Translate this page into:
Amomum villosum Lour. Fruit extract mitigates hyperlipidemia through SREBP-2/LDLR/HMGCR signaling in high-cholesterol diet-fed mice
⁎Corresponding authors at: Department of Diagnostics, Wonkwang University School of Korean Medicine, Iksandaero 460, Iksan, Jeonbuk 54538, South Korea (H.-J. Jung). Ilwonbio Co., Ltd, & Department of Physiology, Wonkwang University School of Korean Medicine, Iksandaero 460, Iksan, Jeonbuk 54538, South Korea (K.-B. Kwon). kendu@wku.ac.kr (Hyun-Jong Jung), desson@wku.ac.kr (Kang-Beom Kwon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Amomum villosum Lour. (Zingiberaceae) is an herbal medicine used in Asian countries for various ailments. In the current experiment, the effect of AV water extract (AVE) on cholesterol-lowering capabilities along with its essential bio-molecular mechanisms have been examined. The efficiency of AVE against high-cholesterol diet (HCD)-generated hyperlipidemia was investigated using C57BL/6 mice. Mice have been divided to six categories: control group (normal diet), high-cholesterol diet (HCD), and HCD treated with AVE at 100, 200, and 500 mg/kg, or simvastatin 40 mg/kg for four weeks. AVE treated animal groups (100, 200, 500 mg/kg) had markedly lessened body mass increase (p < 0.01) and also had reduced liver and epididymal fat weights (p < 0.05) related to HCD alone batch. Hepatic TG levels in AVE 100 mg/kg, 200 mg/kg, and 500 mg/kg treated groups were meaningfully reduced by 13.32%, 14.61%, and 21.78%, respectively; and serum TG decreased by 25.65%, 31.97%, and 32.22%, respectively, compared to the HCD alone group. Serum TC was reduced by 11.17%, 12.48%, and 26.85% with AVE at 100, 200, and 500 mg/kg, respectively; and serum LDL-C declined by 10.08%, 18.50%, and 30.90% (p < 0.05) with AVE at 100, 200, and 500 mg/kg, respectively. Levels of mRNA expression including LDL receptor (LDLR), sterol regulatory element-binding protein 2 (SREBP2), and HMG-CoA reductase (HMGCR) were noticeably amplified by AVE (500 mg/kg) treatment. Similarly, AVE treatment significantly increased Hmgcr protein expression levels in a dose related fashion. These outcomes show that AVE displays a hypolipidemic effect and might function as a novel hypolipidemic therapy, further; they suggest a mechanism of action for this effect.
Keywords
Amomum villosum
LDL receptor
SREBP-2
HMGCR
LDL cholesterol
1 Introduction
Atherosclerosis begins in early life in humans, and increases gradually in later decades, occasionally developing into atherosclerotic cardiovascular disease and myocardial infarction. The role of LDL cholesterol (LDL-C) as a principal cause of atherosclerosis has been established by studies with animal models, epidemiological cohort studies, genetic studies in polygenic and monogenic human disorders, and randomized trials on LDL cholesterol levels (Ference et al., 2017; Müller et al., 2022).
Further research has found that the connection among elevated total cholesterol and ischemic cardiovascular illness and myocardial infarction fluctuates with age, as LDL-related heart disease is considerably more common in younger people than older people (Anum and Adera, 2004; Iversen et al., 2009; Gränsbo et al., 2016; Brunner et al., 2019). LDL cholesterol makes up the majority of total cholesterol. Maintaining the levels of triglyceride (TG) and cholesterol in normal boundaries is crucial for decreasing the possibility of cardiovascular disease. Abnormal levels of lipid in the blood circulation is known as dyslipidemia, with a low level of “good” cholesterol denoted as high-density lipoprotein cholesterol (HDL-C); a high level of “bad” cholesterol denoted as low-density lipoprotein cholesterol (LDL-C); and/or elevated levels of TG.
