Translate this page into:
Ammonia and orthophosphate removal of tilapia cultivation wastewater with Vetiveria zizanioides
⁎Corresponding author. hefni.effendi@apps.ipb.ac.id (Hefni Effendi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Vetiver is an environmentally friendly plant since it has non-invasive characteristic, has a high level of heavy metal tolerance, and could reduce N and P content originated from organic water pollutants. Tilapia (Oreochromis niloticus) cultivation wastewater, containing high concentration of N and P, was treated with vetiver (Vetiveria zizanioides) in aquaponics with NFT technique. Treatment consisted of triplicate of P1 (tilapia without vetiver); P2 (tilapia and 400 g of wet vetiver) and P3 (tilapia and 800 g of wet vetiver). Treatment of fish cultivation wastewater with vetiver was capable of lowering concentration of NH3 (65.16%), NO2 (27.51%), NO3 (25.05%) in day 7, and NH4 (30.17%), PO4 (42.75%) in day 14. More vetiver density removed more N and P of tilapia culture wastewater.
Keywords
Phytoremediation
Vetiver
Tilapia
Ammonia
Orthophosphate
1 Introduction
Aquaculture has been rapidly expanding industry that requires bulk quantities of water. As much as 200–600 m3 water is needed for the production of 1 kg fish. Many aquaculture production facilities operate as flow through or open systems, hence releasing sizeable quantities of nutrient rich water into a receiving water body. Recent innovations such as denitrification reactors, sludge thickening technologies and ozone treatments led to a further decrease in water use, waste discharge and energy use in Recirculating Aquaculture System (RAS) (Kofinas and Kioussis, 2003; Martins et al., 2010).
Waste of aquaculture mostly originates from uneaten food and feces which is normally biodegradable waste. Thus BOD is determined instead of COD. The main constituents of concern from aquaculture include pH, biochemical oxygen demand (BOD), total suspended solids (TSS), turbidity, nitrogen, and phosphorus species (Steicke et al., 2002). In an aquaculture system without water exchange (zero water exchange) such as stagnant water pond, concentration of aquaculture waste such as ammonia (NH3) and nitrite (NO2) will increase rapidly and toxic to the cultured organisms. Aquaculture waste resulting from metabolic activity contains ammonia (Purwandari et al., 2017; Effendi, 2003). Out of several forms of water-soluble nitrogen, ammonia (NH3) is the most harmful to fish, and most tropical fish species are generally more sensitive to ammonia (Effendi et al., 2015a; Wang and Leung, 2015). Meanwhile PO4 is not harmful to fish, but causing eutrophication of water environment when available in an excessive concentration.
Feed as the main source of ammonia in the cultivation system because fish is only able to absorb 20–30% of nutrients derived from feed while the rest is excreted into the environment in the form of ammonia and organic protein (Avnimelech, 2006). Residual feed and feces discharged into waters has the potential to be organic contaminants in the form of N and P that can affect fertility levels and quality of water. Aquaponic system can be used as an alternative solution as fish farming waste treatment which effectively reduce total ammonia (Effendi et al., 2015b). Feed impact on the environment may also be reduced by selecting ingredients from a low trophic level (e.g. proteins and lipids from phytoplankton rather than from fish), provided feed digestibility does not decrease (Martins et al., 2010).
Phytoremediation is the utilization of plant to remove and accumulate contaminants from environment, including the use of plants to mitigate, transfer, stabilize or degrade pollutants in soil, sediments and water (Ojoawo et al., 2015).
Vetiver (Chrysopogon zizanioides (L) Roberty), a medicinally important perennial plant, known to control soil erosion, tolerates a wide range of pH and elevated levels of toxic metals (Gautam and Agrawal, 2017). Vetiver is hydrophilic terrestrial plant which has physiological characteristics like the ability to absorb dissolved nutrients such as N and P, reduce BOD, COD, TSS, oil spill, accumulate heavy metals, batik production wastewater, tofu production wastewater, and high tolerance to herbicides and pesticides (Effendi et al., 2015d, 2017a; Seroja et al., 2018; Truong et al., 2011; Tambunan et al., 2018).
Tilapia (Oreochromis niloticus) can be cultured in aquaponic systems (Delis et al., 2015; Liang and Chien, 2013; Love et al., 2015; Wang et al., 2016). Tilapia has a good level of tolerance to various environmental conditions, is able to be cultivated in aquaponic system with vegetables (Effendi et al., 2015a), and has a high economic value (Diver, 2006). This study was aimed to analyze the effectiveness of vetiver in removing nitrogen and phosphorous of tilapia cultivation waste water in recirculation systems of aquaponics by comparing treatment of different vetiver planting density.
