Translate this page into:
Ammonia sensing of silver nanoparticles synthesized using tannic acid combined with UV radiation: Effect of UV exposure time
⁎Corresponding author at: Department of Materials Science, Faculty of Science, Chulalongkorn University, Phayathai Rd., Pathumwan, Bangkok 10330, Thailand. vimolvan.p@chula.ac.th (Vimolvan Pimpan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Green synthesis of silver nanoparticles were done using silver nitrate as a silver precursor and tannic acid as a reducing agent and a stabilizer with the assistance of UV radiation at room temperature and neutral condition. The aim of this research was to study the effect of UV exposure time on the synthesis and ammonia sensing ability of the obtained nanoparticles; therefore, UV exposure times were varied from 15, 30, 45, 60, 90 to 120 min. The intensities of the characteristic peak at around 370 and 425 nm in UV–vis spectra increased with increasing UV exposure time. The spherical nanoparticles having the diameters in the range of 8–30 nm were revealed by transmission electron microscope and its crystal structure was revealed by HRTEM. After adding ammonia, the color of all synthesized nanoparticles colloids changed from orange-yellow to yellowish-green which can be preliminary observed by the naked eyes. They also exhibited the absorbance peaks at around 320 nm and a decrease in the intensities of the absorbance peaks at 370 and 425 nm. The results suggested that UV exposure time of 60 min used in this system was suitable for synthesizing silver nanoparticles with high sensing ability to ammonia and the detection limit of 100 ppm.
Keywords
Silver nanoparticles
Tannic acid
UV radiation
UV exposure time
Ammonia
Sensing
1 Introduction
Ammonia is occurred from the decomposition of organic compounds, the gas exchange with the atmosphere and nitrogen fixation processes (Ip et al., 2001). It’s widely used in many industries. Ammonia gas is toxic to eyes, nose, throat and lungs. Ammonia solution having concentration higher than 1000 mg/L can cause burn to skin and membranes. The United States Environment Protection Agency (US EPA) sets the national criteria for ammonia in fresh water at 17 mg TAN/L for an acute criterion and 1.9 mg TAN/L for a chronic criterion at pH 7 and 20 °C (US EPA, 2013). Due to its toxic nature to human and animals, the detection of ammonia levels in the environment is necessary (Dubas and Pimpan, 2008; Timmer et al., 2005). Several ammonia sensors have been developed based on the reactions such as Nessler reaction (Molins-Legua et al., 2006) and luminol-hypochlorite reaction (Li and Dasgupta, 1999). However, there are many drawbacks. For example, toxic chemicals are used in these reactions and additional equipment are needed for the detection process.
In recent years, silver nanoparticles have been used for detecting many chemicals via colorimetric sensing due to its good optical characteristic known as surface plasmon resonance (SPR) (Wiley et al., 2005). SPR is an optical phenomenon observed when the electromagnetic radiation excites the surface conducting electrons of silver nanoparticles resulting in a coherent resonance oscillation around a particle (Willets and Van Duyne, 2007). When the surrounding environment is changed, especially the change in the reflective index of the surrounding medium and the adsorption of the molecules on the metal surface, the change in the absorbance of the visible and near-infrared wavelength regions can be observed by UV–vis spectroscopy and sometimes by the naked eyes (Zeng et al., 2014).
Many researchers reported the colorimetric sensing of silver nanoparticles for many chemicals. Yang et al. (2015) synthesized the silver nanoparticles using peach gum polysaccharide. The obtained nanoparticles showed the color change from brown to colorless for hydrogen peroxide (H2O2) with the detection limit at 0.035 mM. Manivel and Ilanchelian (2016) synthesized silver nanoparticles using Ficus amplissima leave extract under sunlight irradiation. These nanoparticles were then used for mercury ion sensing. Yellowish silver nanoparticles colloids became colorless in the presence of Hg2+ with wide range pH from 3.2 to 8.5. Song et al. (2016) prepared silver nanoparticles using formamidinesulfinic acid and soluble starch. The synthesized silver nanoparticles showed the colorimetric sensing for metal ions. Their colors changed from yellow to orange for Cr3+, pink for Al3+ and to colorless for Fe3+ and Hg2+ ions, respectively.
Few studies involved ammonia detection. For example, Dubas and Pimpan (2008) studied the ammonia sensing of silver nanoparticles reduced by sodium borohydride and stabilized by poly(methacrylic acid). Their color changed from purple to yellow after adding ammonia solution with the detection limit of 5 ppm. Detsri and Popanyasak (2015) presented the composite thin films of silver nanoparticles and polyaniline prepared by the Layer-by-Layer (LbL) deposition technique. The films were exposed to ammonia and exhibited the color change from orange-red to yellow with the detection limit of 0.2038 m. However, in these works, some chemicals used in the synthesis of silver nanoparticles were harmful to the environment and excess chemicals were needed in order to complete the reaction. Therefore, natural products especially those derived from plants are of interest. For example, natural biopolymers are playing an important role in nanotechnology and show potential in many application such as adsorption, sensors, antimicrobial activity, catalysis, etc. (Pandey, 2016, 2017; Pandey and Ramontja, 2016a,b,c,d,e; Pandey and Nanda, 2016) using “green” chemicals such as sugar, polysaccharides, ascorbic acid and plant extracts (Ganaie et al., 2014) that can act as both reducing agent and stabilizer is a benefit to the synthesis. However, the disadvantage of using these chemicals is that a long reaction time is needed to complete the reduction at room temperature for 20 h (Raveendran et al., 2003).
Tannic acid was selected to be used in this work because it is a chemical substance that can be derived from plants such as Tara spinosa pods (Tara spinosa), Aleppo Oak (Quercus infectoria) and Sicilian Sumac (Rhus coriaria L.) which are renewable resources. It contains a branched structure with phenolic form showing a potential to act as a reducing agent and stabilizer. Its solubility in water also allow the system to employed water in the process which is safe for the environment. Currently, it has been used as both reducing agent and stabilizer for synthesizing silver nanoparticles (Cataldo et al., 2013; Yi et al., 2011). However, long reaction times such as 5 h (Yi et al., 2011) or 12 h (Tian et al., 2007) must be employed in order to obtain the desired products. Furthermore, it was also found that higher formation of silver nanoparticles was obtained with increasing the reaction time (Raja et al., 2014). Therefore, microwave radiation (Chen et al., 2008; Melendrez et al., 2015; Yin et al., 2004), sunlight (Pourjavadi and Soleyman, 2011; Yang et al., 2015) and UV radiation (Dubas and Pimpan, 2008; Spadaro et al., 2010) have been applied for reducing the reaction time.