Approximately 60–70% of circulating serum cholesterol is in the LDL form (Talbert, 2005). LDL carries cholesterol to the peripheral tissues from the liver. An elevated LDL-C level causes arterial wall injury and atherosclerotic plaque formation. In contrast to other lipoproteins, individual LDL particles include a single large protein called apolipoprotein B (apoB), which is essential for LDL particle binding to its receptor called LDL receptor (LDLR). LDL particles in circulation are continuously removed through LDLR in the liver. Nearly 70% of LDL is removed by the liver by means of this mechanism, releasing free cholesterol (FC) (Malloy and Kane, 2004).
Hypercholesterolemia refers to an elevated LDL level in the circulation. Amomum villosum Lour. (AV) is a medicinal plant growing all over southeast Asia. Similar to cardamom, AV is cultivated for its fruit, which after maturity dries into pods and is covered with aromatic seeds. Previous studies indicated that AV has pharmacological activity on diarrhea, inflammation, ulcers, and pain (Huang et al., 2014). Earlier studies also showed that volatile oil of AV inhibits nonalcoholic fatty liver disease (Lu et al., 2018) and inflammatory bowel disease in animals (Chen et al., 2018). Studies also demonstrated that AV water extract (AVE) treatment on healthy individuals is effective in reducing postprandial glycemia (Kim et al., 2020), and it counteracts high- fat diet (HFD) generated obesity in mouse models (Kim et al., 2021). Furthermore, AVE showed a significant inhibitory effect on α-glucosidase activity (Kim et al., 2022).
In this exploration, we make an attempt to elucidate the effect of AVE on HCD-induced hyperlipidemia model in mice with a role of LDL receptor (LDLR), sterol regulatory element-binding protein 2 (SREBP2), and HMG-CoA reductase (HMGCR) expression.
2 Materials and methods
2.1 Production of crude AV extract
AV extraction has been carried out based on the previous reports (Kim et al., 2020; Kim et al., 2021).
2.2 Animals
In vivo testing were accompanied under procedures conveyed by the Institutional Animal Care and Use Committee of Wonkwang University (permit number WKU 20–01). Eight-week-old C57BL/6 mice (male) were obtained from Charles River Technology, Orient Bio Inc., Gapyung-Gun, Gyeonggi-Do, South Korea and acclimatized for a week under light (12 h) and dark (12 h) cycle at 20 ± 2 °C, with standard food (Table1) and water ad libitum. After a week of acclimatization, mice were distributed into the six groups (n = 10). Group 1 was the control, fed a standard diet, given 1× phosphate buffered saline (PBS; PH 7.4) p.o.; group 2 was the HCD, given 1× phosphate buffered saline (PBS) p.o. as the control for AVE; and groups 3, 4, 5, and 6 were treatment groups given AVE 100 mg/kg (p.o.), AVE 200 mg/kg (p.o.), AVE 500 mg/kg (p.o.), or simvastatin (S0509 TCI, Tokyo, Japan) 40 mg/kg (p.o.) (Araújo et al., 2015; Irani et al., 2016), respectively. The experimental drugs were dissolved in 1× PBS buffer solution and administered orally to mice via gavage. All the treatments were started on the same day and carried out for 28 days. On the final day of experiment, blood samples were collected from euthanized mice, and centrifuged at 4 °C for 10 min at 2500 rpm to separate serum from the blood sample. Tissues (liver, epididymal fat) were surgically removed and weight measured. Both serum and liver tissues were saved at −70 °C for further biochemical investigations. Composition of the HCD (D12079B) is shown in Table 2. *Anhydrous milk fat typically contains approximately 0.3% cholesterol, so D12079B contains approximately 0.21% cholesterol. Formulated by E. A. Ulman, Ph.D., Research Diets, Inc., October 12, 1995. Diet formulated to match Teklad Western Diet #TD88137, except that 1% Corn oil replaces 1% butter fat.
