active compounds such as sterols and tocols, phytosterols, polyphenols and tocopherols were also studied (Benkaci-Ali et al., 2012). Besides, other compounds: p-cymene, monoterpenes, thujene, octenol with substantial amounts of flavouring agents including cineole and thymoquinone (Rao et al., 2007). Meanwhile, several studies have investigated the content similarities in triacylglycerols (TAG) and fatty acids of the seed oil, in which complexity in the compounds was varied based on the extraction techniques or place where seeds were grown (Gharby et al., 2015).
Nigella sativa seed oil demonstrated many pharmacological activities such as antiparasitic, antihypertensive, analgesic, antineoplastic, antibacterial properties against hepatotoxicity and nephrotoxicity due to the presence of many active components (Abedi et al., 2017, Ketenoglu et al., 2020).
The comprehensive data on Nigella sativa from Saudi have been limited. (Ramadan, 2002) was studied some aspects of the physicochemical properties of Nigella sativa from the center and south of Saudi. Therefore, the current study is aimed to examine the effect of the extraction methods on the physicochemical properties of Saudi’s black cumin oils using different solvents with different polarity. The outcome of this study is important for processing and utilizing both fixed and volatile oils and by-products.
2 Constituents and procedures
2.1 Constituents
Nigella sativa seeds were obtained from Al-Qassim bazaar, Saudi. The seeds were subjected to cleaning, washing, and air drying before being transported to Malaysia.
2.2 Chemicals and reagents
All chemicals and solvents were used without any further modification. The 99.5% acetonitrile and 99.5% acetone of high-performance liquid chromatography grade were procured from John Townson Baker Chemical Company. The derivative of transesterification of fats and methanol were obtained from BDH and Fluka medical laboratories.
3 Procedures
The oil extraction and preservation of the Nigella sativa seed was conducted using cold pressing (CP), and Soxhlet methods tested with 6 solvents, which had different polarity values.
3.1 Cold pressing method
A mechanical pressing method without any heating treatment was used on the seeds at 25 °C. The separation of the oil phase from fibers was conducted by storing the crushed seeds for one night at ambient temperature. Subsequently, the oil was further filtered using Whatman qualitative filter paper No. 4.
3.2 Soxhlet extraction method
The American Oil Chemists Society (AOCS) method No. 963.15 was applied using the following solvents: hexane, tetrahydrofuran (THF), ethanol, dichloromethane, methanol and methanol–water binary system, for 6 h.
4 Physicochemical property
4.1 Free fatty acid contents
The AOCS scheme Ca 5a-40 was used as a determinant of FFAs. The defused isopropyl alcohol was then combined with a total of 5 g oil sample placed in an Erlenmeyer flask and heated to 40 °C and titrated vs. a standard sodium hydroxide solution. Eqs. (1) and (2) were used to calculate the FFA% and acid value, respectively.
where, V = ml of NaOH; N = NaOH normality (Eq/l); W = sample weight (g) and 2.81 is the number used to change the unit of oleic acid.
4.2 Iodine value (IV)
The AOCS official method Cd 1–25 surplus, of about 0.5 g oil sample was put in a 500 ml flask, with addition of 15 ml alicyclic hydrocarbon, and 25 ml of iodine monochloride solution, which was used to calculate IV of each oil sample. Subsequently, the vacuum flask was placed in a dim area for 60 min following an hour of incubation; then 20 ml 10% KI with 150 ml of boiled steam water was added to the mixture. Later, 1 ml of 1% of starch solution was added, followed by a continuous titration until the cerulean colour disappeared.
where, N = Na2S2O3 normality (Eq/l); Vb = ml of Na2S2O3 used for blank test; VS = ml of Na2S2O3 used for the sample; W = sample weight (g).
4.3 Saponification value
The SV of each oil sample was measured. The mixture in the boiling flask connected to a condenser was boiled for 60 min and 1 ml of phenolphthalein was added. SV was calculated using Eq. (4):
where, Vb = ml of blank; Vs = ml of titrant; W = sample weight (g); N = KOH normality (Eq/l)
4.4 Colour
A predefined procedure using a Lovibond tintometer model F/10508 was adopted to determine the colour of each oil sample. This procedure is based on POIM test method that involves the melting of the frozen oil sample in an oven at 60 °C. Once the liquefied samples were obtained, the colour was determined at 28 °C. Finally, the result was obtained by acquiring the red and yellow indices.