This present work mainly focused on the effect of UV exposure time on the synthesis of silver nanoparticles using tannic acid and ammonia sensing ability of the obtained silver nanoparticles. Therefore, other parameters including pH, metal salt concentration and tannic acid concentration were kept constant. In addition, the effect of ammonia concentration on ammonia sensing ability of the obtained silver nanoparticles was also investigated.
2 Materials and methods
2.1 Chemicals
Silver nitrate and tannic acid were obtained from Sigma-Aldrich (USA). Potassium carbonate was purchased from Ajax Finechem (Australia). Ammonia (25% aqueous solution) was a product of Merck (Germany). These chemicals were analytical grade and were used as received without any purification. Deionized water was used for preparing all aqueous solution in the experiments.
2.2 Synthesis of silver nanoparticles
20 ml of 1 mM tannic acid aqueous solution was placed in a beaker and adjusted to pH 7.0 using 0.1 M potassium carbonate. 5 ml of 4 mM silver nitrate aqueous solution was then added to tannic acid solution at room temperature. The mixture was then exposed to a 16 W UV radiation. The exposure times were varied from 15, 30, 45, 60, 90 to 120 min, respectively. After that, the synthesized silver nanoparticles were subjected to characterization and ammonia sensing test. The remaining nanoparticles were then stored in amber bottles and kept in the dark place at room temperature.
2.3 Characterization of the synthesized silver nanoparticles
The optical characteristics of the silver nanoparticles were determined using a UV–vis spectrophotometer (Specord S100, Analytic Jena GmbH) in the wavelength range of 200–600 nm. The morphology of the synthesized silver nanoparticles were observed by transmission electron microscope (TEM) (JEM-2100, JEOL). This machine was operated at 120 keV accelerating voltage in a vacuum chamber. A small drop of silver nanoparticles colloids was dropped on a carbon-coated copper grid for TEM testing. The prepared grid was kept in the desiccator for drying overnight.
2.4 Ammonia sensing test
Ammonia sensing test was done by mixing 1 ml of silver nanoparticles with 1 ml of ammonia aqueous solution. Ammonia concentrations were varied from 0, 100, 200, 300, 400 to 500 ppm, respectively. 25% of ammonia aqueous solution were diluted to the concentration mentioned above. The color change was observed by the naked eyes and the SPR bands of silver nanoparticles were detected using UV–vis spectrophotometer (Specord S100, Analytic Jena GmbH).
3 Results and discussion
3.1 The characteristics of the synthesized silver nanoparticles
After adding silver nitrate solution into tannic acid solution, the colors of the mixtures changed from colorless to orange-yellow as shown in Fig. 1 indicating the formation of silver nanoparticles. Their colors became darker with increasing the exposure time suggesting higher amount of silver nanoparticles were formed.
The appearances of silver nitrate solution and silver nanoparticles colloids synthesized at various times of 15, 30, 45, 60, 90 and 120 min.
It is clearly seen from Fig. 2 that the SPR bands of most synthesized silver nanoparticles are at around 370 nm and 425 nm except for those of the silver nanoparticles synthesized under UV exposure for 15 min that exhibited slightly higher wavelength at 372 and 429 nm, respectively. The higher wavelength of SPR band at around 425 nm is in good agreement with the typical maximum wavelength (λmax) of silver nanoparticles previously reported by Bulut and Ozacar (2009) whereas the lower wavelength of SPR band at around 370 nm suggests the formation of silver nanoparticles with different sizes as previously found by several researchers (Darroudi et al., 2009; Balavandy et al., 2014). On the other hand, tannic acid exhibits the absorbance of hydroxylated polyphenol and benzenoid band at 210 nm and 275 nm, respectively (Cataldo et al., 2013). This is because tannic acid is hydrolyzed into gallic acid and glucose at mild acid or basic condition (Yi et al., 2011). These phenolic groups donate the electrons for the reduction of silver ions into silver atoms and form the quinone groups which could promote the stabilization of the synthesized silver nanoparticles as shown in Fig. 3 as previously observed in other plant extract baring similar functional groups (Edison et al., 2016). UV radiation promotes the reduction by increasing the dissociation of the system (Abedini et al., 2013) Fig. 2 also shows an increase in the absorbance intensities of the silver nanoparticles at 370 nm and 425 nm with increasing UV exposure time due to higher numbers of the nanoparticles formed as a result of higher reduction (Yi et al., 2011). However, the peak intensity of the silver nanoparticles synthesized using UV exposure time of 60 min is comparable to those of silver nanoparticles synthesized using UV exposure times of 90 and 120 min. This suggests that UV exposure time of 60 min may be suitable for synthesizing silver nanoparticles using this system.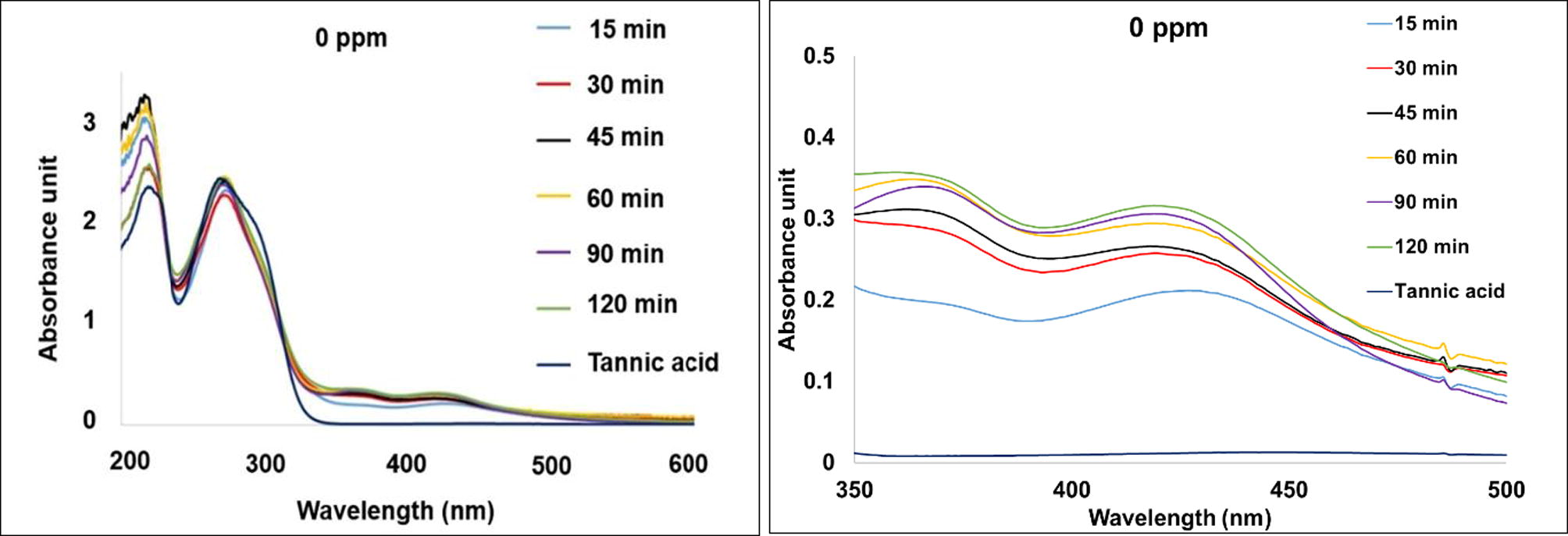
UV–vis spectra of tannic acid solution and the silver nanoparticles colloids synthesized using various UV exposure times (left) and their expansion at wavelength range from 350 to 500 nm (right).