Product Code
5L79
Nutrients
Protein
18%
Fat, ether extract
5.2%
Fat, acid hydrolysis
6.7%
Crude fiber
5.2%
Ash
5.7%
Calcium
0.85%
Phosphorus
0.62%
Certified
Not applicable
Irradiated
Not applicable
Autoclavable
Applicable
Appropriate for:
Rats
Applicable
Mice
Applicable
Hamsters
Applicable
Ingredient
gm (%)
kcal (%)
Protein
20
17
Carbohydrate
50
43
Fat
21
41
Total kcal/gm
4.7
100
Ingredient
gm
kcal
Casein, 80 Mesh
195
780
DL-methionine
3
12
Corn starch
50
200
Maltodextrin 10
100
400
Sucrose
341
1364
Cellulose
50
0
Milk fat, anhydrous*
200
1800
Corn oil
10
90
Mineral mix S10001
35
0
Calcium carbonate
4
0
Vitamin mix V10001
10
40
Choline bitartrate
2
0
Cholesterol, USP*
1.5
0
Ethoxyquin
0.04
0
Total
1001.54
4686
2.3 Serum and liver biochemical analysis
Serum TG (cat. no. AM157S; Asan Pharmaceuticals, Seoul, South Korea), total cholesterol (cat. no. AM202; Asan Pharmaceuticals), LDL cholesterol (cat. no. DG-CHO100; DoGen bio, Seoul, South Korea) and liver TG were analyzed by their respective assay kits based on the manufacturer instructions.
2.4 Real-time PCR
Liver total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Hepatic expression of target genes was analyzed by real-time quantitative PCR (qRT-PCR), using the Applied Biosystems qRT-PCR platforms. All primers were designed based on species-specific genes using the primer3web version 4.1.0 and Primer-blast tool and procured from GenoTech (Daejeon, Korea). The primers were: Srebp2 (sense 5′- TGGGCAGCAACGGGACCATT-3′, anti-sense 5′-GGAGGACCGGTAGCGCTTTTCA-3′); Ldlr (sense 5′-ACCGCAACATCTACTGGACA-3′, anti-sense 5′-GTCCACACCATTCAAACCCC3′); Hmgcr (sense 5′- TGCTGCTTTGGCTGTATGTC-3′, anti-sense 5′- TGAGCGTGAACAAGAACCAG-3′); Gapdh (sense 5′- CGTCCCGTAGACAAAATGGT-3′, anti-sense 5′-TTGATGGCAACAATCTCCAC-3′).
2.5 Immunoblotting
Mouse liver tissues (50 mg) from different animal groups were homogenized in radio immunoprecipitation assay buffer (RIPA buffer) with a protease inhibitor cocktail. Total protein (∼20 μg) was fractionated by SDS-PAGE and relocated into a PVDF membrane. In order to block non-specific antibody binding sites, PVDF membranes were incubated with 5% skimmed milk (w/v) at room temperature for 1 h time duration on shaker followed by overnight incubation with specific primary antibodies of anti-HMGCR (cat. no. SC-271595, Santa Cruz Biotechnology) and mouse anti-β actin (cat. no. SC-47778, Santa Cruz Biotechnology) at 4 °C on shaker. On the following day membranes were incubated for 1 h at room temperature with horseradish peroxidase-coupled secondary antibody (Promega, Madison, WI) to identify immuno-reactive antigen followed by membrane washing using 1X TBST. Finally, membrane was treated with enhanced chemiluminescence (ECL) detection reagent (1:1) (Bio-Rad) and the protein bands were pictured by Imaging System (AE-9300 Ez-capture MG/AE-9160 Ez-capture ST, Tokyo, Japan). The densitometry assessment for HMGCR was normalized to β-actin as an internal standard.
2.6 Statistical analysis
Statistical examination has been performed with Duncan's multiple comparison assessment using SPSS Software Package v. 26.0 (IBM, NY, USA). Results were reported as mean ± standard deviation (SD) and p < 0.05 was measured statistically significant.
3 Results
3.1 Effects of A. villosum extracts on body mass gain, liver, and epididymal fat masses in mice
The HCD (alone) group exhibited significantly higher body weight gain (32.4 g) compared to control diet fed animals (26.6 g; p < 0.01). However, related to the HCD alone fed animals, AVE treatments in the context of the HCD, significantly (p < 0.01) declined body mass increase by 27.4 g (AVE 100 mg/kg), 27.3 (AVE 200 mg/kg), and 27.5 g (AVE 500 mg/kg). Simvastatin (40 mg/kg) with the HCD also showed a significant body weight reduction (25.5 g; p < 0.01) compared to the HCD alone group; this decline rate was greater than the AVE treated groups but it was not statistically altered in the fourth week (Table 3). All data are mean ± SD **p < 0.01 vs. Control; #p < 0.05, ##p < 0.01 vs. cholesterol diet. N = 10.