2 Materials and method
The study applied recirculation aquaculture system of aquaponics with nutrient film technique (NFT). NFT is a hydroponic method with a thin water flow (15.34 ml/s) as high as ±1 cm, so the roots grow in a shallow nutrient layer, while the non-submerged roots can absorb oxygen through diffusion (Rakocy et al., 2006).
Aquarium (80 x 40 x 60 cm3, and 200 L), gutter (80 x 15 x 15 cm3, and 1.2 L), and water tank (80 x 40 x 60 cm3, and 200 L) were used. The system utilized peristaltic pump to control water flow. Water exchange was not performed during the study. The water in the aquarium before usage was aerated for 1 week to enhance dissolve oxygen in the water (Effendi et al., 2017e).
Prior to use, the vetiver was stored in a 40 x 35 x 25 cm3 tank with a floating raft system in 25 L water added 5 ml commercial hydroponic nutrient solution (AB mix) per 1 L media and acclimated to wetland conditions for 1 month. Vetiver height of 10 cm was planted in several pots filled with rockwool and placed in a gutter. Vetiver utilized the available nutrients resulting from decomposition of uneaten fish food and feces.
A total of 20 tilapias (Oreochromis niloticus), average weight of 14 g, average length of 8–9 cm, was used. Density of 20 fish/200 L refers to Sace and Fitzsimmons (2013). There was no addition of artificial nutrients for vetiver during six weeks experiment. Treatment consisted of triplicate of P1 (tilapia without vetiver); P2 (tilapia and 400 g of wet vetiver) and P3 (tilapia and 800 g of wet vetiver) (Fig. 1). 400 and 800 g vetiver as wet weight means life vetiver grass weight as treatment.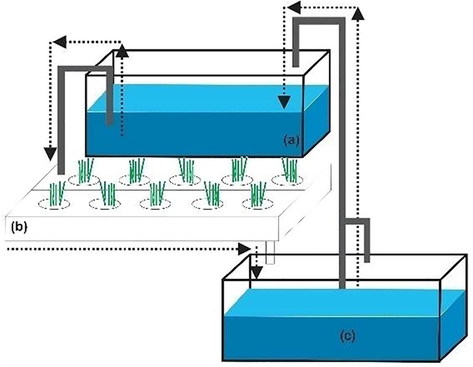
Aquaponic installation (a) Fish tank, (b) Vetiver gutter, (c) Water tank after vetiver treatment.
Seven days fish acclimatization would accumulate organic matter, which later provided nutrient for the growth of vetiver. The fish age was 3 months (with average length of 9 cm and average weight of 14 g). Fishes were fed by pellets three times a day as much as 3% of body weight. Average feed per day: 3.27 g (1st week), 3.88 g (2nd week), 4.43 g (3rd week), 4.79 g (4th week), 5.67 g (5th week), 6.72 g (6th week). Floating pellet, size 2 mm, contained 33% protein with 5.97% N and 1.10% P.
Water quality was measured weekly for six weeks in tank (c). Parameters analyzed were N (TAN, nitrate, nitrite), P (orthophosphate), Dissolved Oxygen (DO), pH, temperature, and turbidity, referring to APHA (2012). All data were analyzed by ANOVA using SPSS (Saltman, 2015). Moreover, varimax factor was determined by comparing all variables to scrutinize correlation among variables.
3 Results and discussion
Initial water quality characteristic and average water quality in each treatment are presented in Tables 1 and 2. Different letters (a and b) in the same row are significantly different at P < 0.05 level.