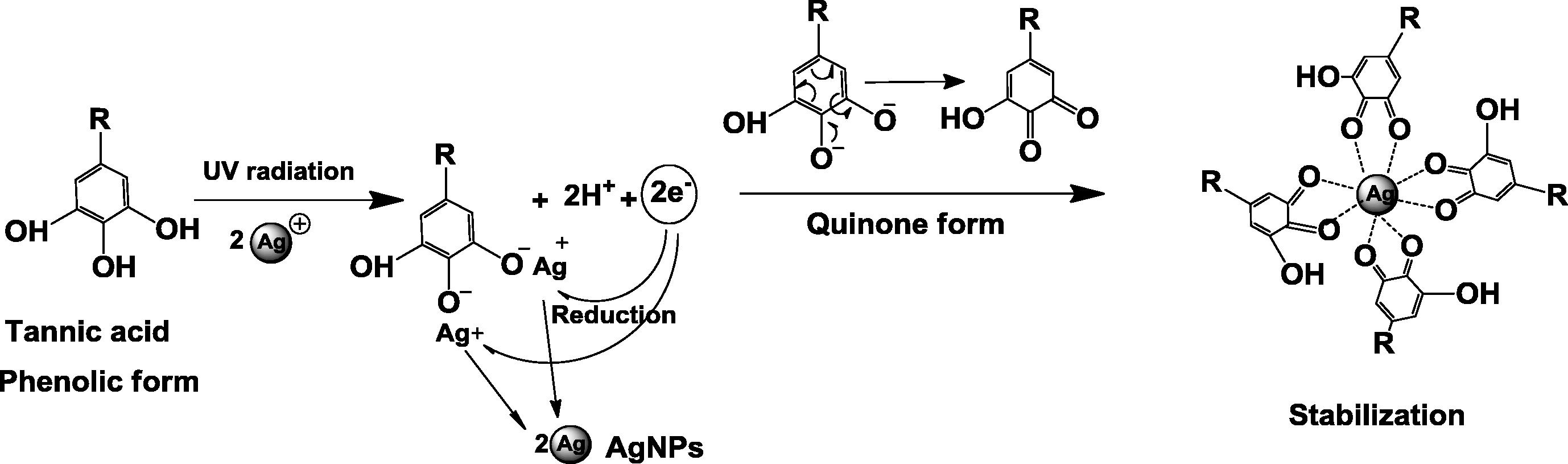
The possible reaction of the synthesis of silver nanoparticles using tannic acid acting as a reducing agent and a stabilizer with the assistance of UV radiation.
An example of TEM photograph as shown in Fig. 4A also confirms the formation of silver nanoparticles. The main morphology of the synthesized silver nanoparticles is spherical with the diameter ranging from 8 to 30 nm. The histogram in Fig. 4B indicates that there are two main sizes of the obtained nanoparticles which are in good agreement with the SPR bands as shown in Fig. 2. This histogram also shows the narrow size distribution of silver nanoparticles. This is because UV radiation can help achieving the narrow size distribution of silver nanoparticles as previously reported by Spadaro et al. (2010). High-resolution TEM image shown in Fig. 4C with the interlayer having a spacing of 0.24 nm reveals that the growth of Ag nanoparticles occurs preferentially on the (1 1 1) plane of FCC silver (Philip, 2010; Lim et al., 2006). EDX spectrum in Fig. 4D also confirms the presence of silver element.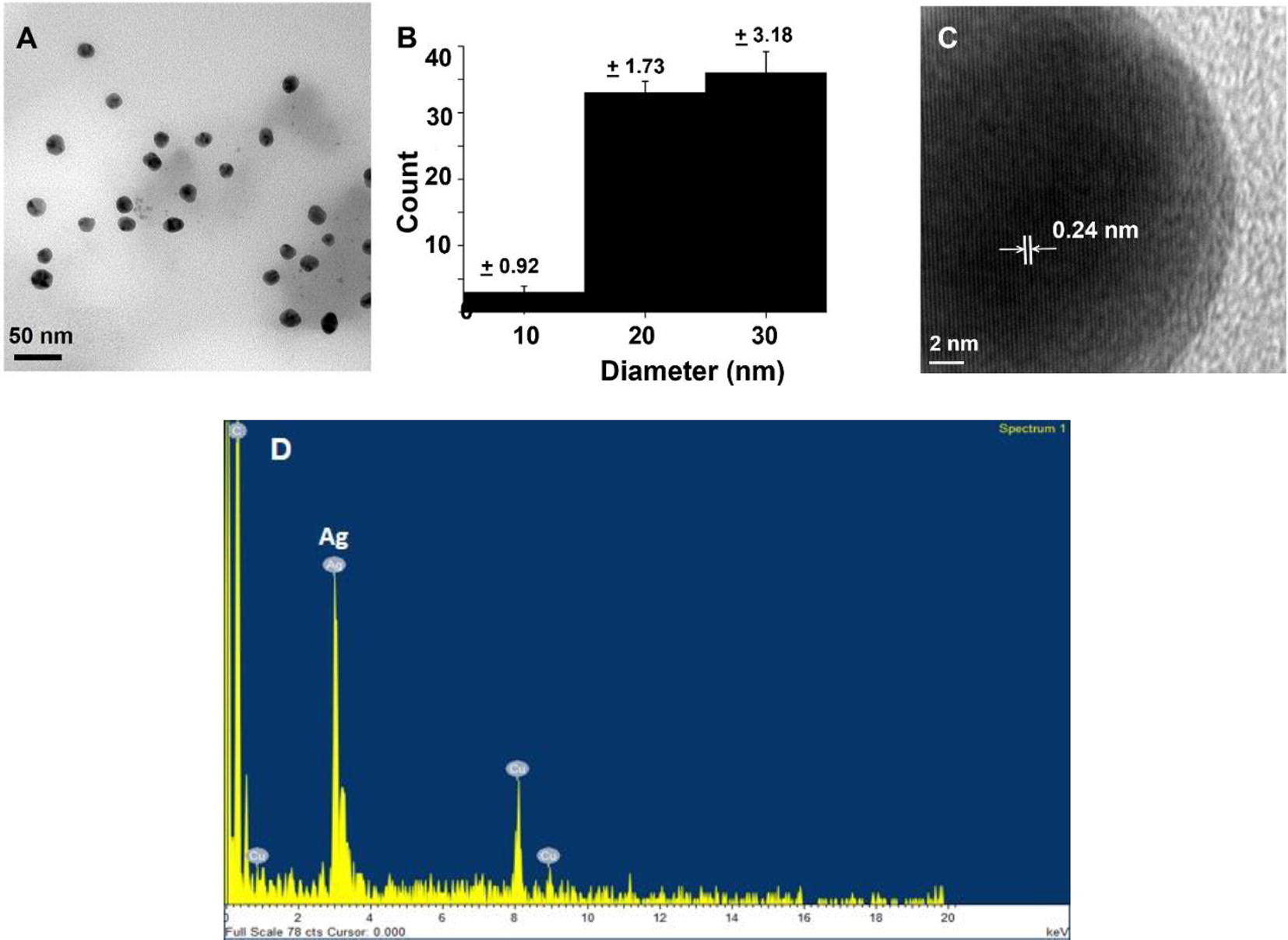
(A) TEM photograph, (B) the histogram (c) HRTEM and (d) EDX spectrum of silver nanoparticles synthesized using UV exposure time of 60 min.
