Translate this page into:
An in-vitro anti-inflammatory and anti-microbial essential on Ni(II), Cd(II) mixed ligand complexes by using 2,4-dinitrophenyl hydrazine and dimethylglyoxime
⁎Corresponding author. muthanayuv22@gmil.com (M. Muthuppalani),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The objective of the present work deals among synthesis as well as characterization of Ni(II), Cd(II) mixed ligand complex were derived from 2,4 di-nitrophenyl hydrazine and dimethyl glyoxime. The mixed ligand metal complexes were prepared via 2,4-dinitrophenyl hydrazine as well as dimethylglyoxime among Ni(II) and Cd(II) in molar radio (M:L1:L1) 1:1:1. CHNO analysis, conductivity measurement, PXRD, SEM, UV–visible, infrared spectrum, and magnetic susceptibility, thermal analysis were used to characterized the synthesized compounds. According to the study of the results, all of the metal complexes have octahedral geometry. The molar conductance value indicate that non-electrolytic character of compounds Ni(II), Cd(II). According to the powder X-ray diffraction (PXRD) patterns, all of the compounds are crystalline character. Moreover the Ni(II), Cd(II) complexes were very stable in terms of thermal stability. Metal complexes have been tested against Gram-positive (S. aureus, B. subtilis and A. niger) and Gram-negative (P. aeruginosa, E. coli and C. albicans) pathogenic bacteria as well as fungus. As a result, the metal complexes have reported strong antimicrobial efficiency.
Conclusions
Furthermore, applications of metal compounds were tested for anti-inflammatory efficiency utilize egg albumin. Result shows that the activities of metal complexes are good.
Keywords
Ni(II)-DNPH-DMG complex
Cd(II)-DNPH-DMG complex
Thermal analysis of mixed ligand metal complexes
Antimicrobial
Anti-inflammatory assay
1 Introduction
Many researchers are focusing on mixed chelating metal complexes because they are more important than ever in pharmacological applications such as antimicrobial and fungal, antitumor, anti-inflammatory, and anticancer (Siddiki et al., 2021). Recent evaluations in the fields of bioinorganic and medicinal chemistry contain enhanced the coordination chemistry of transition metal ions using several verity of ligands (Hossain and Banu, 2019). In an important field of research, transition elements play a vital part in the use of transition metals complexes as medicines for the treatment of numerous disorders (Muthuppalani et al., 2022). Metal complexes that are stable and safe among active metal sites are useful as biological probes (Raman et al., 2012). The environment roughly the metals centre, such as coordination structures, ligands, and donator classes, is one of the most important aspects for metallo enzymes to accomplish their specific physiological function (Sujamol et al., 2010). The 2,4-dinitrophenylhydrazine (DNPH) is an important component in biological and pharmacological processes (Devi et al., 2010). It is a more over essential class of medicines counterpart of H2NNH2 (hydrazine) (Nasiru, 2012). N (Nitrogen) and O (Oxygen) can be brought together since they are both beneficial to bio-inorganic processes (Journal, 2011).Dimethylglyoxime was among the most widely utilised ligand for coordinating nickel ions in priority, which also forms Ni-DMG compounds due to attribute specificity and consistency (Padma Rao et al., 2013). The oxime molecule (>C=N–OH) is amphoteric, with slightly basic nitrogen as well as moderately acidic hydroxyl groups, and is thought to be formed from oxy-imine. Many transition metals in the Periodic Table react on dioxime ligands to generate extremely reliable complexes. Furthermore, the Dioximes can coordinate through the oxime groups' N, N or N, O sites. As a result, some Dioximes compounds showed considerable anti - carcinogenic and therapeutic efficacy (Shaker, 2010).
The current research has developed Ni(II) and Cd(II) compounds utilizing 2,4-dinitrophenylhydrazine (DNPH) with dimethyl glyoxime (DMG),moreover physico-chemical characteristics and also to investigate the biological capability of DNPH with compounds of Ni(II), Cd(II).
2 Experimental
2.1 Materials
All of the chemicals have been obtained from commercial sources and used without additional purified process (NiCl2·6H2O, CdCl2·6H2O and dimethyl glyoxime (DMG) from Merck as well as 2,4-dinitrophenylhydrazine from Sigma Aldrich, and also solvents such as dimethyl sulfoxide (DMSO), dimethyl formaldehyde (DMF), Ethanol, Acetone, as well as Methanol, utilized as an AnalaR grad.
