Translate this page into:
Amomum villosum Lour. fruit extract ameliorates high-fat diet-induced body mass gain and adipogenic pathways in C57BL/6 mice
⁎Corresponding authors at: Ilwonbio Co., Ltd., & Department of Physiology, Wonkwang University School of Korean Medicine, Iksandaero 460, Iksan, Jeonbuk, 54538, South Korea. Tel: +82-63-850-6917; Fax: +82-63-842-3138; (Kang-Beom Kwon). Department of Anesthesiology and Pain Medicine, Wonkwang University School of Medicine, Wonkwang University Hospital, 460 Iksan-daero, Iksan, Jeonbuk 54538, South Korea. Tel: +82-63-859-1560; Fax: +82-63-859-5472; (Hyang-Do Ham). gidehwkd@gmail.com (Hyang-Do Ham), desson@wku.ac.kr (Kang-Beom Kwon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Amomum villosum Lour. is commonly used in Asian countries as an herbal remedy to medicate several diseases including type 2 diabetes. In this animal experiment, we examined the influence of Amomum villosum water extract (AVE) against metabolic variations in high-fat diet (HFD)-induced obesity in C57BL/6 mice. Mice were nourishing with a normal diet (control), high-fat diet (HFD), HFD + AVE 100 mg/kg body weight (b.wt.)/day, HFD + AVE 200 mg/kg b.wt./day, and HFD + AVE 500 mg/kg b.wt./day for 7 weeks. The AVE (100, 200, and 500 mg/kg)-treated animals exhibited substantial decreases in body mass, fat mass, adipocyte hypertrophy, and epididymal white adipose tissue (eWAT) collate to the HFD-fed group. AVE treatment also reduced hepatic triglyceride level and significantly increased the adiponectin expression in adipocytes. Furthermore, AVE treatment significantly inhibited adipogenesis in the AVE group by reducing the mRNA expression of sterol regulatory element-binding protein1 (SREBP1), fatty acid synthase (FAS), CCAAT/enhancer binding protein (C/EBP)-α, peroxisome proliferator-activated receptor (PPAR)-γ, and tumor necrosis factor (TNF)-α agreeing to the HFD-fed animals. These research outcomes recommend that AVE is possibly valuable for the prevention of HFD-induced obesity via modification of various pathways related with adipogenesis and food consumption.
Keywords
Amomum villosum
Adipogenesis
HFD-induced obesity
FAS
SREBP1
PPAR-γ
1 Introduction
Obesity and overweight is a complex disease of energy inequality along with unnecessary storage of lipids within non-adipose tissues (Friedman, 2002). Liver fat accumulation is identified as fatty liver or hepatic steatosis. Intensification of fat in the liver cells and can cause complications in cases of obesity.
Non-alcoholic fatty liver disease (NAFLD) considered a vital origin of long-term hepatic disease in the global population with an incidence of 25% among adults (Younossi et al., 2016). Non-alcoholic steatohepatitis (NASH) is categorized by means of elevated lipid level in the liver (without substantial alcohol intake), which is accompanied by significant histological changes such as hepatocyte damage, fibrosis, and lobular inflammation. The appropriate molecular mechanisms and signaling pathways involved in the development of NASH from NAFLD remains unclear, which significantly hinders the drug discovery efforts against NASH.
Preadipocytes are converted to adipocytes via the process of adipogenesis, which is followed by fat accumulation within the tissues. Peroxisome proliferator-activated receptor γ2 (PPARγ2) and CCAAT/enhancer binding protein α (C/EBPα) are the principal transcriptional regulators of the adipogenic process (Rosen and MacDougald, 2006). Sterol regulatory element-binding protein-1c (SREBP-1c) regulated fatty acid synthase (FAS) is a multi-enzyme protein which is involved in fatty acid synthesis (Shen et al., 2014). Lipid increment in the liver is mediated by fatty-acid synthesis, increased fat uptake, and significantly increased lipid catabolism inhibition. Similarly, excessive triglycerides accumulate in the liver due to augmented hepatic de novo lipid synthesis (Shen et al., 2014).
SREBP-1-activated FAS generates substantial fat mass in tissues. Hence, adipogenic regulators deactivation or blockage of lipogenic genes could help inhibition of fatty liver and obesity. Although numerous research carried out to discover unique anti-obesity drugs with the capacity to regulate lipogenesis in addition to adipogenesis, currently no other accepted treatments are available for NASH except way of life amendment by diet control with physical workout.
