Translate this page into:
Amino-acid amendment of Arginine-325-Tryptophan in rs13266634 genetic polymorphism studies of the SLC30A8 gene with type 2 diabetes-mellitus patients featuring a positive family history in the Saudi population
⁎Corresponding author. imkhan@ksu.edu.sa (Imran Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder with chronic hyperglycemia. Genome-wide association studies (GWAS) have identified many genes and, among them, solute carrier family 30 member 8 (SLC30A8) was one of the important genes linked to the development of T2DM risk. The relationship between T2DM and the SLC30A8 gene is linked through zinc, which plays a key role in the storage and secretion of insulin. The rs13266634 polymorphism includes a strong genetic association in case-control and meta-analysis studies of the global population. The aim of this current study was to scrutinize the genetic relationship between the rs13266634 polymorphism in the SLC30A8 gene with T2DM subjects selected with a family history in the Saudi population. This study involved 120 cases of diagnosed T2DM and 120 confirmed healthy controls that were recruited to screen rs13266634 polymorphisms through a genotyping analysis followed by PCR and RFLP analysis. Baseline characteristics between cases and controls have been evaluated with Student’s t-test. The study results confirmed the genetic association between the allele (p = 0.001), genotypes (CT = 0.005 and TT = 0.03), and various genetic patterns of inheritance (p = 0.001 and p = 0.02). Both analysis of variance (ANOVA) and binary logistic regression analysis revealed non-signiifcant association with T2DM cases and biochemical parameters (p > 0.05). In conclusion, the current results have confirmed the strong genetic association between T2DM cases and controls in the Saudi population with rs13266634 polymorphisms of the SLC30A8 gene.
Keywords
Type 2 diabetes mellitus
SLC30A8-gene
Rs13266634 polymorphism
Genotyping
1 Introduction
Diabetes mellitus (DM) is characterized by chronic hyperglycemia and impaired carbohydrates, lipid and protein metabolism owing to complete or partial incompetence of insulin secretion or insulin action (Nauck and Meier, 2020). Globally, half a billion people are afflicted with diabetes and by 2045, the International Diabetes Federation cautioned that figure will reach 693 million (Zhang et al., 2020). Among different modes of diabetes, type 2 diabetes mellitus (T2DM) is a very common form of the disease that affects the age of onset and has been predicted as a heterogenous group of metabolic and multifactorial disorders (Khan et al., 2019). T2DM is growing epidemic worldwide and is associated with serious complications, which decreases lifespan and quality of life (Blasco-Blasco et al., 2020). The prevalence of adult-onset diabetes or T2DM was found to be 382 million in 2013, and by 2035, the disease will be highly susceptible in 592 million individuals across 130 countries (Athyros et al., 2020). T2DM disease is characterized by chronic hyperglycemia, which increases the risk of cardiovascular disease (Palella et al., 2020).
Obesity is a key aspect in the progression of T2DM, which is aligned with several life-threatening diseases, including gestational diabetes mellitus (GDM), heart disease, hypertension and cancer. Obesity and T2DM originates according to similar criteria for metabolic disorders, illustrating very strong family influences (Sheikhpour et al., 2020). Additionally, T2DM and GDM share similar pathophysiological characteristics of diabetes (Khan et al., 2019). Moreover, genetics and environmental factors play a vital role in disease progression (Khan et al., 2015a). In terms of β-cell activity, the mainstream genes involved play a major part and genetic polymorphisms that affect important proteins involved in glucose metabolism and insulin secretion can also impact susceptibility to T2DM (Witka et al., 2019). Genome-wide association studies (GWAS) have recognized multiple novel susceptibility genes for T2DM. In addition to GWAS, linkage analysis, candidate gene approach along with case-control and hospital-based studies have confirmed the various genes and variants that lead to susceptibility to T2DM (Khan et al., 2015b). In excess of one million single nucleotide polymorphisms (SNPs) have been identified using GWAS and high-throughput technology, which can overcome the limitations of the candidate gene approach (Mtiraoui et al., 2012). The solute carrier member 30, zinc transporter, member 8 (SLC30A8) gene was identified as an rs13266634 polymorphism in the first GWAS study of T2DM in the French population (Sladek et al., 2007). Zinc is an important component of insulin storage and secretion that is presumed to be a critical element underlying the insulin secretion mechanism and may attenuate insulin secretion. SLC30A8 encrypts the zinc carrier protein component-8, which contains eight exons and 369 amino acids, and is considered to be the β-cell zinc homeostasis regulator. The rs13266634 polymorphism is a non-synonymous SNP that causes an amino acid transition from arginine (R/C) to tryptophan (W/T) at position 325. SLC30A8 