2.2 Physicochemical characterization
The Elementar Vario EL III CHNO analyzer was used to accomplish the elemental analysis. A conductivity evaluation became observed from (Equip – Tronics, eq-661A) analyser at room temperature in DMF medium (10−3 M). Infra-red spectrum was obtained utilising Agilent spectrophotometer in the wavelength from 4000 to 400 cm−1 based on the KBr pellet method. UV–visible absorption spectra were collected to use a JASCO V-630 with a 1 nm optical resolution with wavelengths range between 300 and 800 nm, whereas PXRD patterns were obtained using a Shimadzu PW3050/60 with CuK1 = 1.5406 and CuK2 1.5444. Thermal analysis was carried out (Perkin Elmer thermal analyzer) and the SEM photographs were taken with a ZEISS apparatus. Furthermore the magnetic behavior evaluated from (Model MsB-MK1) using Guey’s Balance.
2.3 Preparation of Ni(II), and Cd(II) complexes
Mixed ligand Ni(II) as well as Cd(II) complexes have been obtained via adding a hot DMF solution of (10 mM) DNPH dropwise to an EtOH solution of Nickel(II), Cadmium(II) chloride (10 mM) and ethanol solution of (10 Mm) DMG mixture (1:1:1) ratios. At 60 °C, the reaction mixture was stirred in addition to refluxed for four hours. Finally, a colored complexes Scheme 1 precipitation was obtained and rinsed with EtOH solutions before even being dried under vacuum onto anhydrous CaCl2 desiccators.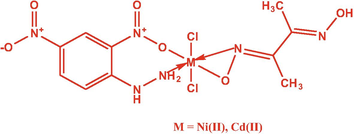
Schematic representation of Ni(II) and Cd(II) mixed ligand complexes.
2.4 In-vitro antimicrobial studies
Disc dispersal was utilized to investigate the antimicrobial potential of Ni(II) and Cd(II) complexes against a variety of gram (positive, negative) bacteria as well as fungals (C. albicans, A. niger, B. subtilis, S. aureus, P. aeruginosa and E. coli). Ni(II), Cd(II) components was diluted in DMSO. Individually, the solution variations (30, 60 µg/ml) were set up. The prepared disks were placed in petri plates containing nutrient medium seeded through each bacterial and fungal serum individually after being dipped in a specific variant of complexes. Every plat was incubated at 37 °C for 24 and 48 h for bacterial and fungal growth, as well as the zone of inhibition value was recorded (Aly and Fathalla, 2020; Shukla and Mishra, 2019).
2.4.1 Microorganisms
The microorganisms species employed in the biological tests included E. coli (MTCC 732), Staphylococcus aureus (MTCC 3160), Pseudomonas aeruginosa (MTCC 424), and Bacillus subtilis (MTCC 441), while the fungal species are Aspergillus niger (MTCC 10180), as well as Candida albicans (MTCC 10183). The Institute of Molecular Biology and biotechnology (IMTECH) in Chandigarh, India, provided the MTCC (microbial variety cultured collections).
2.4.2 Preparation of dried filter paper discs
Filter papers (No. 1) have been used to form disc with a 6 mm diameter that were sterilised in warm air. Upon sterilisation, discs was supplied containing 30 µg/ml and 60 µg/ml from each sample, with a 60 µg/ml chloramphenicol as well as fluconazole standard stock solution and a 60 µg/ml control solution being used correlate the sample solution. They are preserved cold even though they were being used in the test.
2.4.3 In-vitro antimicrobial evaluations
Antimicrobial was accomplished via disc dispersal technique utilising tests. 30 ml of Nutrient agar (NA) medium as well as PDA (Potato dextrose agar) standard was poured into petri plates. Using a micropipette, the experiment organism were injected on consolidated agar platter and distributed for 10 mints before drying. Bacteria from a broth culture were used to isolate the surface areas of the medium. Before evenly inoculating the whole facade of the NA/PDA platter, a sterilized cotton brush is soaked within a standardised microbiological experiment mixture. In a nutshell, inoculums of microbe’s embrace were diffuse on agar platter. The germ-free filter sheets (six millimeter width) comprising disks have been loaded to 30 μg/ml as well as 60 μg/ml of every sample, 60 μg/ml of control, and 60 μg/ml from each sample utilising sterile forceps. A standard solution was placed on the area of the infused agar platter. Bacterial strains were infused on 37 °C at 24 h, whereas fungal strains were incubated for 48 h. Each sample was examined three times.