3.2 Ammonia sensing
Preliminary study of ammonia sensing ability was employed in order to make sure that the results were due to the sensing phenomenon not pH effect. The silver nanoparticles colloids synthesized under UV exposure time of 60 min were used. After adding 200 ppm of ammonia, the pH of the system became 9.20. Therefore, two other nanoparticles colloids were synthesized at pH 9.20 and 10 based on the assumption that if ammonia only increases the pH of the system and causes an increase in the amount of silver nanoparticles but not causing any sensing phenomenon, the observation should be the same. As shown in Fig. 5, it is clearly seen that after adding 200 ppm of ammonia to silver nanoparticles colloids synthesized at pH 7, the new absorbance peak at around 320 nm occurs. However, this peak was not observed in the samples synthesized at alkaline pH. His suggests the sensing ability to ammonia and in good agreement with previous research done by Edison and coworkers (Edison et al., 2016). On the other hand, the color of the colloids synthesized using alkaline conditions are dark brown due to high amount of silver nanoparticles formed but that of the nanoparticles colloids synthesized at pH 7 and 200 ppm of ammonia was added was only yellowish-green.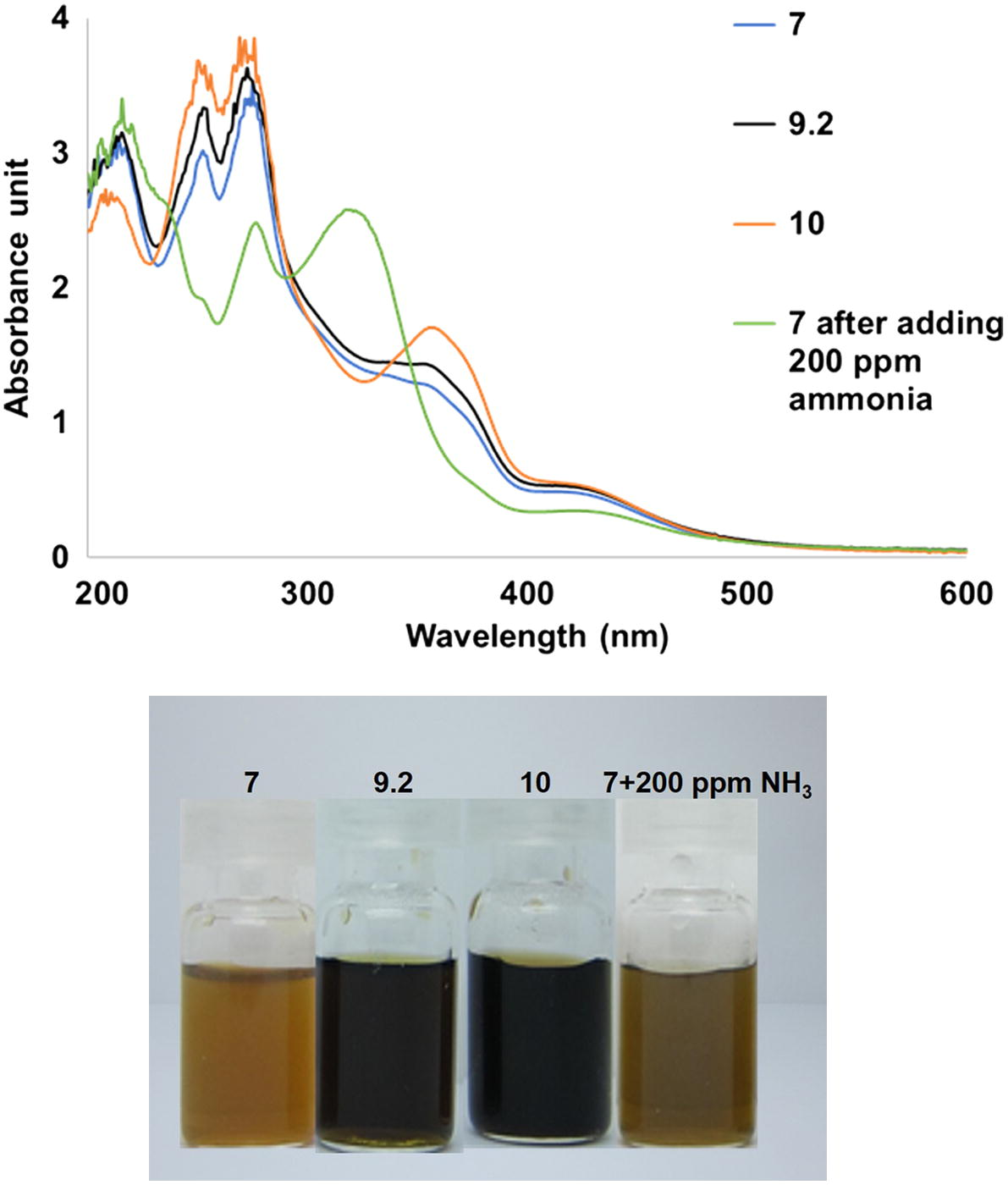
UV–vis spectra (top) and appearances (bottom) of the silver nanoparticles synthesized under 60 min of UV exposure at pH 7.0, 9.20, 10.0 and pH 7.0 after adding 200 ppm of ammonia.