Group/Week
Control
HCD
AVE
100 mg/kgAVE
200 mg/kgAVE
500 mg/kgSimvastatin
40 mg/kg
0
23.6 ± 0.5
23.9 ± 0.4
23.9 ± 1.4
23.5 ± 0.8
23.3 ± 1.3
23.0 ± 0.8
1
23.8 ± 1.5
25.5 ± 0.8
23.9 ± 2.6
23.8 ± 1.7
23.8 ± 1.6
23.2 ± 0.8
2
25.4 ± 1.2
29.0 ± 0.7**
25.7 ± 2.0#
25.5 ± 2.3#
25.6 ± 2.1#
24.0 ± 0.4##
3
26.2 ± 1.2
31.8 ± 0.7**
26.7 ± 2.2#
26.8 ± 3.5#
27.3 ± 2.6#
24.9 ± 1.0##
4
26.6 ± 1.0
32.4 ± 1.0**
27.4 ± 2.5##
27.3 ± 4.1##
27.5 ± 2.8##
25.5 ± 0.8##
Animals in the HCD group had higher epididymal fat weights (1.27 g) than the control animals (0.29 g) (Table 4; p < 0.01). Treatment with AVE at 100, 200, and 500 mg/kg significantly (p < 0.05) decreased epididymal fat weight by 0.73 g, 0.63 g, and 0.63 g respectively compared to the HCD alone fed animals. HCD alone fed animals showed considerably (p < 0.01) higher liver weight (1.23 g) compared to those with a normal diet (1.11 g). Conversely, AVE treatment at 100, 200, and 500 mg/kg significantly (p < 0.05) reduced liver mass by 0.99 g, 1.00 g, and 0.96 g respectively compared to HCD alone. Simvastatin (40 mg/kg) treatment also showed significant reductions in liver (1.01 g) and epididymal fat (0.63 g) weights (p < 0.01). These results indicated that AVE inhibits the growth of the fat pad and decreases liver weight. All data are mean ± SD **p < 0.01 vs. control; #p < 0.05 vs. cholesterol diet. N = 10.
Group/tissue
Control
HCD
AVE
100 mg/kgAVE
200 mg/kgAVE
500 mg/kgSimvastatin 40 mg/kg
Liver
1.11 ± 0.04
1.23 ± 0.08**
0.99 ± 0.15#
1.00 ± 0.18#
0.96 ± 0.08#
1.01 ± 0.12#
Epididymal fat
0.29 ± 0.03
1.27 ± 0.22**
0.73 ± 0.23#
0.63 ± 0.22#
0.63 ± 0.09#
0.63 ± 0.09#
3.2 Effects of A. villosum extract on serum and liver lipid levels
Liver TG (Fig. 1A) and serum TG (Fig. 1B), TC (Fig. 2A), and LDL-C (Fig. 2B) intensities in the HCD fed animals were statistically greater than control diet fed animals (liver TG: 439.5 mg vs. 205.4 mg; p < 0.01; serum TG: 568.5 mg vs. 217.3 mg; p < 0.01; serum TC: 1557 mg vs. 609.9 mg; p < 0.01, and LDL-C: 40.63 mg vs. 19.16 mg; p < 0.01). Thus, the hyperlipidemia condition appeared to have been established in the mouse model successfully. Hepatic lipid levels in AVE 100 mg/kg, AVE 200 mg/kg, and AVE 500 mg/kg treated animals were markedly lesser than HCD alone fed animals, with TGs being reduced by 13.32%, 14.61%, and 21.78%, respectively; serum TG decreased by 25.65%, 31.97%, and 32.22%, respectively; serum TC reduced by 11.17%, 12.48%, and 26.85%, respectively, and serum LDL-C decreased by 10.08%, 18.50%, and 30.90%, respectively. These results demonstrated that AVE reduces serum and hepatic lipid levels in a dose related fashion.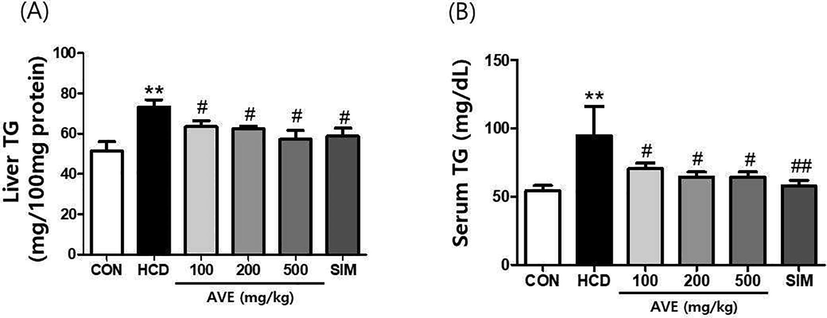
Liver TG and serum TG levels in C57BL/6 mice fed a high-cholesterol diet supplemented with different doses of Amomum villosum water extract (AVE). **p < 0.01 comparing HCD with control diet; #p < 0.05, ##p < 0.01 comparing HCD + AVE with HCD alone. All data are mean ± SD (n = 10).