2.4.4 Measurement of inhibition zones
The antibacterial property of the evaluation substances had been calculated using the mean diameter of the inhibition zones around the disc in mm. A millimeter scale was used to assess the inhibition zones of the microorganisms evaluated by the substances.
2.5 Anti-inflammatory properties of metal complexes
2.5.1 Inhibition of albumin denaturation
0.2 ml new hen's egg albumin, 2.8 ml phosphate buffered saline (PBS, pH 6.4), as well as 2 ml of different metal compound ratios (100, 200, 300, 400, and 500 μg/ml) were included in the reaction mixture (5 ml). As a control, similar amounts of double-distilled water were used. The mixes were then heated on 70°Celsius for five minutes behind being incubated at (37 °C) in an incubator for 15 min. After cooling, the absorbance was in use at 660 nm to use a solution as a control. For the purposes of evaluating absorbance, diclofenac sodium was used as a reference drug, with final concentrations ranging from 100 to 500 μg/ml.The percentage inhibitions of protein denaturation were determined using the method (Hasan, 2019):
Vc = absorbance of control.
Vt = absorbance of experiment sample.
Three different tests were conducted in triplicate. The quantity of sample required inhibiting concentration by 50%, or IC50, was graphically calculated using Ms-Windows based graph pad Instate software using a linear regression technique. The result was represented graphically as average standard deviation.
3 Result and discussion
The physical characteristics of metal complexes, together with their analytical dates, are given through Table 1. A Ni(II), Cd(II) compounds were also stabilised at room temperature. The complexes are solubility using DMF and DMSO, but not in other organic solvents. The empirical values of C.H.N.S. analysis of ligand and metal compounds are in good agreement with theoretical computed values, with complexes ratios of 1:1:1 between metal and ligands.
Complexes
M.F
MW
Color of the complexes
% of yield
Elemental analysisFount/(calculated)
%
%C
%H
%N
%O
%Cl
% Metal
[Ni(DNPH)(DMG)Cl2]
C10H13NiCl2N6O6
442.85
Red
84
27.12
27.242.96
2.9318.98
18.8821.68
21.6416.01
16.0313.25
13.26
[Cd(DNPH)(DMG)Cl2]
C10H13CdCl2N6O6
496.56
brown
72
24.19
24.152.64
2.6816.92
16.8719.33
19.3114.28
14.2622.64
22.59
3.1 Molar conductance
Molar conductance values of the metal complex were measured at 1 × 10−3 M accumulation of DMF solvent as well as entire compounds showed conductance in the range of 25.47–20.39 Ω−1 cm2 mol−1 at 37 °C was shown in (Table 2). The lowest conductivity value suggested that Ni(II) and Cd(II) complexes were non-electrolytes (Manimaran et al., 2021).
Complex
λmax
(nm)Band assignments
Geometry
Magnetic Moment (B.M)
Λm
Ω−1cm2mole−1
[Ni(DNPH)(DMG)Cl2]
384
465n–π*LMCT
(3A2g–3T1g(P))Octahedral
5.1
25.48
[Cd(DNPH)(DMG)Cl2]
379
452n–π*
LMCTOctahedral
Diamagnetic
19.39
3.2 FT-IR spectra analysis of nickel(II) and Cd(II) mixed ligand complexes
FTIR spectrum of the Ni(II) and Cd(II) mixed ligand complexes, to investigated mode of bonding DNPH and DMG ligands to Ni(II), Cd(II) ion. Fig. 1 shows the characteristic bands of a infrared spectra of mixed ligand metal complexes. FTIR spectrum of DNPH (2,4-dinitrophenyl hydrazine)revealed sharp peaks at 3324 cm−1 due to ν(N–H) wavelength shifted to the a higher frequency on 3350–3310 cm−1 shows nitrogen was involved in the coordination of the center metal atom. The ν(NO) band was shows on 1320 cm−1 were observed from DNPH, furthermore these band was shift toward the lower in 25–21 cm−1 in the complexes. These shifts be able to explained by the ligand was coordinated with metal ions through oxygen (Anantha Lakshmi et al., 2008). At lower wavelength 3100–3092 cm−1 the wide band about 3211 cm−1, which was assigned to v(O–H) stretching modes in DMG, was detected on metal complexes spectrum. The impact of coordination of the oxygen atom of the OH group following deprotonation was attributed to this observation (Devi et al., 2018). The lower frequency some new band appear in metal complexes observed at 475–416 cm−1 and 559–530 cm-1that has related to M–N and also M–O significantly (Adly et al., 2020; Ferraz et al., 2009).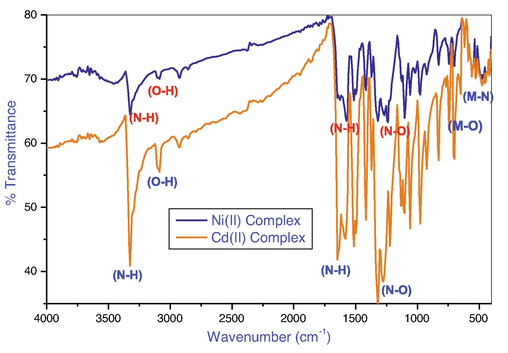
FTIR Spectrum of Ni(II) and Cd(II) mixed ligand complexes.