Amomum villosum Lour. (AV) is an herbal plant. Prior pharmaceutical research has revealed that Amomi Fructus showed significant pharmacological activities against inflammation, diarrhea, and ulceration (Huang et al., 2014). Clinical trials with AV extract (AVE) supplementation reported positive effect on insulin secretion and postprandial glycemia in healthy individuals (Kim et al., 2020). Hence, in the current investigation, we examined the activity of AVE against the high fat diet (HFD)-induced obesity model in mouse.
2 Materials and methods
2.1 Crude AV extract preparation
A. villosum Lour. (AV) was validated by G. Lee, a Herbology specialist, and a voucher specimen was located at the Department of Herbology, Wonkwang University Korean Medical School. AV extracts preparation was reported in our previous study (Kim et al., 2020).
2.2 Animals, diet, and study design
All in vivo experiments were conducted in agreement with Institutional Animal Care and Use Committee of Wonkwang University (permit number WKU 20–31). Eight-week-old adult male C57BL/6 mice (Orient Bio Inc., Charles River Technology, Gapyung-Gun, Gyeonggi-Do, Republic of Korea) were kept in a 12-h light/12-h dark phase, with standard rodent diet and water available ad libitum. The surrounding environment was kept at 20 °C ± 2 °C. The mice were acclimatized to this environment for one week prior to the experiment.
After a 7 days of adaptation to the laboratory conditions, animals were fed with either a HFD (D12492: 60% kcal% fat; 34.9% fat, 26.2% protein, and 26.3% carbohydrate; 5.24 kcal/g) from Research Diets (New Brunswick, NJ, USA) or a control diet (5L79; 13.6% fat, 21.0% protein, and 65.3% carbohydrates; 3.43 kcal/g) from Orient Bio Inc. (Gapyung-Gun, Gyeonggi-Do, Republic of Korea). The mice were allocated into the following five categories (n = 8 per group) and were fed with their corresponding diets: control, HFD + vehicle (1X phosphate buffered saline (PBS)), HFD + AVE (100 mg/kg), HFD + AVE (200 mg/kg), and HFD + AVE (500 mg/kg). PBS and AVE (100, 200, and 500 mg/kg/b. wt.) were treated orally at 200 µl every day, early in the morning, for seven weeks. At the completion of the study, the animals were killed and blood was collected via retro-orbital bleeding without topical anesthesia; the blood was then admitted to clot by allowing it uninterrupted at room heat for 30 min, followed by centrifugation at 2500 rpm for 10 min at 4 °C. Tissue samples were collected and kept at −70 °C or in 10% formalin for additional biochemical and histological investigates respectively (Figarola et al., 2013).
2.3 RNA extraction and quantitative RT-PCR
Total RNA samples from mice liver and eWAT tissues were extracted with the TRIzol reagent (Invitrogen, CA), and the complimentary DNA (cDNA) was then synthesized using the PrimeScript™ RT Reagent Kit (TaKaRa Bio, Japan). Real-time PCR was executed with the SYBR Green PCR master mix and the real-time PCR System (Applied Biosystems, CA, US), using the primers displayed in Table 1. All reactions were conducted minimum in triplicate and the results were averaged. The mRNA levels of genes were measured using the relative quantification (RQ) mean levels with GAPDH as the internal control.
Gene name
Sequence for Primers
Accession no.
GAPDH
Forward: CGTCCCGTAGACAAAATGGT
Reverse: TTGATGGCAACAATCTCCACNM_008084
FAS
Forward: GCGATGAAGAGCATGGTTTAG
Reverse: GGCTCAAGGGTTCCATGTTNM_007988
SREBP1
Forward: GGAGCCATGGATTGCACATT
Reverse: GGCCCGGGAAGTCACTGTNM_011480
Adiponectin
Forward: ACGTCATCTTCGGCATGACT
Reverse: CTCTAAAGATTGTCAGTGGATCTGNM_009605
TNFα
Forward: AGGGTCTGGGCCATAGAACT
Reverse: CCACCACGCTCTTCTGTCTACNM_013693
PPARγ
Forward: GAAAGACAACGGACAAATCACC
Reverse: GGGGGTGATATGTTTGAACTTGNM_011146
C/EBPα
Forward: TTGTTTGGCTTTATCTCGGC
Reverse: CCAAGAAGTCGGTGGACAAGNM_007678
2.4 Histological examination
Tissues fixed in 10% buffered formalin were embedding with paraffin, 5-mm sections on the microscope slides were deparaffinized via xylene and rehydration through a sequence of downward concentrations of ethanol. Sections were stained with hematoxylin and eosin (H&E). Photomicrographs were taken on a microscopic slide. All the stained slides were observed in a blinded manner by a professional pathologist.