4.5 Refraction index (RI)
The AOCS official method (Cc7-25) was used to determine the RI of each seed oil sample, using a TAGO Co. Ltd.
4.6 Moisture content
The MX-50 moisture analyzer model was used to examine the dampness content of each oil sample. A total of 5 g of the oil sample was poured into a moisture bowl and dehydrated in the moisture analyzer for 30 min at 101 °C.
4.7 Viscosity
The viscosity was measured using a Brookfield model RV DV-I+ (U.S.A), with a Spindle (size 5). The viscosity was read in centipoises directly from the viscometer, which was maintained at 1 min and 100 rpm at 25 °C
4.8 Fatty acid composition
Gas chromatography purchased from Shimadzu equipped with a capillary column was used to measure the content of the fatty acid combined with a programmed column temperature of 120 °C, with a subsequent 3 °C increment per min for 57 min. The injector and detector temperature were set at 260 °C and 280 °C, correspondingly. Helium carrier gas was used with a flow measurement of 0.3 ml/min.
FAME was prepared with base-catalyzed for transesterification oil samples and the higher FAME coating remained was carefully decanted. Approximately 2 g of the sample was dissolved in 1 ml of toluene, followed by the addition of 7.5 ml of methanol and 1.5 ml of the reagent solution.
Following the cool off, 10 ml of hexane combined with 10 ml of water was added to the sample mixture to deduct the fatty acid methyl esters contained in the hexane phase. The hexane phase was dried with anhydrous Na2SO4. The fatty acid content of each sample was determined using their respective FAME and was combined with an analytical separation using gas chromatography (Bahadi et al., 2019).
4.9 Triacylglycerol profile
The analyte in a solvent comprised a mixture of acetone and acetonitrile with 63.5% and 36.5% ratio, fixed at a rate of 1 ml/min. The seed oil sample preparation entailed the dilution of 0.1 ml sample with 1.5 ml acetone to form an acetonitrile mixture. The HPLC was immersed in the mixture and auto-injected with an overall operation time of 35 min (Mohammed et al., 2016).
4.10 GC–MS quantification method
Nigella sativa seed oil constituents were identified by using a GC–MS and DB-Wax column. The oven temperature was programmed at 70 °C for 5 min, then upraised to 230 °C at 2 °C/min and seized for 10 min at 230 °C. EI mass spectra were recorded at 70 eV ionization voltage over the mass range 40–400 u.
5 Results and discussion
5.1 Oil extraction methods
Table 1 illustrates the yields and physicochemical parameters of Nigella sativa seed oil extracted via CP and Soxhlet methods using 6 solvents of different polarity values. The extracted seed oil using ethanol had the highest yield of 40.16% while methanol–water binary system produced the lower oil yield (18.41%). This may be due to the superior ability of ethanol, compared to other solvents, to extract lipid components from the seed. The common yield of Nigella sativa seed oil was in the range of 28–40% (Benkaci-Ali et al., 2012).
CP
hexane
THF
CH3CH2OH
CH2Cl2
CH3OH
mix.
Yield (%)
21.73
32.90
29.19
40.16
23.20
21.16
18.41
Colour
R3Y9
R6Y70
R7Y60B2
R10Y60B2
Y9Y70B7
R10Y60B6
R9Y60B10
FFA (as oleic)%
7.84
13.18
15.63
13.92
16.04
19.28
8.40
PV (meq/kg)
9.42
8.14
11.14
9.17
12.14
13.72
18.13
IV (g I2/100 g oil)
121
123
105
112.1
117
101
87
SV
209.2
188.27
192.15
205.15
198.17
208.18
211.75
RI 25 °C
1.472
1.468
1.470
1.476
1.469
1.454
1.394
Moisture (%)
0.57
1.97
0.83
1.33
1.92
8.47
19.72
Viscosity (MPA S)
75
65
76
71
74
50
29
5.2 Physicochemical properties of Nigella sativa seed oil
The results listed in Table 1, demonstrated a substantial effect of solvent polarity, where low FFAs value were obtained using CP method (7.84%) while methanol, a highly polar solvent yielded a high FFAs value (19.28%). Because polar solvents induce TAGs hydrolysis and saponification reactions that facilitate FFAs formation in vegetable oils (Gharby et al., 2015).