3.3 Electronic spectral data and magnetic moments
The electronic spectrum of Ni(II) and Cd(II) mixed ligand complexes data illustrated in Table 2 and Fig. 2. The electronic spectra of the Ni(II) complex exhibits a strong absorption band at 384, 465 nm which is according to n → π* and 3A2g–3T1g(P). Transitions imply that the metal ion is surrounded by an octahedral geometry. The magnetic moment of the Ni(II) complex measured 3.14B.M, confirming the metal complex's octahedral geometry(Anantha Lakshmi et al., 2008).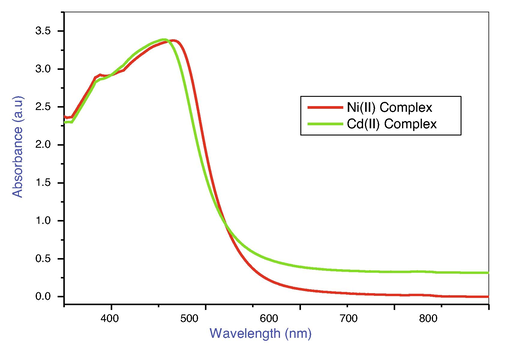
Electronic Spectrum of Nickel(II) and Cadmium(II) mixed ligand complexes.
The absorption band at 379, 452 nm in the electronic spectra of the Cd(II) complex may be ascribed to the n → π* and LMCT. Transitions imply that the metal ion is surrounded by an octahedral geometry. The diamagnetic nature of the Cd(II) complex may be seen in its magnetic moment value(Sulekh Chandra and Monika Tyagi, 2009).
3.4 PXRD analysis
Fig. 3a and b shows the PXRD (powder X-ray diffraction) study of Ni(II), Cd(II) compounds in the range of 2ϴ = 10–800. It’s described as prominent crystalline peaks seen in metal complexes that reveal the complexes’ crystalline nature. Debye Scherrer’s equation was used to compute the crystallite size of the complexes (Netalkar et al., 2014). where
Constant as 0.9, λ = (Cu Kα = 1.5406 Å) wavelength of X-ray radiation, β = Full-width half maximum (FWHM) and θ = diffraction angle. The average crystallite sizes of the Ni(II) and Cd(II) complexes are around 56.74 and 69.34 nm respectively.
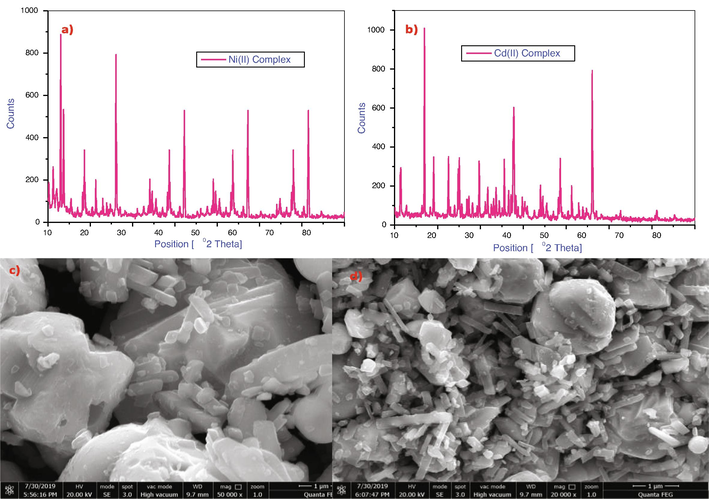
- PXRD patterns of a) Ni(II) complex, b) Cd(II) complex and SEM spectrum of c) Ni(II) complex, d) Cd(II) complexes.