Parameter
Control (P1)
Treatment (P2)
Treatment (P3)
Temperature (°C)
30.17
29.50
29.47
Turbidity (NTU)
1.12
1.08
1.11
pH
6.93
6.83
7.00
DO (mg L−1)
6.10
6.17
6.37
NH3 (mg L−)
0.0034
0.0026
0.0030
NH4(mg L−1)
0.4859
0.5067
0.3813
NO2 (mg L−1)
0.05
0.05
0.05
NO3 (mg L−1)
0.13
0.13
0.15
PO4 (mg L−1)
0.04
0.04
0.03
Parameter
Control (P1)
Treatment (P2)
Treatment (P3)
Limit
Temperature (°C)
30.34 ± 0.39
29.50 ± 0.32
29.86 ± 0.27
11–42 °C (FAO, 2012)
Turbidity (NTU)
7.13 ± 4.17a
5.57 ± 3.43a
5.26 ± 3.38a
–
pH
6.00 ± 0.28a
6.04 ± 0.22b
6.04 ± 0.23b
6–9 (Popma and Masser, 1999)
DO (mg L−1)
5.24 ± 0.63a
5.42 ± 0.69 a
5.45 ± 0.77a
≥5 (Lloyd, 1992) 3–5 (Anita and Pooja, 2013)
NH3 (mg L−)
0.026 ± 0.08a
0.020 ± 0.03b
0.015 ± 0.05b
0.05 (Lawson, 1995) 0.1 max.tolerable level
(Pillay and Kutty, 2005)
NH4(mg L−1)
1.486 ± 0.15a
1.48 ± 0.33 a
1.35 ± 0.05b
0.2–2 (Boyd,1998)
NO2 (mg L−1)
0.41 ± 0.06a
0.33 ± 0.03 a
0.32 ± 0.03a
0.5 (Swann, 1997) ≤1 (Pillay and Kutty, 2005)
NO3 (mg L−1)
0.79 ± 0.05a
0.77 ± 0.07 a
0.72 ± 0.03a
≤10 (Pillay and Kutty, 2005)
PO4 (mg L−1)
1.29 ± 0.06a
1.23 ± 0.04b
1.19 ± 0.04b
0.03–2 (Anita and Pooja, 2013)
3.1 Temperature, Turbidity, pH, and Dissolved oxygen
Treatment without plants (P1), vetiver of 400 g (P2), and vetiver of 800 g (P3) had relatively similar temperature, pH and Dissolved Oxygen (DO). Moreover, turbidity and inorganic nutrient content including Total Ammonia Nitrogen (TAN), Ammonia (NH3), Ammonium (NH4), Nitrite (NO2) in P3 tended to be lower than those in P1 and P2.
Turbidity in P3 was lower than in P2 and P1 (Table 2). Furthermore, turbidity in each treatment was not significantly different (p > 0.05). Time of observation had significant impact on turbidity (p < 0.05).
Value of pH was significantly different among treatments (p < 0.05) and decreased at the end of observation. During observation period, pH ranged 6.00–6.04. Process of organic matter breakdown in waters produces CO2 which causes acidic water. According to DeLong et al. (2009), optimum pH for tilapia growth is 6–9, while optimum pH for the growth of aquatic plants is <7 (Owens et al., 2005). For aquatic plants, pH influences the metabolic process as well as the absorption of nutrients and carbon (Mitchell, 1974). Moreover, fish living in environments with low pH (<5.0) may die from a decrease in plasma ion and osmoregulation process failure (Evans and Claiborne, 2006). Thus, maintaining the pH in the range of 6–7 in this system is necessary. In addition to optimize the growth of fish and plants, pH in the range of 6–7 can maintain ammonia in the form of NH4+, thus lowered toxicity level of NH3 (Goldman and Horne, 1983).
DO is one of the important parameters and a limiting factor for fish life. Low DO will disrupt the lives of fish cultured since DO is not only needed by the fish, but also required by microbes in oxidizing organic materials. Nitrifying bacteria are aerobic and require oxygen to produce NO3 in nitrification process (Henriksen et al., 1981). Average DO for all treatments was >5 mg L−1 with a range of 5.24 to 5.45 mg L−1. DO in P1, P2, and P3 did not show any significant differences, whereas observation duration significantly affected the DO (p < 0.05).
3.2 Ammonia, ammonium, nitrite, and nitrate
NH3 in water is usually measured as total ammonia nitrogen/TAN (NH3+ NH4). The toxicity of NH3 is primarily attributable to the un-ionized form (NH3), as opposed to the ionized form (NH4). In general, more NH3 and greater toxicity exist at higher pH. Toxicity increases as pH increases and as temperature increases. Plants are more tolerant of NH3 than animals, and invertebrates are more tolerant than fish (Anonymous, 2014).
NH3 in all treatments increased from day 0 to day 14 due to accumulation of uneaten feed and fish feces. NH3 ranged from 0.03 to 0.91 mg L−1. NH3 in P3 was lower than that in P2 and P1 (Fig. 2). Meanwhile a research by Effendi et al. (2017b) on aquaponics of guoramy and romaine lettuce found that NH3 ranged 0.02–0.04 mg L−1. Tilapia cultivation resulted in more NH3. In addition, Effendi et al. (2017c) reported that NH3 removal of catfish cultivation wastewater using vetiver ranged 0.2657–2.8648 mg L−1. Therefore, catfish cultivation produced more NH3 than tilapia and gouramy cultivation. TAN fluctuations in each treatment had similar pattern. Concentration of NH3 sharply declined on day 21 of observation and continued to decline until day 42. This sharp decline of NH3 was followed by the increase of NH4 and NO3, suggesting that the convertion of NH3 to NO3 through nitrification process occurred much more intensive (Fig. 3). NH3 contained in P1 tended to be higher than in P2 and P3. Furthermore, NH3 in P1, P2, and P3 were significantly different (p < 0.05) (Fig. 2). Plants can act as phytoremediation to absorb NH4+; thus, toxic NH3 can be reduced through the balance of TAN (Tyson et al., 2011).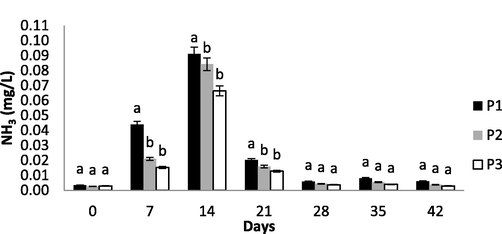
Ammonia (NH3) fluctuation during experiment. Different letters (a and b) are significantly different at P < 0.05 level.