Likewise, lipase enzyme is affected by: the degree of pressing, solvent polarity and the Soxhlet temperature. Furthermore, the solvent boiling temperature influences the lipase enzyme activity (Solati et al., 2014).
The PV results as appeared in Table 1 revealed that the oil sample extracted with hexane had the lowest PV (8.14 meq O2/kg seed oil), however, the PV for solvent-extracted oil samples was greater compared to the CP method (9.42 meq O2/kg oil). The PV for overall oil samples in this research agreed with those reported by (Gharby et al., 2015). Peroxides are unsteady and decay to minor oxidation carbonyls products and are responsible for unfavorable oils flavors.
The lipid with a low IV demonstrates a low unsaturated fatty acid structure and higher oil stability. The IV value of all oils samples were within the reported range except that for oil extracted with methanol–water binary system which was marginally low (87 g I2/100 g oil).
The SV values for all studied seed oil samples were ranged (188.27–211.75) and agreed with (Kiralan et al., 2014) study. The higher SV values suggesting the presence of high TAGs content (Mohammed et al., 2016).
The RI of all the studied samples was within the reported range except the oil sample extracted with the methanol–water binary system that demonstrated a lower value (1.394).
The highest moisture content value (19.72%) was observed for the oil sample extracted with the methanol–water binary system. The other oil samples had moisture% slightly lower than the reported range (5.46–7%). The aforementioned differences may be related to the variants in the polarity of the solvents used.
5.3 Fatty acid composition
Table 2 demonstrates the composition of fatty acids for Nigella sativa seed oil samples. Fig. 1 demonstrates a typical GC-FID chromatogram. The main unsaturated fatty acids were octadecadienoic acid (linoleic) and octadec-9-enoic acid, their combination compose over 70% of the whole fatty acids in each seed oil sample, while hexadecanoic acid and stearic acid were the key saturated fatty acids. The findings were in agreement with (Solati et al., 2014; Piras et al., 2013) studies. The CP method demonstrated a maximum ratio for the total unsaturated fatty acids to the total saturated fatty acids (TU/TS) in oil. This is comparable to the Soxhlet method tested with varied solvents. The TU/TS ratios of oil in this work were higher than the studies of (Ramadan and Mörsel, 2002; and Cheikh-Rouhou et al., 2007). Nevertheless, linolenic acid was not found in the oil sample extracted with methanol–water binary system.