3.5 Morphological observations
The SEM micrographs revealed a Ni(II) and Cd(II) complex are given in Fig. 3c and d, spherical-like structure with particle diameter of estimated 55 and 65 µm, with in the scale bar 1 µm respectively. All these metal complexes surfaced to have showed an aggregated rod-like arrangement and slightly cluster nature.
3.6 Thermal property of metal complexes
TG (Thermogravimetric) as well as DTG (differential thermogravimetric) assessment of Ni(II), Cd(II) complexes curves are shows in Fig. 4a and b. Thermal dissolution of complexes of characteristic [M(L)2] consists of three phases: M = Ni(II), Cd(II), and n = 1, 2, 3. The first process corresponds to the loss of moisture in the temperature varied 30–100 °C; the important phase of decomposition takes place among 200 and 157 °C, with a mass reduction of estimated 75.4%, 80.3% of the ligand; and the final phase occurs up to 201–340 °C and 201–400, with the residual ligands being dropped. After several stages of decomposition, the complexes were transformed to their correlating metal oxides at extreme temps.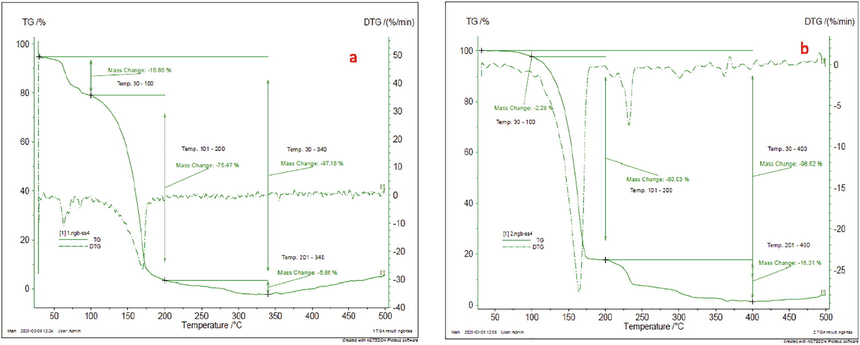
TG/ DTG Cure of a) Ni(II) complex and b) Cd(II) complex.
3.7 In-vitro antimicrobial efficiency of nickel and cadmium complexes
The result of antimicrobial behavior of Ni(II) and Cd(II) complexes by using disc diffusion method is summarized Table 3. Synthesized metal complexes good active screened against gram positive and gram negative bacterial and fungal. The Ni(II) complex good active against the bacteria B. subtilis and E. coli in 30 as well as 60 µg/ml and less active against fungal species. Furthermore, the Cd(II) complex high activities against bacteria p. aeruginosa and fungi C. albicans in 60 µg/ml. The complexes had stronger antimicrobial property in higher concentration are shows in Fig. 5a. The higher activities of metal compounds are explain using the chelation theory (Gupta and Fahmi, 2016; Manimohan et al., 2020), which states that chelation reduces the polarity of metal ions by exchanging a portion of their positive charge by the ligand. This enhances lipid solubility, making it easier for it to permeate into microorganisms normal cells (Saddam Hossain, 2018; Tadavi et al., 2020). Organic ligands have donor sites which are active against the bio-potential activities the enhanced biological activities due to the presence of oxygen as well as nitrogen donor sites in DMG, DNPH.