2.5 Statistical analysis
A statistical investigation was executed using the Duncan’s multiple comparison test. All data are labelled as mean ± standard deviation (SD) with statistical significance fixed at p < 0.05.
3 Results and discussion
Fatty liver (steatosis) with its interconnected provocative condition (non-alcoholic steatohepatitis (NASH)) are the prevalent liver problems of metabolic disorders and obesity. high fat diet-induced lipid accumulation as well as dyslipidemia establish the fatty liver extension, and could headway to non-alcoholic steatohepatitis, cirrhosis, fibrosis and, eventually, hepatocellular carcinoma (Angulo, 2002). Uncontrolled hepatic triglyceride accretion in hepatocytes is consider as a main factor for NAFLD, which crucially accompanies insulin resistance in liver (Postic and Girard, 2008).
In this experimental research, we assessed the anti-obesity efficiency of AVE in high fat diet-induced obesity in animal model. Throughout six weeks of HFD feeding, only a small increase in body weight was detected in AVE prescribed groups matched with the HFD group. AVE oral consumption significantly decreased epididymal WAT fat mass. Results shown in Fig. 1 indicate the effect of AVE (100, 200, and 500 mg/kg) on the mice body weight nourished with high fat diet for a duration of seven weeks. AVE-treated groups showed considerable reduction of mean body weight compared with the high fat diet-induced control group after six weeks of oral feeding, while there was no substantial variance in food consumption. Average body weight of AVE treated groups as follows (32.22 ± 7.13 g vs. 28.48 ± 5.22 g (AVE 100 mg/kg); 32.22 ± 7.13 g vs. 28.02 ± 4.36 g (AVE 200 mg/kg); 32.22 ± 7.13 g vs. 26.78 ± 3.97 g (AVE 500 mg/kg)). AVE-fed groups showed lesser body weight gain than the HFD-fed animals by 11.60% (AVE 100 mg/kg), 13.03% (AVE 200 mg/kg), and 16.88% (AVE 500 mg/kg) (Fig. 1, p < 0.05). Moreover, the weights of epididymal WAT (2.48 ± 0.43 g vs. 2.18 ± 0.22 g (AVE 100 mg/kg); 2.48 ± 0.43 g vs. 1.89 ± 0.02 g (AVE 200 mg/kg); 2.48 ± 0.43 g vs. 1.45 ± 0.51 g (AVE 500 mg/kg)) and liver (1.56 ± 0.22 g vs. 1.15 ± 0.06 g (AVE 100 mg/kg); 1.56 ± 0.22 g vs. 1.16 ± 0.05 g (AVE 200 mg/kg); 1.56 ± 0.22 g vs. 1.25 ± 0.05 g (AVE 500 mg/kg)) in the AVE treated animals were considerably reduced related with the high fat feed fed animals (Fig. 2A and B, p < 0.01).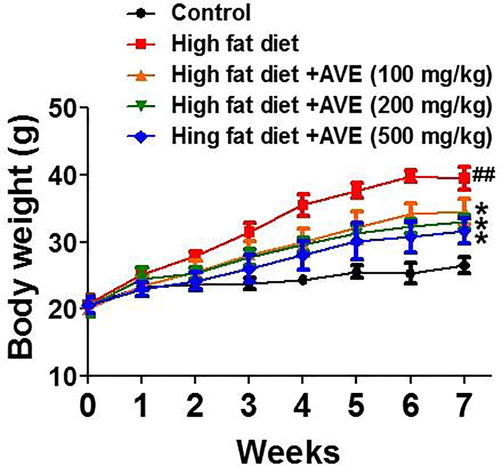