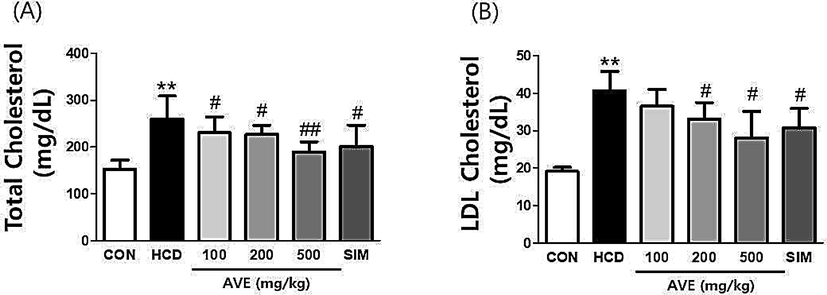
Serum total cholesterol and LDL cholesterol levels in C57BL/6 mice fed a high-cholesterol diet supplemented with different doses of Amomum villosum water extract (AVE). **p < 0.01 comparing HCD with control diet; #p < 0.05, ##p < 0.01 comparing HCD + AVE with HCD alone. All data are mean ± SD (n = 10).
3.3 Effect of A. villosum extract on the expressions of lipid metabolism target genes in liver
As shown in Fig. 3, hepatic Srebp2, Ldlr, and Hmgcr mRNA levels were significantly (p < 0.01) lower in the HCD alone fed animals related to control mice. However, AVE treatment (500 mg/kg) significantly increased Ldlr, Srebp2 and Hmgcr levels compared to the HCD alone group (Fig. 3). We also evaluated the HMGCR protein level by immunoblotting. Hepatic HMGCR protein expression level was noticeably (p < 0.01) decreased in the HCD alone fed animals compared to the normal diet fed group. However, AVE treatment significantly increased the HMGCR protein expression level in a dose- dependent fashion, compared to the HCD alone group (Fig. 4).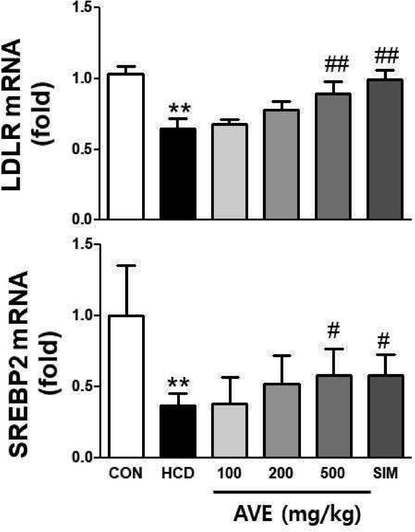
Hepatic mRNA expression level of LDLR and SREBP2 in C57BL/6 mice fed a high-cholesterol diet supplemented with different doses of Amomum villosum water extract (AVE). **p < 0.01 comparing HCD with control diet; #p < 0.05, ##p < 0.01 comparing HCD + AVE with HCD alone. All data are mean ± SD (n = 10).