Organism Name
Standard
(60 µg/ml)Ni(II) complexes
Cd(II) complex
30 µg/ml
60 µg/ml
30 µg/ml
60 µg/ml
Bacillus subtilis
24
15
17
12
18
Staphylococcus aureus
23
10
15
10
20
Pseudomonas aeruginosa
21
11
13
–
12
Escherichia coli
34
11
19
13
16
Aspergillusnigre
15
9
10
10
12
Candida albicans
25
2
5
15
18
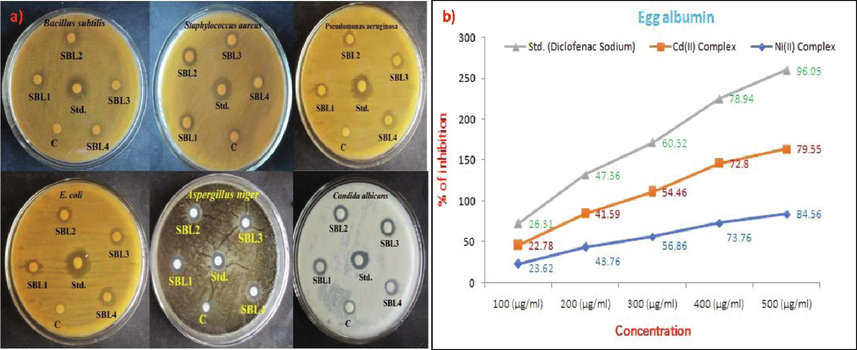
a) Antimicrobial Activities of Ni(II) and Cd(II) complexes and b) anti-inflammatory activity of metal complexes.
3.8 In-vitro anti-inflammatory activities
An anti-inflammatory behavior of Ni(II), Cd(II) compounds was investigated in protein (bovine serum albumin) denaturation technique used with various concentration (100, 200, 300, 400 & 500 μg/ml), diclofenac sodium used as a reference drug. Table 4 as well as Fig. 5b indicate the % of anti-inflammatory efficiency was computed moreover compared to the reference drug. Three part trial were carried out as well as IC50 was calculated. The results indicating the diclofenac sodium (96.05%) IC50 is 230.75. A Nickel compound (84.56%) IC50 value is 257.31 superior protein denaturation compound compare to the Cadmium complex (79.55%) IC50 value is 270.83. the metal complexes lower activity comparing to standard drug. Due to the occurrence of donor (‘N’, ‘O’) places into the DNPH and DMG contained in the compounds which demonstrate the good potential of complexes. The order of anti-inflammatory activity diclofenac sodium > Ni(II) complex > Cd(II) complexes (Szczepaniak and Fichna, 2019).
Samples
% of inhibitions
IC50 value (µg/ml)
100 (µg/ml)
200 (µg/ml)
300 (µg/ml)
400 (µg/ml)
500 (µg/ml)
Ni(II) Complex
23.62 ± 0.23
43.76 ± 0.10
56.86 ± 0.05
73.76 ± 0.09
84.56 ± 0.11
257.31
Cd(II) Complex
22.78 ± 0.20
41.59 ± 0.22
54.46 ± 0.37
72.80 ± 0.14
79.55 ± 0.35
270.83
Std. (Diclofenac Sodium)
26.31 ± 1.84
47.36 ± 3.31
60.52 ± 4.23
78.94 ± 5.52
96.05 ± 6.72
230.75
4 Conclusion
In this artwork, Ni(II), Cd(II) mixed ligand complexes were satisfactorily synthesised and characterised employing a multitude of physicochemical approaches. All complexes exhibited 1:1 electrolyte in essence and metal complexes was initiate to have an octahedral geometry based on magnetic moment, colour, UV–vis spectral, TG inferences. And powder XRD analysis, which shows that the average crystalline diameters are between 56.74 and 69.34 nm. The mixed ligand complexes were extremely stable in terms of thermal stability. Thus the study found that the mixed ligand Ni(II) and Cd(II) complexes have significant antibacterial action when compared to conventional antibiotics. Furthermore the Ni (II), Cd(II) complexes have showed excellent inflammation properties compared to the standard diclofenac.
Acknowledgements
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project, under grant no. (KEP-40-130-42).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, spectroscopic, X-ray diffraction, antimicrobial and antitumor studies of Ni(II) and Co(II) complexes derived from 4-acetyl-5,6-diphenyl-3(2H)-pyridazinone and ethylenediamine. J. Mol. Struct.. 2020;1219:128607
- [CrossRef] [Google Scholar]
- Preparation, characterization of some transition metal complexes of hydrazone derivatives and their antibacterial and antioxidant activities. Arab. J. Chem.. 2020;13:3735-3750.
- [CrossRef] [Google Scholar]
- Synthesis and structural studies of first row transition metal complexes of N-(2-nitro)-benzilidine-3-hydrazino quinoxaline-2-one. Bull. Chem. Soc. Ethiop.. 2008;22
- [CrossRef] [Google Scholar]
- Synthesis and characterization of transition metal(II) Schiff bases complexes derived from 2,5-dihalosalicylaldehyde and 4-methyl-3-thiosemicarbazide. Asian J. Chem.. 2018;30:2445-2449.