Effect of Amomum villosum water extract (AVE) treatment on body weight in high-fat diet (HFD)-fed C57BL/6 mice. ##P < 0.01 compare high-fat diet (HFD) with control; *P < 0.05 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
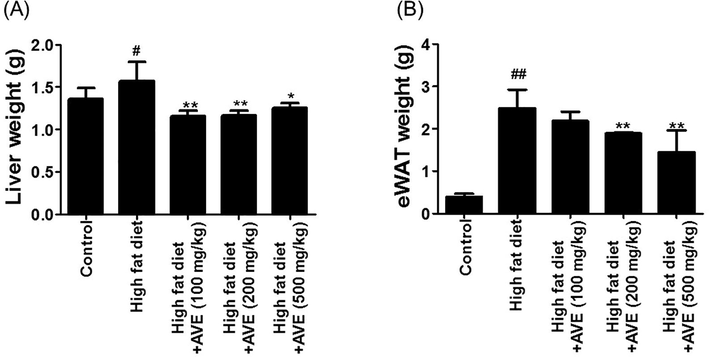
Effect of Amomum villosum water extract (AVE) treatment on the liver and epididymal white adipose tissue (eWAT) weight changes in high-fat diet (HFD)-fed C57BL/6 mice. #P < 0.05, ##P < 0.01 compare high-fat diet (HFD) with control; *P < 0.05, **P < 0.01 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
During the development of adipogenic process, PPARγ and C/EBPα, which are crucial transcriptional factors, were elevated and mediate the transcription of marker genes involved in terminal adipocyte differentiation process (Schadinger et al., 2005). In this study, the most noticeable differences were detected in epididymal WAT (Fig. 3) fat deposits, which were responsible for the bulk of the adiposity and weight increase. Epididymal WAT was markedly bigger in the HFD mice group than the AVE-treated groups, which was in agreement with observations of the histological results. Liver tissue sections stained with hematoxylin and eosin (H&E) from HFD mice indicated significant fat droplet accumulation compared with the AVE-treated groups (Fig. 4A), which was consistent with a notable rise in hepatic triglyceride content in the high fat diet group than the AVE treated animals (100, 200, and 500 mg/kg) (Fig. 4B, p < 0.05).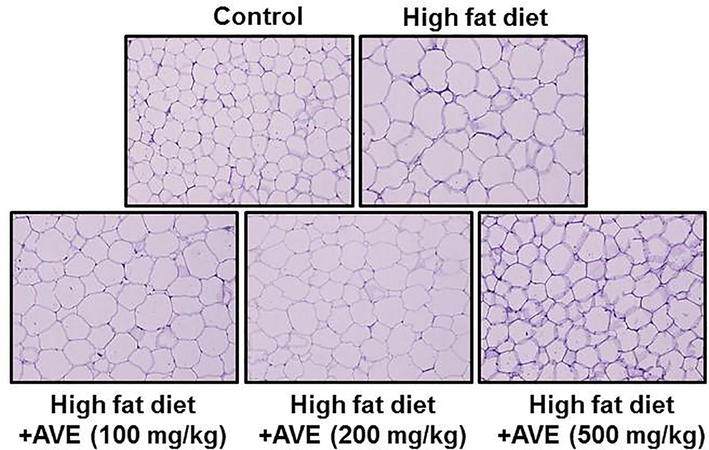
Effect of Amomum villosum water extract (AVE) treatment on morphological changes in epididymal adipose tissues in high-fat diet (HFD)-fed C57BL/6 mice.
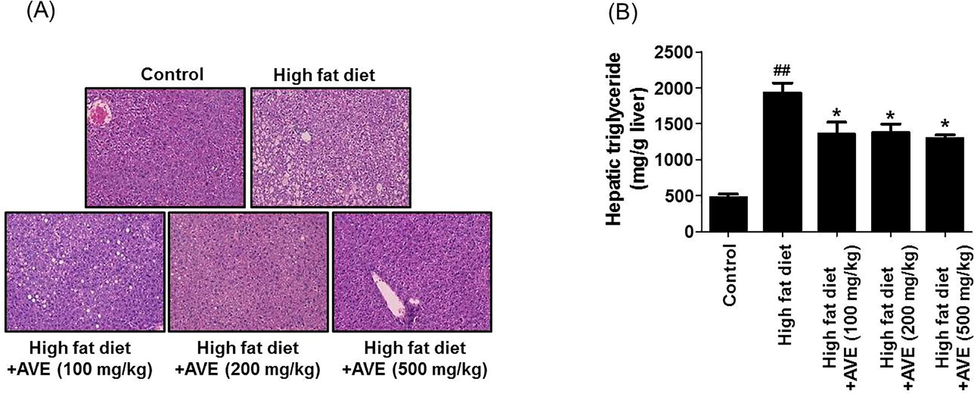
Effect of Amomum villosum water extract (AVE) treatment on lipid storage level in the liver evaluated by H&E staining (A) and hepatic triglyceride level (B) in HFD-fed obese C57BL/6 mice. ##P < 0.0-1 compare high-fat diet (HFD) with control; *P < 0.05 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