Fatty Acid Composition
CP
Hex
THF
C2H5OH
CH2Cl2
CH3OH
mix
Literature Rohman et al. (2019)
Miristic Acid (C14:0)
0.21
0.24
0.18
0.299
0.171
0.278
0.506
0.13–0.16
Palmitic Acid (C16:0)
13.43
14.51
12.88
15.71
12.31
14.50
18.18
12.90–13.25
Palmitoleic Acid (C16:1)
0.24
0.23
0.220
0.946
0.262
0.243
0.366
0–0.60
Heptadecanoic Acid (C17:0)
0.11
1.16
0.326
0.371
0.252
–
–
0.3–3.29
Stearic Acid (C18:0)
3.11
3.07
3.271
2.941
3.227
2,989
2.442
2.56–2.80
Oleic Acid (C18:1)
22.63
23.36
24.12
22.77
24.07
24.26
22.86
22.63–24.51
Linoleic Acid (C18:2)
56.32
54.14
54.04
54.96
56.28
54,55
54.17
58.90–61.20
Archidic Acid (C20:0)
0.36
0.22
0.234
0.214
0.234
0.268
–
0.13–0.15
Linolenic Acid (C18:3)
0.20
0.17
0.203
0.19
0.203
0.167
–
0.21–0.28
Eicosenoic Acid (C20:1)
0.38
2.08
0.312
–
0.312
0.221
–
0.27–0.35
Eicosadienoi c Acid (C20:2)
2.57
0.22
1.581
4.576
2.487
1.719
1.279
1.86–9.40
Behenic Acid (C22:0)
0.28
0.11
–
–
–
0.115
–
–
Eicosatrienoic Acid (C20:3)
0.11
2.147
–
2.35
0.156
0.239
–
0.30 – 1.10
TUSFAs
83.66
73.54
81.47
84.48
81.128
77.647
68.36
–
TSFAs
15.47
14.94
16.7
13.677
17.643
20.496
18.28
–
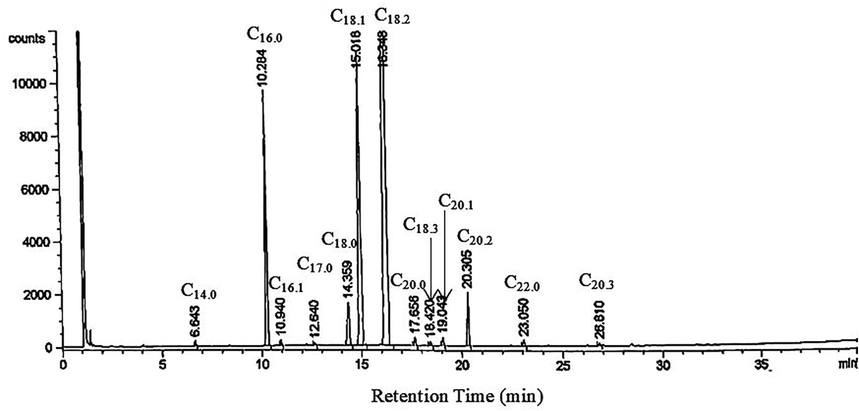
A Typical GC-FID Chromatogram for Nigella sativa Seeds Oil.
5.4 Triacylglycerol profile
Table 3 illustrates the TAG values of each seed oil sample obtained through HPLC, while Fig. 2 illustrates the common TAG profile. The results indicated that seed oil composition had LLL ranging from 6.09 to 17.96% as the key detected TAG followed by OLL and finally PLL. The results were comparable to the results of the GC-FID as reported in Table 2 that demonstrates the composition of fatty acid for Nigella sativa seed oil samples.
Triacylglycerol species
CP
Hex
THF
C2H5OH
CH2Cl2
LLL
17.96
10.55
6.09
9.41
8.63
OLL
2.80
9.48
Nd
8.56
7.62
OLL
15.28
Nd
0.51
Nd
7.92
PLL
15.74
Nd
0.68
0.73
Nd
SLL
1.66
7.47
Nd
2.92
3.47
OOL
5.51
6.72
Nd
0.69
5.29
OOL
9.60
0.89
Nd
Nd
0.63
POL
1.52
Nd
Nd
Nd
Nd
OOO
2.30
1.78
Nd
0.92
0.68
OOO
2.68
Nd
Nd
Nd
0.74
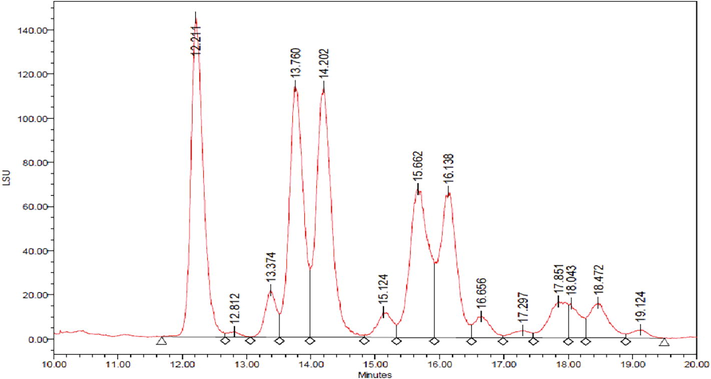
Typical TAG Profile for Oil Sample Extracted with Hexane.
The values of TAG were lower compared to that reported by Khoddami et al. (2011), which in the range of 19.90–20.60% for LLL, (16.07–16.97%) for OLL and (12.40–18.51%) for PLL. Moreover, the oil contains small quantities of SLL, POL and OOO. Similar results were reported by Mazaheri et al. (2019). Alternatively, TAGs were not detected in the oil samples extracted with methanol and the methanol–water binary system. This may be due to the effect of high solvent polarity that may induce TAGs hydrolysis.