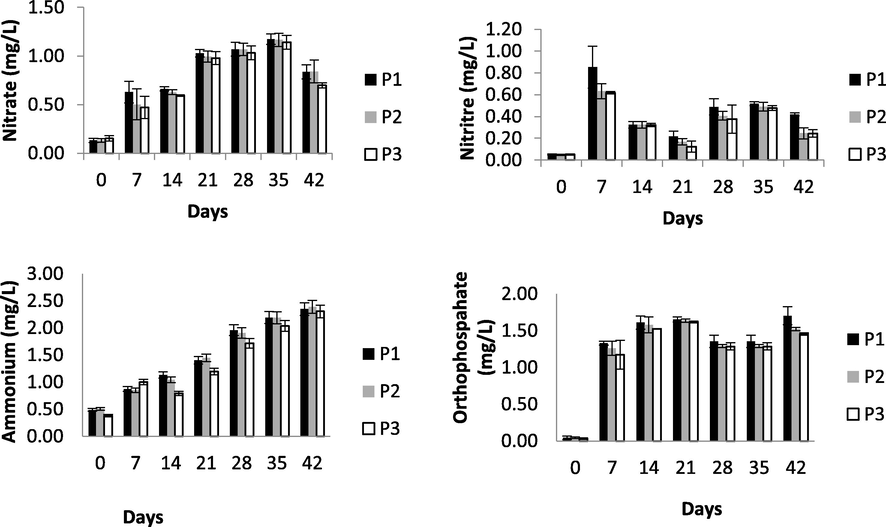
Nitrate, nitrite, ammonium, and orthophospahte fluctuation during experiment. No significant difference among treatment.
At the beginning, NH3 is oxidized to nitrite by ammonia oxidizing bacteria (AOB), later converted to nitrate by nitrite oxidizing bacteria (NOB) (Hu et al., 2015). Removal of NH3 concentration greater than NH4, NO2 and NO3 was likely associated with much faster growth of AOB than NOB. This is supported by Yamamoto et al. (2008), growth of AOB population will be faster than NOB when the temperature is above 25 °C.
Changes in temperature and pH during observation period affected the equilibrium of NH3 and NH4. At the beginning of experiment (day 0), pH reached 7.0 and affected the equilibrium of TAN. Therefore, concentration of NH3 on day 0 tended to be normal. Later, pH decreased to 6.0 on day 14, caused NH3 in all treatments to decline sharply. P3 had lower average concentration of NH3 than that of P2 and P1 (Table 2). NH3 in P2 and P3 were significantly different (p < 0.05) and observation time significantly affected NH3 (p < 0.05).
NH4 ranged from 0.38 to 2.38 mgL−1. At the end of the experiment, NH4 in P1 (1.48 ± 0.15 mg L−1) tended to higher than in P2 (1.48 ± 0.33 mgL−1) and P3 (1.35 ± 0.22 mg L−1). Furthermore, NH4 in all treatments increased until the end of observation. P3 had lower NH4 than P2 and P1 (Table 2). NH4 in P2 and P3 was significantly different, observation time also had significant impact on ammonium (p < 0.05). Plants play as biofiltration by absorbing NH4. Meanwhile nitrification bacteria reduce NH3 concentration through oxidation and converting NH3 to NO3 (Tyson et al., 2011).
Average NO2 in P3 was lower than that in P2 and P1 (Table 2). Concentration of NO2 during observation period ranged from 0.05 to 0.85 mg L−1. Concentration of NO2 tended to rise and only decreased on day 42 of observation. NO2 in P2 and P3 was not significantly different (p > 0.05), while observation time significantly affected NO2 (p < 0.05). NO2 is the intermediate product of nitrification process. Hence NO2 concentration is generally lower than NH3 and NO3. Plants do not use nitrite as nutrient source and high concentration of nitrite leads to poisoning in fish. Thus, nitrite concentration should not exceed 5 mg L−1 (DeLong et al., 2009).