Expression of PPARγ, C/EBPα, and TNF-α mRNA were suppressed and adiponectin mRNA level was induced by AVE intervention. C/EBPα (110.6-fold (AVE 100 mg), 5530-fold (AVE 200 mg), and 1382.5-fold (AVE 500 mg)), PPARγ (87-fold (AVE 100 mg), 4350-fold (AVE 200 mg), and 639.7-fold (AVE 500 mg)), and proinflammatory cytokine TNF-α (1.47-fold (AVE 100 mg), 1.50-fold (AVE 200 mg), and 1.52-fold (AVE 500 mg)) mRNA levels were markedly declined matched with the HFD group. Adiponectin mRNA level was increased in the AVE-treated groups (1.72-fold (AVE 100 mg), 1.88-fold (AVE 200 mg), and 1.59-fold (AVE 500 mg)) compared to the high fat diet group (Figs. 5 and 6).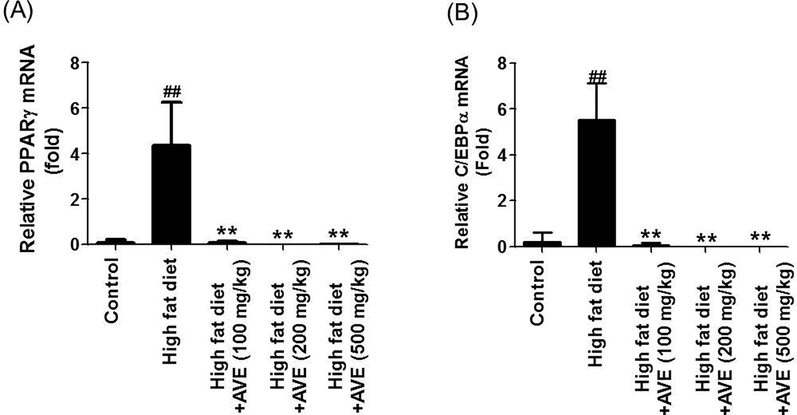
Effect of Amomum villosum water extract (AVE) treatment on the expression levels of epididymal adipose tissue-derived PPARg and C/EBPα in high-fat diet (HFD)-fed C57BL/6 mice. ##P < 0.01 compare high-fat diet (HFD) with control; **P < 0.01 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
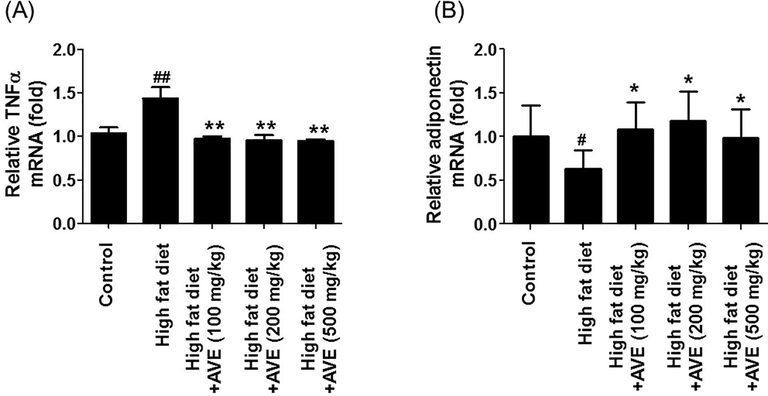
Effect of Amomum villosum water extract (AVE) treatment on the expression levels of epididymal adipose tissue-derived TNFα and adiponectin in high-fat diet (HFD)-fed C57BL/6 mice. #P < 0.05, ##P < 0.01 compare high-fat diet (HFD) with control; *P < 0.05, **P < 0.01 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
In agreement through previous reports (Schadinger et al., 2005), PPARγ and C/EBPα mRNA levels were considerably upregulated in epididymal WAT in the HFD group. However, AVE-treated groups (100, 200, and 500 mg/kg) displayed significantly declined PPARγ and C/EBPα mRNA intensities compared with the HFD group. Hence, the decrease in body weight and epididymal WAT pads is due to oral treatment with AVE, which consequently leads to the reduction of eWAT and the inhibition of adipocyte hypertrophy.
Previous research has shown that the, quantity of plasma adiponectin is inhibited in diabetic animal model and obese individuals (Lagathu et al., 2006; Kern et al., 2003). However, decline of adipose tissue mass level and enhancement of insulin resistance were observed after adiponectin supplementation (Berg et al., 2002).