- [CrossRef] [Google Scholar]
- Structural and optical characterization studies on 2, 4- dinitrophenylhydrazine single crystal. J. Miner. Mater. Charact. Eng.. 2010;09:321-330.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and biological activity studies of mixed ligand complexes with Schiff base and 2,2’-bipyridine. Int. J. Appl. Sci. - Res. Rev. 2019;6:1-7.
- [Google Scholar]
- Copper(II) complexes with 2-pyridineformamide-derived thiosemicarbazones: Spectral studies and toxicity against Artemia salina. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2009;73:140-145.
- [CrossRef] [Google Scholar]
- Co(II) and Ni(II) complexes with Schiff base ligands: Synthesis, characterization, and biological activity. Russ. J. Gen. Chem.. 2016;86:1182-1190.
- [CrossRef] [Google Scholar]
- Evaluation of in vitro and in vivo therapeutic efficacy of Ribes alpestre Decne in Rheumatoid arthritis. Braz. J. Pharm. Sci.. 2019;55
- [CrossRef] [Google Scholar]
- The preparation and characterization of some metal complexes with tridentate ONO ligand derived from phenyl hydrazine. Baghdad Sci. J.. 2011;8:796-805.
- [CrossRef] [Google Scholar]
- M.R.K. and S.L.B. Padma Rao. Ch. V., Praveen K., Kishorebabu B., Padma M., Anna Sudheer K., Sandhya Rani K., Koteswarao K., Suseelabai G., V.R.B., 2013. Zinc dimethylglyoxime complexes. Der Pharma Chem. 5, 280–284.
- Synthesis, spectral characterization and biological activities of Co(II) and Ni(II) mixed ligand complexes. Molecules. 2021;26:823.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterisation and biological activities of novel biomaterial/N, N, O donor tridentate Co (II), Ni (II) and Zn (II) complexes of hydrazide based biopolymer Schiff base ligand. J. Inorg. Organomet. Polym. Mater.. 2020;30:4481-4495.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and bio-potential activities of Co(II) and Ni(II) complexes with O and N donor mixed ligands. Crystals. 2022;12:326.
- [CrossRef] [Google Scholar]
- Complexes of cobalt(Ii), nickel(Ii) and zinc(Ii) with Schiff bases derived from 4-anisaldehyde. Int. J. Pharm. Sci. Res.. 2012;3:5116-5120.
- [Google Scholar]
- Nickel(II) complexes of thiosemicarbazones: synthesis, characterization, X-ray crystallographic studies and in vitro antitubercular and antimicrobial studies. Transit. Met. Chem.. 2014;39:519-526.
- [CrossRef] [Google Scholar]
- DNA binding mode of novel tetradentate amino acid based 2-hydroxybenzylidene-4-aminoantipyrine complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2012;96:698-708.
- [CrossRef] [Google Scholar]
- Metal complexes as potential antimicrobial agent: A review. Am. J. Heterocycl. Chem.. 2018;4:1.
- [CrossRef] [Google Scholar]
- Preparation and spectral properties of mixed-ligand complexes of VO(IV), Ni(II), Zn(II), Pd(II), Cd(II) and Pb(II) with dimethylglyoxime and N -acetylglycine. E-Journal Chem.. 2010;7:S580-S586.
- [CrossRef] [Google Scholar]
- Metal complexes used as anti-inflammatory agents: Synthesis, characterization and anti-inflammatory action of VO(II)-complexes. Arab. J. Chem.. 2019;12:1715-1721.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterization, thermal behavior and biological activities study of ternary metal complexes of alanine and 1,8-diaminonapthalene with Co(III), Ni(II), Cu(II), Zn(II) and Cd(II) Mater. Today Proc.. 2021;46:6374-6381.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization, electrochemical behaviour and thermal decomposition studies of some transition metal complexes with an azo derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2010;75:106-112.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activities of n-s donor ligand and its transition metal complexes. Int. J. Chem. Sci. 2009;7:116-124.
- [Google Scholar]
- Organometallic compounds and metal complexes in current and future treatments of inflammatory bowel disease and colorectal cancer—a critical review. Biomolecules. 2019;9:398.
- [CrossRef] [Google Scholar]
- Synthesis, crystal structures and antimicrobial activity of palladium metal complexes of sulfonyl hydrazone ligands. Eur. J. Chem.. 2020;11:377-384.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102114.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