Increased NO3 occurred over time. NO3 in all treatments increased until day 35 of observation then decreased until day 42. NO3 concentration ranged from 0.13 to 1.17 mg L−1. P3 had lower average of NO3 than P2 and P1 (Table 2). Concentrations of NO3 in P2 and P3 were not significantly different. Time of observation significantly affected NO3 concentration (p < 0.05). NO3 is relatively non toxic to most of fish, and does not cause any health hazard except at exceedingly high levels (>90 mg L−1) (Stone and Thomforde, 2004). NO3 toxicity for tilapia may occur if the concentration exceeds 300–400 mg L−1 (DeLong et al., 2009).
3.3 Orthophosphate
Phosphorus in the form of orthophosphate (PO4) is an essential plant nutrient, resulting from decomposition of tilapia cultivation wastewater. Concentration of PO4 in P3 was lower significantly than in P2 and P1. Low PO4 in P3 might be attributable to usage by vetiver for their growth. Eichornia crassipes could reduce 63.3% TP in water (Wang et al., 2011). Maximum P reduction in this research was 42.75%. According to Li et al. (2013), phosphorus (P) accumulates in plant root tissues. Therefore, PO4 concentration of tilapia cultivation wastewater underwent much more reduction in P3 (800 g vetiver) than in P2 (400 g vetiver) and P1 (control, without vetiver) (Table 2).
3.4 Correlation of water quality parameter
In aquaponics system, turbidity had strong positive correlation (0.907) with other parameters in component 1 (45.186% of total variance) and negatively correlated with NH3, DO and pH (−0.079, −0.533, −0.579) (Table 3), suggesting that high turbidity might hinder DO penetration. pH in P3 was lower than in P2 and P1. Strong loading* ≥0.75, moderate loading (0.5–0.75) and weak loading 0.5–0.3) (Liu et al., 2003).
System
P1
P2
P3
Component
1
2
3
1
2
3
1
2
3
NO3
.832*
−.294
.051
.690
−.478
−.121
.504
−.643
.289
NO2
.808*
−.441
−.130
.792*
.125
.002
.690
−.424
−.126
TAN
.922*
−.142
−.179
.853*
−.343
.320
.921*
−.261
.176
NH3
−.200
.965*
.095
−.014
.963*
.100
−.079
.948*
.117
NH4
.917*
−.185
−.181
.844*
−.376
.313
.915*
−.289
.170
PO4
.787*
−.200
−.025
.655
−.479
−.244
.808*
−.315
−.103
DO
−.720
.319
.168
−.611
.633
−.047
−.533
.666
−.230
pH
−.453
.831*
.221
−.393
.843*
−.092
−.579
.742
.060
Temperature
.014
.210
.942*
.033
.053
.963*
.014
.008
.951*
Turbidity
.635
−.005
−.675
.750*
−.264
−.068
.907*
−.122
.004
% Explained variance
48.216
21.439
15.131
40.627
28.434
12.274
45.186
27.516
11.438
Cumulative%
48.216
69.655
84.786
40.627
69.060
81.335
45.186
72.702
84.140
Turbidity (0.907) strongly correlated with TAN (0.921), NH4 (0.915), PO4 (0.808) in P3 component 1, suggesting that high turbidity might be associated with high concentration of those three parameters, but correlated negatively with NH3. The same pattern occurred in P2 component 1. In control without vetiver (P1, component 1) positive correlation of turbidity was not only with three parameters but also with NO3, likely indicating that the available nitrate as a result of decomposition of organic matter was not utilized, as did in P2 and P3.
In P3, DO was positively correlated (0.666) in component 2 (27.516% of total variance) with NH3 (0.948), pH (0.742), suggesting that temperature and pH increment will shift the equilibrium of TAN into NH3, which is a more toxic element. Shifting TAN to NH3 was proved by negative correlation of DO with TAN, NO3, NO2, NH4, PO4 and turbidity (−0.261, −0.643, −0.424, −0.289, and −0.122, respectively) (Table 3).
3.5 N and P removal
Percentage of nutrient removal was calculated by comparing nutrient in treatment and control. Percentage of nutrient removal in P2 and P3 for ammonia (NH3), ammonium (NH4), nitrate (NO3), nitrite (NO2), and orthophosphate (PO4) fluctuated during the observation period (Table 4).