5.5 GC–MS analysis
The chemical compositions of Nigella sativa seed oil samples were identified with GC–MS and results were presented in Table 4. The results demonstrated that each oil sample contains different constituents. The highest compounds were obtained with the CP method while the methanol–water binary system yielded the lowest compounds. This may be due to the solvent polarity.
Compound
CP
Hex
THF
C2H5OH
CH2Cl2
CH3OH
mix
αthujane
0.07
–
–
–
–
–
–
Thymoquinone
0.58
0.44
0.36
0.15
0.68
0.15
–
2-Decenal
0.20
–
0.07
0.02
–
–
–
2,4-Decadienal
0.23
0.06
0.05
0.02
0.05
–
–
Thymol
0.14
0.09
0.07
0.03
0.08
0.05
–
2,4-nonadienal
0.07
–
0.02
–
–
–
Longifolene
0.07
0.12
0.09
0.04
0.13
0.05
–
Naphthalene, 2-butyldecahydro
0.07
Phenol, 2-methyl-5-(1-methylethyl)-
–
–
0.02
0.04
–
–
–
Dodecanoic acid
0.08
–
0.22
–
–
–
–
1,9-Tetradecadiene
0.19
–
0.04
–
–
–
–
Dimethylcyclohexanol
0.40
–
–
–
–
–
–
Methyl stearate
–
–
0.03
0.02
0.06
1.16
0.86
3-octadecyne. 7-Hexadecyne
1.89
1.41
1.33
0.44
1.32
0.79
15.8
Indole
0.04
1H-Pyrido[4,3-b]indole, 2,3,4,4a
0.09
2-Methyl-Z,Z-3,13-octadecadienol
0.08
Isopropyl linolate
–
–
–
–
0.24
–
–
Glycerol 1-palmitate
0.21
0.22
0.11
0.09
0.39
9,17-Octadecadienal
0.81
4.75
1.30
0.69
0.47
1.83
–
9-Octadecenal
0.39
0.41
0.53
0.32
0.33
0.67
22.9
The oil samples extracted with CP, hexane, THF, ethanol, CH2Cl2 and methanol produced different amounts of thymoquinone: 0.58, 0.44, 0.36, 0.15, 0.68 and 0.15%, respectively. The results were in agreement with studies by Kokoska et al. (2008) and Nickavar et al. (2003). But the results of this study were lower than those recorded by Krishnaven and Saranya (2016) and Ghahramanloo et al. (2017). Furthermore, other constituents detected in oil samples in different percentages: 2-Decenal (0.02–0.2%), 2,4-Decadienal (0.02–0.23%), Thymol (0.03–0.14%), Longifolene (0.04–0.13%), Indole (0.04%) and 9-Octadecadienal (0.39–22.9%).
6 Conclusion
This study revealed the viability in the composition and physical and chemical properties of Nigella sativa seeds oil from Al-Qassim, KSA. Whereby, the aforementioned properties are affected by the polarity of the solvent used during the extraction process. Soxhlet method using ethanol produced the highest yield (40.16%) of FFA. All oil samples demonstrated a high ratio of unsaturated per saturated fatty acids. The major fatty acids detected were linoleic and oleic acid in the seeds oil samples. Moreover, the samples were rich with unsaturated TAG but low in saturated TAG. The oil samples extracted with dichloromethane had the highest amounts of thymoquinone at 0.68%. The results of the current study could aid in the selection of the ideal solvents for the extraction of Nigella sativa seeds oil for industrial applications. Moreover, the presence of many bioactive compounds in oil samples suggesting the medicinal uses of the Nigella sativa seed oil. Notably, additional studies are required to explore new applications of this plant.
Acknowledgements
One of us (Moneer Alrashidi) is glad to acknowledge the Community College, Taibah University, Medina, Saudi Arabia for granting a scholarship for PhD studies. Authors also thank Laboratory for Biolubricant, Biofuels and bioenergy Research, Centre for Advanced Materials & Renewable Resources, Faculty of Sciences and Technology, Universiti Kebangsaan Malaysia, 43600, Bangi, Selangor for providing laboratory facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Microwave-assisted extraction of Nigella sativa L. essential oil and evaluation of its antioxidant activity. J. Food Sci. Technol.. 2017;54(12):3779-3790.