Furthermore, to determine a detection limit, various concentrations of ammonia were added to the silver nanoparticles colloids synthesized under 60 min of UV exposure at pH 7. Fig. 6 shows that the peak at 320 nm start clearly appearing at ammonia concentration of 100 ppm. This suggests that 100 ppm may possibly be the detection limit for this system. Therefore, the lowest concentration of ammonia used in further experiment was 100 ppm.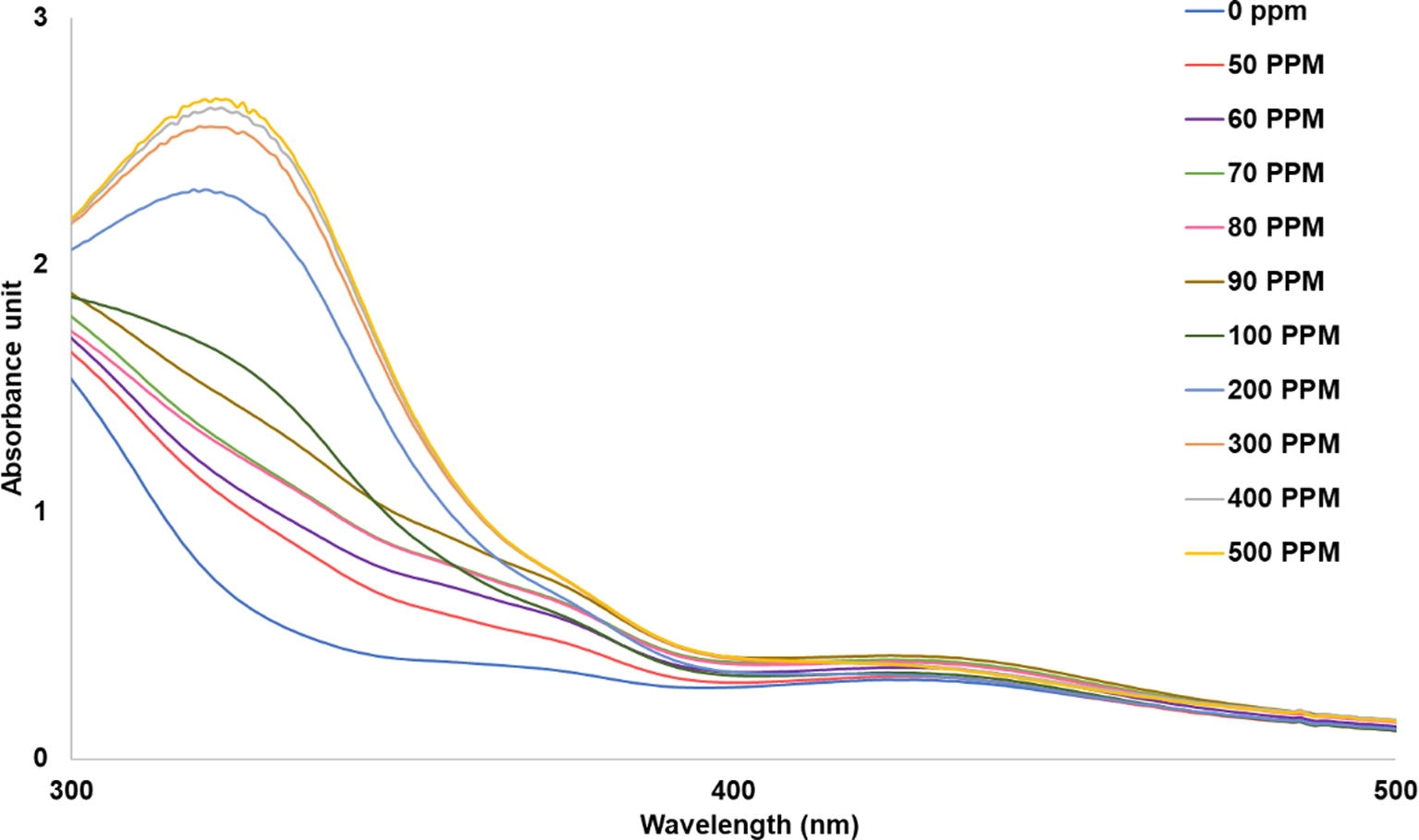
UV–vis spectra of the silver nanoparticles synthesized at pH 7 under 60 min of UV exposure after adding various amount of ammonia.
Fig. 7 shows the new absorbance peaks appeared at around 320 nm after adding ammonia into all silver nanoparticles colloids while the intensities of the peaks at around 370 and 425 nm decreased. As UV exposure time increases, higher intensities of the absorbance peaks at around 320 nm are observed indicating higher sensing ability of the system. This is because higher amount of silver nanoparticles were formed at longer UV exposure time.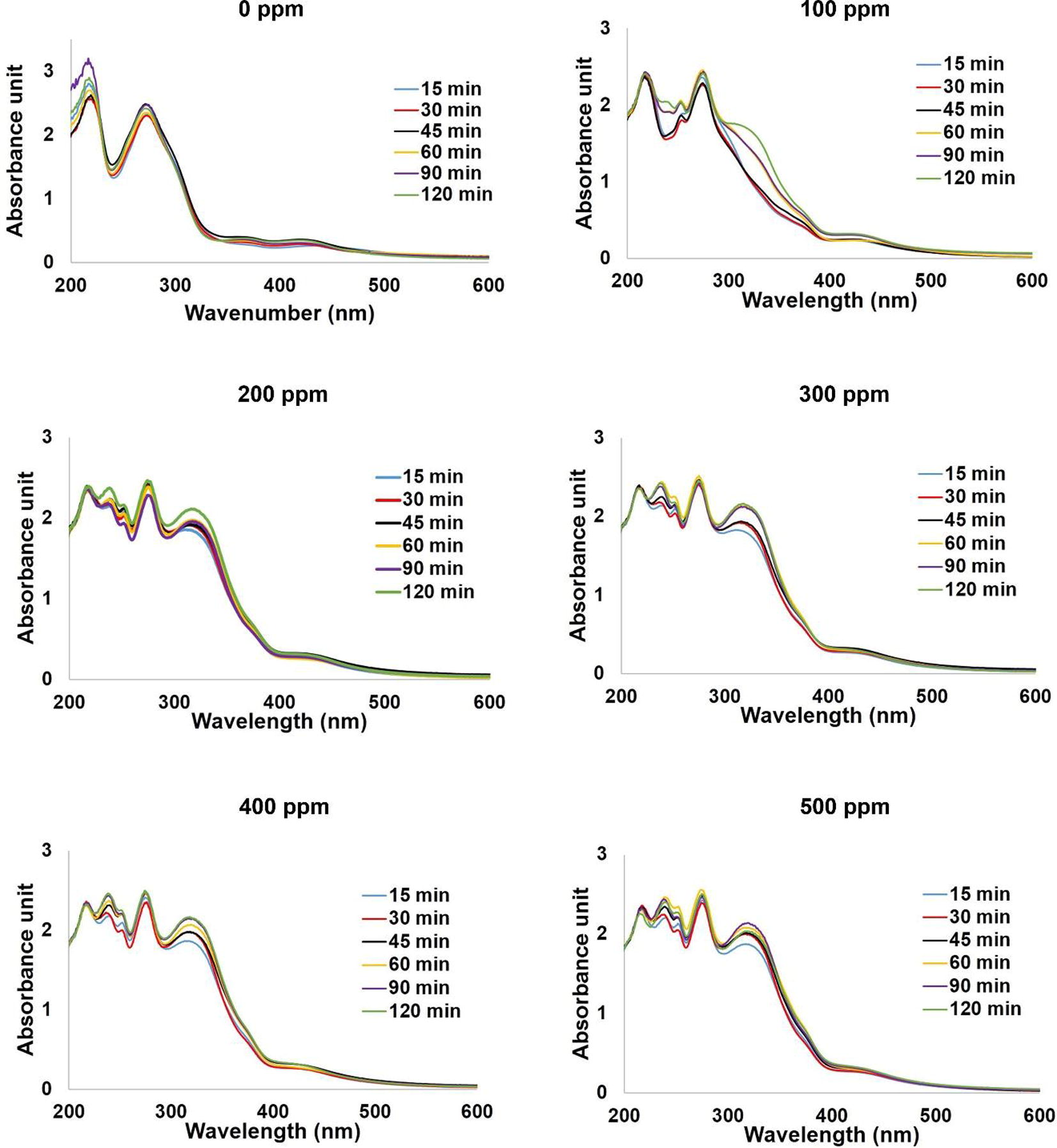
Effect of UV exposure time on the sensing ability of silver nanoparticles colloids.