SREBP-1 is an important adipogenesis-related transcription factor, which acts as a vital regulator of glucose and energy metabolism along with preadipocyte differentiation and lipid biosynthesis (Sahagun et al., 2012; Kim et al., 1998; Tontonoz and Spiegelman, 2008).
In adipose tissue insulin resistance and fatty acid synthesis were increased by lipogenesis mediated by FAS (Latasa et al., 2003). We have conducted further experiments to identify the efficiency of AVE on lipid storage in liver and lipid metabolism-associated gene expression levels (Fig. 7A and B). Assessment of mRNA level in liver showed that AVE treatment considerably decreased FAS and SREBP1, which provided evidence of AVE-derived antilipogenic effect.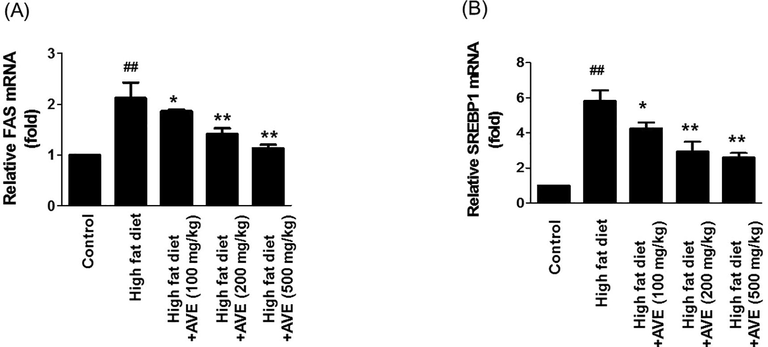
Effect of Amomum villosum water extract (AVE) treatment on the expression of hepatic FAS and SREBP1 in high-fat diet (HFD)-fed C57BL/6 mice. ##P < 0.0-1 compare high-fat diet (HFD) with control; *P < 0.05, **P < 0.01 compare AVE (100, 200, 500 mg/kg) with high-fat diet (HFD).
Herein, we have described numerous mechanisms essential for the anti-obesity activity of AVE; however, it is necessary to further investigate the active compounds that are involved in this activity. Collectively, our experimental results provide confirmation that AVE treatment has an anti-obesity property and further blocks fatty liver via modifying the lipid metabolism related gene expressions.
Author contributions
H.-R. K., Y.-S. K., H. D. H., and K.-B. K. designed the research. H.-R. K., P. A., Y.-S. K., and Y.-G. K., performed the research. D.-G. R., Y.-R. L., G. L., H. D. H., and K.-B. K. analyzed the data. H.-R. K., P. A., and K.-B. K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Acknowledgement
This present study was supported by Wonkwang University, South Korea in 2019.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends. Endocrinol. Metab.. 2002;13(2):84-89.
- [Google Scholar]
- COH-SR4 reduces body weight, improves glycemic control and prevents hepatic steatosis in high fat diet-induced obese mice. PLoS ONE. 2013;8(12):e83801.
- [Google Scholar]
- SNP typing for germplasm identification of Amomum villosum lour. based on DNA barcoding markers. PLoS One. 2014;9(12):e114940.
- [Google Scholar]
- Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779-1785.
- [Google Scholar]
- Acute effects of Amomum villosum Lour. fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebo-controlled, crossover study in healthy subjects. Saudi J. Biol. Sci.. 2020;27(11):2968-2971.
- [Google Scholar]
- Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig.. 1998;101(1):1-9.
- [Google Scholar]
- Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162-2173.
- [Google Scholar]
- Occupancy and function of the -150 sterol regulatory element and -65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell. Biol.. 2003;23(16):5896-5907.
- [Google Scholar]
- Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig.. 2008;118(3):829-838.
- [Google Scholar]
- Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol.. 2006;7(12):885-896.
- [Google Scholar]
- Modulation of PPAR-gamma by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Res.. 2012;2012:318613
- [Google Scholar]
- PPARγ2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab.. 2005;288(6):E1195-E1205.
- [Google Scholar]
- Hepatic differentiated embryo-chondrocyte expressed gene 1 (DEC1) inhibits sterol regulatory element-binding protein-1c (SREBP-1c) expression and alleviates fatty liver phenotype. J. Biol. Chem.. 2014;289(34):23332-23342.
- [Google Scholar]
- Fat and beyond: the diverse biology of PPARgamma. Ann. Rev. Biochem.. 2008;77:289-312.
- [Google Scholar]
- Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
- [Google Scholar]