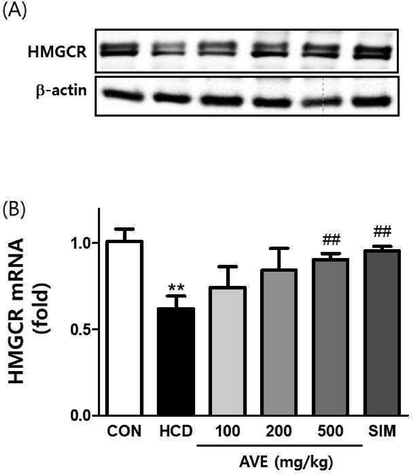
Hepatic protein and mRNA expression of HMGCR in C57BL/6 mice fed a high-cholesterol diet supplemented with different doses of Amomum villosum water extract (AVE). **p < 0.01 comparing HCD with control diet; ##p < 0.01 comparing HCD + AVE with HCD alone. All data are mean ± SD (n = 10).
4 Discussion
Sterol regulatory element-binding proteins (SREBPs) stimulate the expression of genes involved in cholesterol production, such as HMG-CoA reductase (HMGCR). SREBPs stimulate the expression of the LDL receptor (LDLR), which aids in cholesterol absorption. Because cholesterol and triglycerides are so important to all cells, it's no accident that SREBPs are closely regulated, with a lot of interaction and some redundancy. SREBP2 has long been estimated to be associated for the activation of genes involved in cholesterol production. (Madison, 2016). Five types of lipoprotein circulate in the extracellular environment of the human body: low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), intermediate-density lipoprotein (IDL), and chylomicrons. The liver is the major organ for the metabolism and regulation of cholesterol. VLDL is produced in the liver, contains apolipoprotein and fatty acids, and carries cholesterol. In the blood, lipoprotein lipase and cholesteryl ester transfer protein (CETP) convert circulating VLDL into cholesterol enriched species, primary IDL, and then LDL. LDL is the main carrier of cholesterol in the body, transporting cholesterol into the circulation. These lipoproteins are regulated and cleared by the liver via LDL receptors present on the surfaces of hepatocytes (Feingold, 2021).
A deficiency in LDL receptors leads to elevated levels of LDL-C in the circulatory system. If too high, LDL-C particles in circulatory system enter the endothelium of arterial walls, stimulate inflammation, injure the smooth muscle (Epstein and Ross, 1999), and lead to lipid-rich plaques. Experiments in LDL receptor- deficient mice demonstrated that elevated LDL-C leads to severe atherosclerosis (Véniant et al., 2000). In contrast, mice lacking LDL-C could not progress to atherosclerosis regardless of coronary heart disease (CHD) risk factors and diet (Lieu et al., 2003).
AVE acts as a potential anti-obesity agent in mice, as we reported previously (Kim et al., 2021). In this research work, we presented that AVE medication efficiently relieved the hyperlipidemia-associated symptoms in the HCD mouse model, reducing body weight gain, liver mass, epididymal fat mass, and lipid levels in hepatic tissue and serum. Correlate to the HCD alone treated animals, the administration of AVE for a four-week time period led to noticeable decreases in serum TC, LDL and TG levels, suggesting that AVE had beneficial effects on liver function, and decreased cholesterol deposits produced by a HCD. The present study suggested that AVE can scavenge circulating blood LDL and promote cholesterol homeostasis of the liver. We suggest that AVE can reduce lipid levels through upregulation of Srebp2, Ldlr, and Hmgcr gene expression.
During low-cholesterol diet consumption (normal pellet diet), hepatic cholesterol levels are decreased, and as a result endoplasmic reticulum (ER) resided SREBP-2 enters into the nucleus, where it activates the HMG-CoA reductase, the LDL receptor, and other genes necessary for cholesterol synthesis. Sufficient numbers of LDL receptors on plasma membranes keep the blood LDL level down. However, an HCD causes significant cholesterol accumulation on hepatic cell ER membranes, inhibiting srebp-2 processing, reducing LDL receptor production, and raising blood LDL levels. Our results were consistent with a previous study (Goldstein and Brown, 2015).