Day
Percentage of reduction
Ammonia (NH3)
Ammonium (NH4)
Nitrite (NO2)
Nitrate (NO3)
Orthophosphate (PO4)
P2
P3
P2
P3
P2
P3
P2
P3
P2
P3
7
52.10
65.16
2.77
−14.70
25.81
27.51
20.01
25.05
14.50
21.77
14
7.57
27.04
7.60
30.17
−0.02
0.68
5.39
10.48
28.50
42.75
21
21.58
36.53
−3.17
14.68
22.50
42.73
3.17
4.76
26.25
31.25
28
23.90
36.71
2.66
12.46
16.87
22.99
0.00
3.36
4.87
5.17
35
33.61
50.84
0.11
7.10
5.26
7.84
0.47
2.58
15.66
20.31
42
37.28
50.49
−1.79
1.76
41.34
41.85
−0.42
16.59
10.66
14.60
The highest removal percentage was found in the early experiments on P3, namely 65.16%, 27.51% and 25.05% for NH3, NO2 and NO3, respectively. Meanwhile NH3 removal of tilapia culture wastewater by butterhead lettuce was 45.49% (Effendi et al., 2017d). The highest NH4 removal percentage was 30.17% which was obtained in P3 at day 14 (Table 4). P3 had higher rate of inorganic nutrients removal and was more effective than P2 for NH3, NH4, NO2 and NO3.
Growth performance of tilapia and vetiver was also better in P3 compared to that in P2 and elaborated elsewhere. Therefore, increasing number of vetiver population or plant density can be applied to increase the absorption of pollutants. NH3 and NO3 of crayfish culture wastewater was reduced 84.6% and 34.8% by spinach (Effendi et al. 2015c), 91.5% and 23.3% by lettuce (Effendi et al., 2015b). Meanwhile Wahyuningsih et al. (2015) found reduction of tilapia cultivation wastewater of 91.50%, 34.41%, 22.86%, and 49.74% for TAN, NO2 and NO3, respectively by lettuce and added bacteria.
NH4, NO2 and NO3 are the main form of the element absorbed by most plants (Liu et al., 2014). Kennedy and Murphy (2004) stated that increase in plant density affected the decrease in nitrogen concentration. According to Garnett et al. (2003), various different types of plants in the form N source preferred to be absorbed depends on resources available. Fang et al. (2007) also stated that nitrogen intake is done by plant roots and leaves, if both N sources are available, plant prefers to take NH4. Plants have the ability to take up several chemical forms of nitrogen. The most common are ammonium (NH4+), which has a positive charge; nitrate (NO3–), which has a negative charge; and urea, ((NH2)2CO), which has no charge (Mattson et al., 2009). Tea (Camellia sinensis (L.) O. Kuntze) prefers ammonium (NH4+) over nitrate (NO3−) as an inorganic nitrogen (N) source (Yang et al., 2013).
Fish farming wastewater treatment by using economically valuable crops such as vetiver is expected to provide value added. Fish farming waste treatment methods using aquaponics system is an efficient method because waste treatment can be done in one cycle of recirculation by applying this method. Moreover, apart from being used in organic waste treatment, there are several other advantages of using recirculation method including efficiency of water use, efficiency of space usage and production as well as double advantages of harvesting fish and plants (Datta, 2015).
4 Conclusion
Treatment of fish cultivation wastewater with vetiver was capable of reducing the concentration of NH3 (65.16%), NO2 (27.51%), NO3 (25.05%) in day 7, and NH4 (30.17%), PO4 (42.75%) in day 14. More vetiver density removed more N and P of tilapia cultivation wastewater. Optimization of fish and plant density as well as searching the best fish and plant type combination in phytoremediation are our subsequence work.
Acknowledgement
This study was supported by Center for Environmental Research, Bogor Agricultural University (PPLH-IPB), Indonesia.
References
- Anonymous, 2014. Ammonia in Groundwater, Runoff, and Streams. https://www.water-research.net/index.php/ammonia-in-groundwater-runoff-and-streams (accessed 17 Febuary 2018).
- Water quality guidelines for the management of pond fish culture. 2013;3(6):1980-2009.
- Standard methods for the examination of water and wastewater (22nd edition). Washington: American Public Health Association; 2012.
- Bio-filters: the need for a new comprehensive approach. Aquaculture Eng.. 2006;34:172-178.
- [Google Scholar]
- Boyd, C.E., 1998. Water Quality for Pond Aquaculture. Research and Development Series No. 43. International Center for Aquaculture and Aquatic Environments, Alabama Agricultural Experiment Station, Auburn University, Alabama.
- Treatment of aquaculture wastewater using Vetiveria zizanioides (Liliopsida, Poaceae) AACL Bioflux. 2015;8:616-625.