Fig. 8 reveals that at every UV exposure times, the strong intensities of the absorbance peaks at around 320 nm are comparable when ammonia concentrations are at 200–500 ppm with a slightly increase as the ammonia concentration increases. On the other hand, the intensities of those exposed to 100 ppm of ammonia are weaker. This suggests that the ammonia concentration of 100 ppm may be too low for qualitative analysis even though it is a detection limit and its color change can be preliminary observed by the naked eye as evidenced in Fig. 9. Fig. 9 also shows that the color of all nanoparticles colloids changed from orange-yellow to yellowish-green at every concentration of ammonia.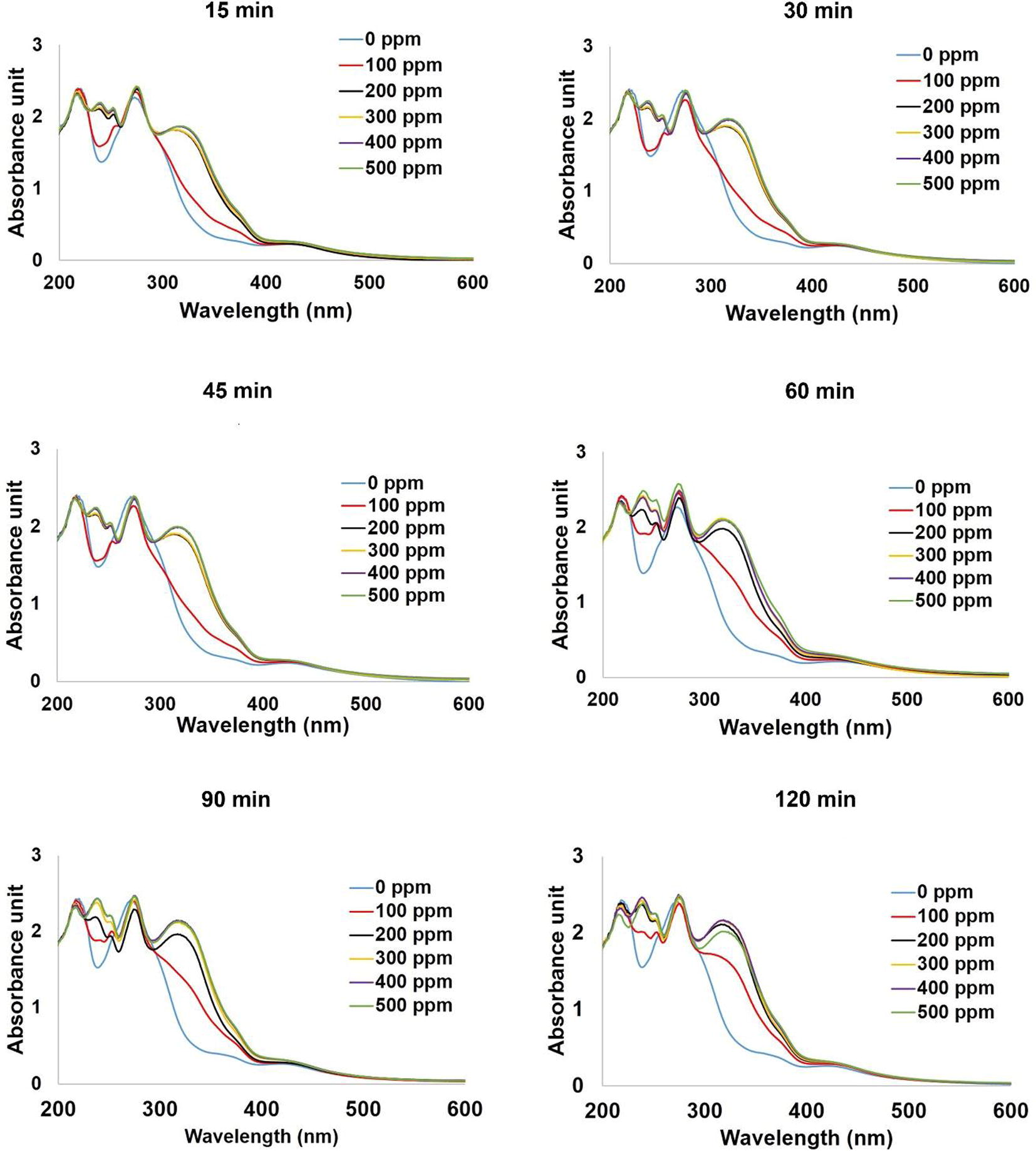
Effect of ammonia concentration on the sensing ability of silver nanoparticles colloids.

The appearances of silver nanoparticles colloids synthesized using UV exposure time of 60 min after exposing to ammonia at the concentrations of 0, 100, 200 300, 400 and 500 ppm.
The results from Figs. 7 and 8 also suggest that UV exposure time of 60 min employed in the synthesis step is suitable for achieving the silver nanoparticles which have high sensing ability to ammonia since their intensities of the absorbance peak at around 320 nm are comparable to those of the silver nanoparticles synthesized using UV exposure time of 90 and 120 min at almost ammonia concentrations.