5 Conclusion
In summary, the treatment of a AVE on HCD-induced hyperlipidemia in mice model lead to the restoration of liver, epididymal fat, and body weight, along with serum TG, TC, LDL-C levels, and in the hepatic TG level, and the levels of different genes facilitated in lipogenesis and adipogenesis processes as well as HMGCR protein expression levels were parallel to those of the control group, emphasizing its promising anti-hyperlipidemic effects.
Acknowledgement
This work was supported by project for Collabo R&D between Industry, Academy, and Research Institute funded by Korea Ministry of SMEs and Startups in 2020 (Project No. S2853447).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hypercholesterolemia and coronary heart disease in the elderly: a meta-analysis. Ann. Epidemiol.. 2004;14(9):705-721.
- [Google Scholar]
- Renoprotective Effects of Vitex megapotamica (Spreng.) Moldenke in C57BL/6 LDLr-null mice undergoing high fat diet. Evid.-Based Complement. Altern. Med.. 2015;2015:1-10.
- [Google Scholar]
- Application of non- HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet. 2019;394(10215):2173-2183.
- [Google Scholar]
- Therapeutic Effect of Amomum villosum on inflammatory bowel disease in rats. Front. pharmacol.. 2018;9:639.
- [Google Scholar]
- Feingold, KR., Introduction to Lipids and Lipoproteins. 2021. In: Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., Kovacs, C.S., Kuohung, W., Laferrère, B., Levy, M., McGee, E.A., McLachlan, R., Morley, J.E., New, M., Purnell, J., Sahay, R., Singer, F., Sperling, M.A., Stratakis, C.A., Trence, D.L., Wilson, D.P., editors. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 26247089.
- Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J.. 2017;38:2459-2472.
- [Google Scholar]
- A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161-172.
- [Google Scholar]
- Risk factor exposure in individuals free from cardiovascular disease differs according to age at first myocardial infarction. Eur. Heart J.. 2016;37(25):1977-1981.
- [Google Scholar]
- SNP typing for germplasm identification of Amomum villosum lour. Based on DNA barcoding markers. PLoS One. 2014;9(12):e114940.
- [Google Scholar]
- MicroRNA- 30c mimic mitigates hypercholesterolemia and atherosclerosis in mice. J. Biol. Chem.. 2016;291(35):18397-18409.
- [Google Scholar]
- Hypercholesterolaemia and risk of coronary heart disease in the elderly: impact of age: the Copenhagen City Heart Study. Eur. J. Intern. Med.. 2009;20(2):139-144.
- [Google Scholar]
- Amomum villosum Lour. fruit extract ameliorates high- fat diet-induced body mass gain and adipogenic pathways in C57BL/6 mice. J. King Saud Univ. Sci.. 2021;33(5):101473.
- [Google Scholar]
- Inhibitory effect of Amomum villosum water extracts on α-glucosidase activity. Physiol. Mol. Plant Pathol.. 2022;117:101779.
- [Google Scholar]
- Acute effects of Amomum villosum Lour. Fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebocontrolled, crossover study in healthy subjects. Saudi J. Biol. Sci.. 2020;27(11):2968-2971.
- [Google Scholar]
- Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107(9):1315-1321.
- [Google Scholar]
- Volatile oil of Amomum villosum inhibits nonalcoholic fatty liver disease via the gut-liver axis. Biomed. Res. Int.. 2018;2018:1-16.
- [Google Scholar]
- Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov.. 2022;21(3):201-223.
- [Google Scholar]
- Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res.. 2016;57(3):333-335.
- [Google Scholar]
- Malloy, M.J., Kane, J.P., 2004. Agents used in hyperlipidemia; in Katzung BG (ed.): Basic and Clinical Pharmacology, ed 9. New York, McGraw-Hill, pp 561–575.
- Talbert, R.L., Hyperlipidemia; in DiPiro, J.T., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G., Posey, L.M., (eds.) 2005. Pharmacotherapy: a Pathophysiologic Approach, ed., 6. McGraw-Hill, New York.
- Defining the atherogenicity of large and small lipoproteins containing apolipoprotein B100. J. Clin. Invest.. 2000;106(12):1501-1510.
- [Google Scholar]