- [Google Scholar]
- Aquaponics-integration of hydroponics with aquaculture. Butte: National Center for Appropriate Technology (NCAT); 2006. p. :28.
- Effendi, H., 2003.Assessing of water quality for water resources and environmental management. Kanisius, Jakarta, p. 258 (In Indonesian).
- Combination of water spinach (Ipomea aquatica) and bacteria for freshwater cryfish red claw (Cherax quadricarinatus) culture wastewater treatment in aquaponic system. J. Adv. Biol.. 2015;6(3):1072-1077.
- [Google Scholar]
- The performance of nile tilapia (Oreochromis niloticus) and vetiver grass (Vetiveria zizanioides) concurrently cultivated in aquaponic system. Adv. Environ. Biol.. 2015;9(24):382-388.
- [Google Scholar]
- Wastewater treatment of freshwater crayfish (Cherax quadricarinatus) culture with lettuce (Lactuca sativa) Int. J. Appl. Environ. Sci.. 2015;10(1):409-420.
- [Google Scholar]
- Phytoremediation of freshwater crayfish (Cherax quadricarinatus) culture wastewater with spinach (Ipomoea aquatica) in aquaponic system. AACL Bioflux. 2015;8(3):421-430.
- [Google Scholar]
- Crude oil spilled water treatment with Vetiveria zizanioides in floating wetland. Egypt. J. Aquat. Res.. 2017;43:185-193.
- [Google Scholar]
- Effendi, H., Purwandari, Y., Wardiatno, Y., 2017b. Wastewater of gourami (Osphronemus goramy) cultivation treatment by romaine lettuce (Lactuca sativa L. var. longifolia). In: The JSFS 85th Anniversary-Commemorative International Symposium “Fisheries Science for Future Generations” Symposium, Tokyo, 22–24 September 2017.
- Ammonia removal of catfish (Clarias sp) cultivation wastewater using vetiver grass (Vetiveria zizanioides) Pollut. Res.. 2017;36(3):419-427.
- [Google Scholar]
- Removal of nitrogen and phosphorus of tilapia farming waste (Oreochromis niloticus) by butterhead lettuce (Lactuca sativa l. var. capitata). Asian Journal of Microbiology, Biotechnology, and Environmental. Science. 2017;19(4):67-72.
- [Google Scholar]
- The use of nile tilapia (Oreochromis niloticus) cultivation wastewater for the production of romaine lettuce (Lactuca sativa L. var. longifolia) in water recirculation system. Applied Water. Science. 2017;7(6):3055-3063.
- [Google Scholar]
- The physiology of fishes (3rd Edition). Boca Raton: CRC Press, Taylor & Franches Group; 2006. p. :601.
- FAO, 2012. Cultured aquatic species information programme. Oreochromis niloticus. Cultured aquatic species information programme. Text by Rakocy, J. E. In: FAO Fisheries and Aquaculture Department. Rome.
- Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot.. 2007;99(2):365-370.
- [Google Scholar]
- Kinetics of ammonium and nitrate uptake by eucalypt roots and associated proton fluxes measured using ion selective microelectrodes. Funct. Plant Biol.. 2003;30:1165-1176.
- [Google Scholar]
- Gautam, M., Agrawal, M., 2017. Phytoremediation of metals using vetiver (Chrysopogon zizanioides (L.) Roberty) grown under different levels of red mud in sludge amended soil. J. Geochem. Explor. 182(B), 218–227.
- Rates of nitrification, distribution of nitrifying bacteria, and nitrate fluxes in different types of sediment from Danish waters. Mar. Biol.. 1981;61(4):299-304.
- [Google Scholar]
- Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol.. 2015;188:92-98.
- [Google Scholar]
- Indicators of nitrate in wetland surface and soil-water: Interactions of vegetation and environmental factors. Hydrol. Earth Syst. Sci.. 2004;8(4):663-672.
- [Google Scholar]
- Reactive phosphorus removal from aquaculture and poultry productions systems using polymeric hydro gels. Environ. Sci. Technol.. 2003;37:423-427.
- [Google Scholar]
- Fundamentals of Aquacultural Engineering. New York: Chapman and Hall; 1995.
- Growth characteristics of six wetland plants and their influences on domestic wastewater treatment efficiency. Ecol. Eng.. 2013;60:382-392.
- [Google Scholar]
- Effects of feeding frequency and photoperiod on water quality and crop production in a tilapia-water spinach raft aquaponics system. Int. Biodeterioration Biodegradation 2013
- [CrossRef] [Google Scholar]
- Application of factor analysis in the assessment of groundwater quality in a black foot disease area in Taiwan. Sci. Total Environ.. 2003;313:77-89.