From Fig. 10, it is clearly seen that using UV exposure time of 60 min was enough for producing silver nanoparticles with high sensitivity and the results were repeatable as shown in Fig. 11.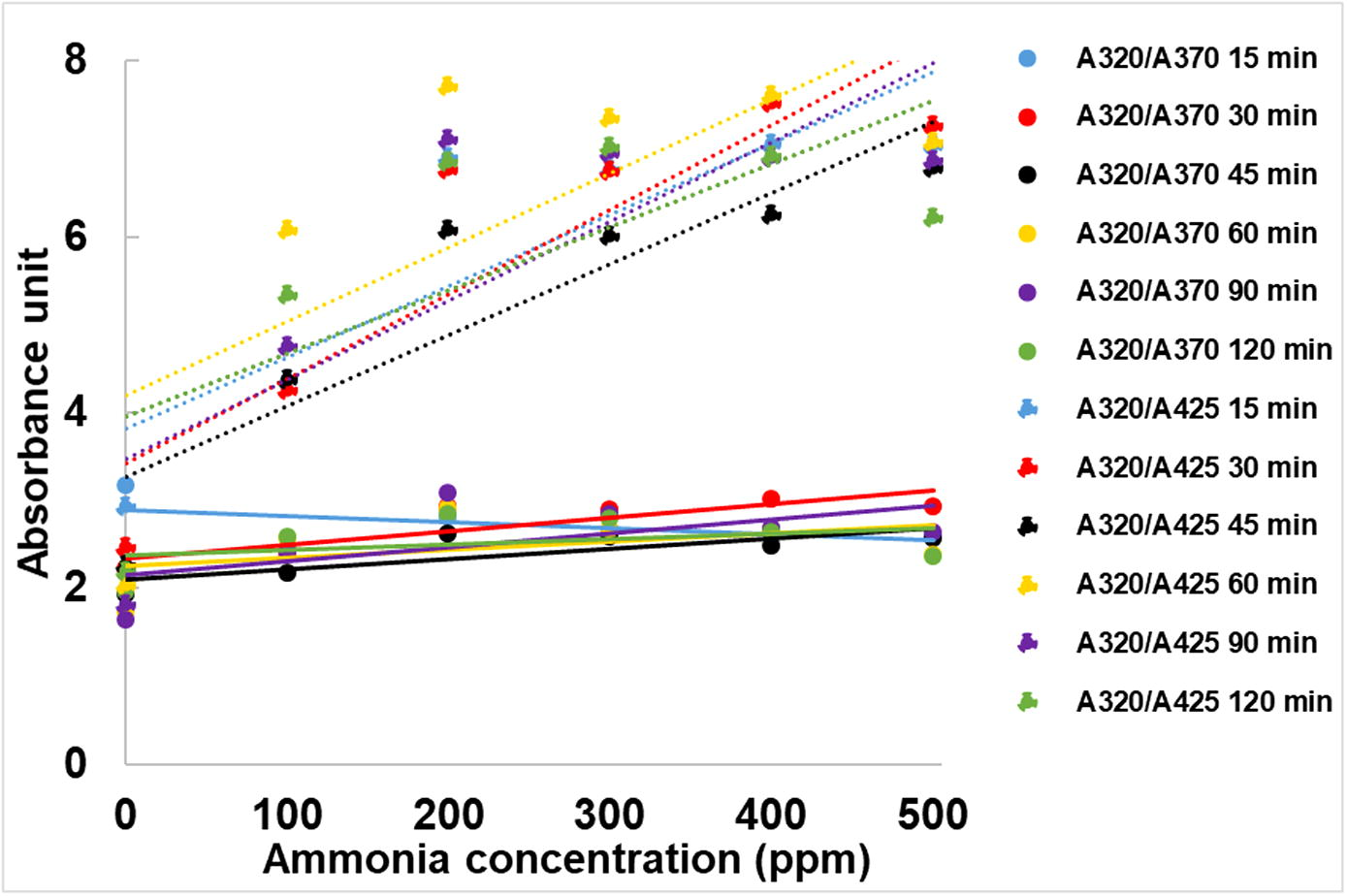
Ratio of absorbance peaks of the silver nanoparticle after adding various concentrations of ammonia.
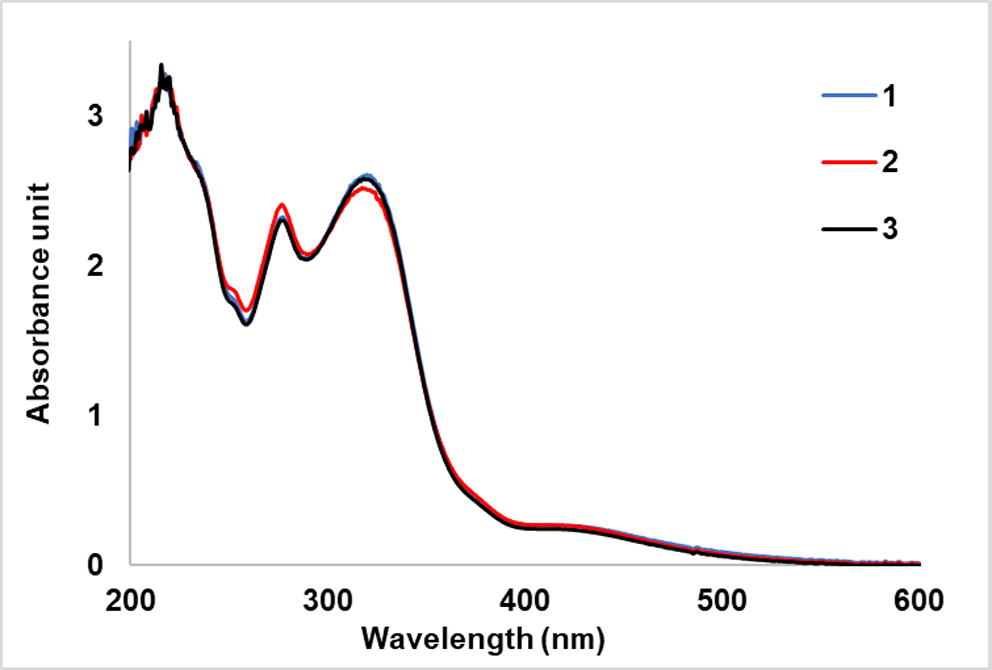
Repeatable results of UV–vis spectra of the silver nanoparticles colloids synthesized at UV exposure time of 60 min and added with 200 ppm of ammonia.
4 Conclusion
Spherical silver nanoparticles were synthesized by a “green” method using tannic acid combined with UV radiation at room temperature and neutral condition. The above results indicate that a greater amount of silver nanoparticles were formed with increasing UV exposure time as shown in Figs. 1 and 2. All synthesized silver nanoparticles exhibited ammonia sensing ability. However, due to the difference concentrations of the silver nanoparticles obtained at each UV exposure time, their sensing ability was different. In addition, this sensing ability also affected by ammonia concentration. Since the color change of silver nanoparticles colloids after exposing to ammonia can be preliminary detected by the naked eye, the application of these nanoparticles as an ammonia sensor is possible.