- [Google Scholar]
- Physicochemical characteristics of Malaysian crude palm kernel oil. Malaysian J. Chem.. 2019;21:17-27.
- [Google Scholar]
- Chemical composition and physicochemical characteristics of fixed oils from Algerian Nigella sativa seeds. Chem. Nat. Compd.. 2012;47(6):925-931.
- [Google Scholar]
- Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem.. 2007;101:673-681.
- [Google Scholar]
- Comparative analysis of essential oil composition of Iranian and Indian Nigella sativa L. extracted using supercritical fluid extraction and solvent extraction. Drug Des. Devel. Ther.. 2017;2221–2226
- [Google Scholar]
- Chemical investigation of Nigella sativa L. seed oil produced in Morocco. J. Saudi Soc Agric. Sci.. 2015;14(2):172-177.
- [Google Scholar]
- Optimization of microwave-assisted extraction of thymoquinone from Nigella sativa L. seeds. Maced. J. Chem. Chem. Eng.. 2016;35(2):209.
- [Google Scholar]
- Cold pressed black cumin (Nigella Sativa L.) seed oil. Cold Pressed Oils 2020:53-64.
- [Google Scholar]
- Physicochemical characteristics of Nigella sativa seed oil as affected by different extraction methods. J. Am. Oil Chem. Soc.. 2011;88(4):533-540.
- [Google Scholar]
- Physico-chemical characteristics of released and improved black cumin (Nigella sativa) World Scientific Res.. 2020;7(1):1-4.
- [Google Scholar]
- Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind. Crops Prod.. 2014;57:52-58.
- [Google Scholar]
- Blends of cold pressed black cumin oil and sunflower oil with improved stability: A study based on changes in the levels of volatiles, tocopherols and thymoquinone during accelerated oxidation conditions. J. Food Biochem.. 2017;41(1):1-10.
- [Google Scholar]
- Comparison of chemical composition and antibacterial activity of Nigella sativa seed essential oils obtained by different extraction methods. J. Food Protect.. 2008;71(12):2475-2480.
- [Google Scholar]
- phytoconstituent analysis of Nigella sativa seeds using analytical techniques. Bull. Environ. Pharmacol. Life Sci.. 2016;5:25-38.
- [Google Scholar]
- Characterization of secondary volatile profiles in Nigella sativa seeds from two different origins using accelerated solvent extraction and gas chromatography-mass spectrometry. Biomed. Chromatogr.. 2012;26(10):1157-1162.
- [Google Scholar]
- Acomprehensive review of the physiochemical, quality and nutritional properties of nigella sativa oil. Food Rev. Int.. 2019;35(4):342-362.
- [Google Scholar]
- The effects of different extraction methods on antioxidant properties, chemical composition, and thermal behavior of black seed (Nigella sativa L) Oil: Evidence-based complement. Altern. Med. 2016:1-10.
- [Google Scholar]
- Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Zeitschrift für Naturforschung C.. 2003;58(9–10):629-631.
- [Google Scholar]
- Chemical composition and in vitro bioactivity of the volatile and fixed oils of Nigella sativa L. extracted by supercritical carbon dioxide. Ind. Crops Prod.. 2013;46:317-323.
- [Google Scholar]
- Ramadan MF, Mörsel JT., 2002. Characterization of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nah Food 46:240–244.
- Comparative evaluation of SFE and solvent extraction methods on the yield and composition of black seeds (Nigella sativa) J. Liq. Chromatogr. Relat. Technol.. 2007;30(17):2545-2555.
- [Google Scholar]
- Nigella sativa oil: Physico-chemical properties, authentication analysis and its. Food Res.. 2019;3:628-634.
- [Google Scholar]
- Antioxidant property, thymoquinone content and chemical characteristics of different extracts from Nigella sativa L. seeds. J. Am. Oil Chem. Soc.. 2014;91(2):295-300.
- [Google Scholar]