- [Google Scholar]
- Effects of nitrogen fertilizers on the growth and nitrate content of lettuce (Lactuca sativa L) Int. J. Environ. Resour. Public Health. 2014;11(4):4427-4440.
- [Google Scholar]
- Pollution and freshwater fish. Oxford, UK.: Fishing News Books; 1992. p. :192.
- Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquacult. Eng.. 2015;68:19-27.
- [Google Scholar]
- New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquacult. Eng.. 2010;43:83-93.
- [Google Scholar]
- Mattson, N., Leatherwood, R., Peters, C., 2009. Nitrogen: All Forms Are Not Equal. Cornell University Cooperative Extension.
- Aquatic Vegetation and Its Use and Control. France: Unesco; 1974. p. :135.
- Phytoremediation of phosphorus and nitrogen with Canna x generalis reeds in domestic wastewater through NMAMIT constructed wetland. Aquatic Procedia. 2015;4:349-356.
- [Google Scholar]
- Effects of pH on growth of Salvinia molesta Mitchell. J. Aquatic Plant Manage.. 2005;43:34-38.
- [Google Scholar]
- Aquaculture, Principles and Practices (2nd Edition). Oxford, UK: Blackwell Publishing Ltd; 2005. p. :630.
- Popma, T., Masser, M., 1999. Tilapia Life Story and Biology. Southern Regional Aquaculture Center Publication No. 283.
- The use of gouramy (Osphronemus goramy) rearing wastewater for growing romaine lettuce (Lactuca sativa l. var. longifolia) in aquaponic system. Asian Journal of Microbiology, Biotechnology, and Environmental. Science. 2017;19(2):359-366.
- [Google Scholar]
- Rakocy, J.E., Masser, M.P., Losordo, T.M., 2006. Recirculating aquaculture tank production systems: aquaponics-integrating fish and plant culture. Southern Regional Aquaculture Center, United States Department of Agriculture, Cooperative State Research, Education, and Extension Service.
- Recirculating aquaponic systems using Nile tilapia (Oreochromis niloticus) and freshwater prawn (Macrobrachium rosenbergii) polyculture and the productivity of selected leafy vegetables. Merits Res. J.. 2013;1(1):011-029.
- [Google Scholar]
- Saltman, H.J., 2015. Experimental Design and Analysis. http://www.stat.cmu.edu/∼hseltman/309/Book/Book.pdf (accessed 30 March 2018).
- Tofu wastewater treatment using vetiver grass (Vetiveria zizanioides) and zeliac. Appl. Water Sci.. 2018;8:2.
- [CrossRef] [Google Scholar]
- Steicke, C.R., Jegatheesan, V., Zeng, C., 2002. Recirculating aquaculture systems - a review. Water Wastewater Treatment Technol. 14.
- Understanding your fish pond water analysis report. Cooperative Extension Program, University of Arkansas at Pine Bluff Aquaculture/Fisheries; 2004. p. :4.
- Swann, L.D., 1997. A Fish Farmer’s Guide to Understanding Water Quality, Aquaculture Extension Illinois, Purdue University, Indiana Sea Grant Program Fact Sheet AS-503.
- Phytomerediating batik wastewater using Vetiver Chryspogon zizanioides (L) Polish J. Environ. Stud.. 2018;27(3):1281-1288.
- [Google Scholar]
- Vetiver system applications. Technical Reference Manual. The Vetiver Network International; 2011. p. :127.
- Opportunities and challenges to sustainability in aquaponic systems (reviews) Hort Technol.. 2011;21(1):6-13.
- [Google Scholar]
- Nitrogen removal of aquaculture wastewater in aquaponic recirculation system. AACL Bioflux. 2015;8(4):491-499.
- [Google Scholar]
- An application of phytoremediation to river pollution remediation. Procedia Environ. Sci.. 2011;10:1904-1907.
- [Google Scholar]
- Effects of unionized ammonia on tropical freshwater organisms: implications on temperate-to tropic extrapolation and water quality guidelines. Environ. Pollut.. 2015;205:240-249.
- [Google Scholar]
- The performance of coupling membrane filtration in recirculating aquaponic system for tilapia culture. Int. Biodet. Biodeg.. 2016;107:21-30.
- [Google Scholar]
- Long-term stability of partial nitritation of swine wastewater digester liquor and its subsequent treatment by anammox. Bioresour. Technol.. 2008;99:6419-6425.
- [Google Scholar]
- Characterization of ammonium and nitrate uptake and assimilation in roots of tea plants. Russ. J. Plant Physiol.. 2013;60(1):91-99.
- [Google Scholar]