Funding
This work was funded by the Department of Materials Science, Faculty of Science, Chulalongkorn University, the Center of Excellence on Petrochemical and Materials Technology and the authors’ personal fund.
Conflict of interest
The authors certify that there is no actual or potential conflict of interest in relation to this manuscript.
Acknowledgement
The authors would like to acknowledge the Department of Materials Science, Faculty of Science, Chulalongkorn University and the Center of Excellence on Petrochemical and Materials Technology for financial and equipment supports.
References
- A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett.. 2013;8(1):474.
- [CrossRef] [Google Scholar]
- Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem. Cent. J.. 2014;8(1):11-20.
- [CrossRef] [Google Scholar]
- Rapid, facile synthesis of silver nanostructure using hydrolyzable tannin. Ind. Eng. Chem. Res.. 2009;48:5686-5690.
- [CrossRef] [Google Scholar]
- A green synthesis of colloidal silver nanoparticles and their reaction with ozone. Eur. Chem. Bull.. 2013;2:700-705.
- [CrossRef] [Google Scholar]
- Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater. Chem. Phys.. 2008;108:421-424.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of UV-irradiated silver/montmorillonite nanocomposites. Solid State Sci.. 2009;11(9):1621-1624.
- [CrossRef] [Google Scholar]
- Fabrication of silver nanoparticles/polyaniline composite thin films using Layer-by-Layer self-assembly technique for ammonia sensing. Colloids Surf. A. 2015;467:57-65.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles for ammonia sensing. Talanta. 2008;76:29-33.
- [CrossRef] [Google Scholar]
- Optical sensor for dissolved ammonia through the green synthesis of silver nanoparticles by fruit extract of Terminalia Chebula. J. Cluster Sci.. 2016;27:683-690.
- [CrossRef] [Google Scholar]
- Biomimetic synthesis of silver nanoparticles using the amphibious weed ipomoea and their application in pollution control. J. King Saud Univ. Sci.. 2014;26:222-229.
- [CrossRef] [Google Scholar]
- Ammonia toxicity, tolerance, and excretion. In: Patricia W., Paul A., eds. Fish Physiology. Academic Press; 2001. p. :109-148.
- [Google Scholar]
- Chemiluminescence detection with a liquid core waveguide – determination of ammonium with electrogenerated hypochlorite based on the luminol-hypochlorite reaction. Anal. Chim. Acta. 1999;398:33-39.
- [CrossRef] [Google Scholar]
- Synthesis of Ag nanospheres particles in ethylene glycol by electrochemical-assisted polyol process. Chem. Phys. Lett.. 2006;420(4):304-308.
- [CrossRef] [Google Scholar]
- Selective and sensitive colorimetric detection of Hg2+ at Wide pH range using green synthesized silver nanoparticles as probe. J. Cluster Sci.. 2016;1–18
- [CrossRef] [Google Scholar]
- Quality and high yield synthesis of Ag nanowires by microwave-assisted hydrothermal method. Nanoscale Res. Lett.. 2015;10:48.
- [CrossRef] [Google Scholar]
- A guide for selecting the most appropriate method for ammonium determination in water analysis. TrACTrends Anal. Chem.. 2006;25:282-290.
- [CrossRef] [Google Scholar]
- Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: a comprehensive review. J. Sci. Adv. Mater. Dev.. 2016;1:431-453.
- [CrossRef] [Google Scholar]
- A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq.. 2017;241:1091-1113.
- [CrossRef] [Google Scholar]
- Au nanocomposite based chemiresistive ammonia sensor for health monitoring. ACS Sens.. 2016;1:55-62.
- [CrossRef] [Google Scholar]
- Guar gum-grafted poly(acrylonitrile)-templated silica xerogel: nanoengineered material for lead ion removal. J. Anal. Sci. Technol.. 2016;7:24-37.
- [Google Scholar]
- Natural bentonite clay and its composites for dye removal: current state and future potential. Am. J. Chem. Appl.. 2016;3:8-19.
- [Google Scholar]
- Recent modifications of bentonite clay for adsorption applications. Focus Sci.. 2016;2(4):1-10.
- [Google Scholar]
- Sodium alginate stabilized silver nanoparticles–silica nanohybrid and their antibacterial characteristics. Int. J. Biol. Macromol.. 2016;93:712-723.
- [CrossRef] [Google Scholar]
- Turning to nanotechnology for water pollution control: applications of nanocomposites. Focus Sci.. 2016;2(2):1-10.
- [Google Scholar]
- Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta Part A. 2010;75(3):1078-1081.
- [CrossRef] [Google Scholar]
- Novel silver nano-wedges for killing microorganisms. Mater. Res. Bull.. 2011;46:1860-1865.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using tannins. Mater. Sci.-Poland. 2014;32:408-413.
- [CrossRef] [Google Scholar]
- Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc.. 2003;125:13940-13941.
- [CrossRef] [Google Scholar]
- Preparation of silver nanoparticles reduced by formamidinesulfinic acid and its application in colorimetric sensor. J. Cluster Sci.. 2016;27:1203-1212.
- [CrossRef] [Google Scholar]
- Synthesis of PMA stabilized silver nanoparticles by chemical reduction process under a two-step UV irradiation. Appl. Surf. Sci.. 2010;256:3812-3816.
- [CrossRef] [Google Scholar]
- A facile aqueous-phase route for the synthesis of silver nanoplates. Mater. Lett.. 2007;61:130-133.
- [CrossRef] [Google Scholar]
- Ammonia sensors and their applications – a review. Sens. Actuators B. 2005;107:666-677.
- [CrossRef] [Google Scholar]
- United States Environment Protection Agency (US EPA), 2013. The national criteria for ammonia in fresh water.
- Shape-controlled synthesis of metal nanostructures: the case of silver. Chem. Eur. J.. 2005;11:454-463.
- [CrossRef] [Google Scholar]
- Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem.. 2007;58:267-297.
- [CrossRef] [Google Scholar]
- Sunlight irradiation induced green synthesis of silver nanoparticles using peach gum polysaccharide and colorimetric sensing of H2O2. Mater. Lett.. 2015;154:21-24.
- [CrossRef] [Google Scholar]
- Green, effective chemical route for the synthesis of silver nanoplates in tannic acid aqueous solution. Colloids Surf. A. 2011;392:131-136.
- [CrossRef] [Google Scholar]
- Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater. Chem. Phys.. 2004;83:66-70.
- [CrossRef] [Google Scholar]
- Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev.. 2014;43:3426-3452.
- [CrossRef] [Google Scholar]