gene expression significantly increases in T2DM pancreatic islets in patients with CT/TT genotypes. The gene expression levels in SLC30A8 in pancreatic islets of T2DM patients carrying a risk allele is increased by approximately 2.5-fold compared to a non-diabetic control group. The TT genotypes with rs13266634 polymorphisms have the maximum level of gene expression (Faghih et al., 2014, Khan et al., 2015a, Khan et al., 2019, Khan et al., 2015b). Similar polymorphisms have been replicated in numerous case-control studies in T2DM throughout the globe and, based on case-control studies, meta-analysis studies have also been published. However, no full-fledged investigation has been tied to the Saudi population with a minimum three-digit sample size, which is the basic criteria for carrying out a case-control study either in T2DM cases or healthy controls. Therefore, the present study was designed to investigate the rs13266634 polymorphism in the SLC30A8 gene in diagnosed T2DM subjects with a family history of diabetes in the Saudi population
2 Materials and methods
2.1 Sample selection
In this case-control study, 120 T2DM cases and 120 healthy controls were recruited from outpatient clinics in various regions of primary health clinics in the capital city of Saudi Arabia. An ethical grant (E-19-3694) was obtained from the institutional review board (IRB) at the College of Medicine at King Saud University (KSU). This study was in full compliance with Helsinki Declaration standards. The inclusion criteria for T2DM cases were based on World Health Organization (WHO) guidelines (126 mg/dL or >7.0 mmol/L) within Saudi nationals. The exclusion criteria for T2DM cases were based on Al-Daghri et al. (2012). The inclusion criteria of healthy control subjects had normal glucose tolerance (FBG < 7.0 mmol/L) without any family history of any form of diabetes within the family pedigree. The exclusion criteria were patients confirmed with diabetes (FBG > 7.0 mmol/L).
2.2 Anthropometric measurements
Age was recorded in years and gender was documented either as male or female. The details surrounding body mass index (BMI) were obtained based on the measurement of height in centimeters (cms) and weight in kilograms (kg). A validated mercurial sphygmomanometer was used to record the values of hypertension (HTN) of each participant one half-hour after completion of rest. Finally, waist and hip ratios were also determined for all participants.
2.3 Biochemical measurements:
A total of 3 mL of coagulant blood was collected and serum was used to measure fasting blood glucose (FBG) values; which were obtained based on WHO criteria and lipid-profile parameters, such as total-cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) and triglycerides (TG).
2.4 Molecular analysis
Using a Norgen DNA extraction kit (Norgen Biotec Corp., Canada), genomic DNA was extracted with 2 mL of peripheral blood collected in an EDTA vacutainer on the basis of the kit protocol. A NanoDrop Spectrophotometer was used to measure the concentration of genomic DNA. Both from T2DM and healthy controls, DNA samples were stored at −80 °C until genotyping was performed according to a polymerase chain reaction (PCR). Complete molecular analysis was carried out at the male campus of the Department of Clinical Laboratory Sciences, College of Applied Medical Sciences at the KSU premises of the genetics laboratory (G-141/1).
2.5 Amendment of amino acids in the R325W mutation of the SLC30A8 gene
The rs13266634 polymorphism was selected from the genes involved in T2DM recognized by GWAS. Genotyping was performed with PCR and then followed with a restriction fragment-length polymorphism (PCR-RFLP) analysis. Initially, PCR was carried out for a 50-µL reaction involving 50–100 ng of genomic DNA, 10 pmoles of +/- primers, Norgen master mix including 10x buffer, 25 mM of MgCl2, 0.5 mM dNTPs and 10 units of Taq DNA polymerase and distilled water. PCR was conducted with initial denaturation (95 °C-5 min), denaturation (95 °C-30 s), annealing (60 °C-30 s), extension (72 °C-45 s) and final extension for 72 °C-5 min holding at 4 °C after the completion of reactions. PCR analysis was followed for 35 cycles in a thermal cycler. Sense and antisense sequences of the primers were adapted from Khan et al. (Khan et al., 2015a).
Next, the HpaII restriction enzyme was used to digest the PCR products to reach the C↓CGG sequence to cut the amendment of the nucleotide sequence from C-T i.e., C↓CGG to C↓TGG (Fig. 1). A total of 15 µl of PCR product was employed for the digestion along with the 5 µl of distilled water, 4 µl of 10x buffer and 1 µl of 10 U/µL of HpaII restriction enzyme (Thermo Fisher Scientific, USA), incubated at 37 °C for a couple of hours. Both the undigested and digested PCR products were run on 2% and 3% horizontal agarose gel electrophoresis; at 100 V, 12 W, 90 mA for a minimum of 60 min and maximum of 100 min stained with ethidium bromide and visualized on a UV transilluminator. Table 1 lists the primer sequences and SNP data in detail. Fig. 2 consists of undigested and digested band sizes of the rs13266634 polymorphism in the SLC30A8 gene.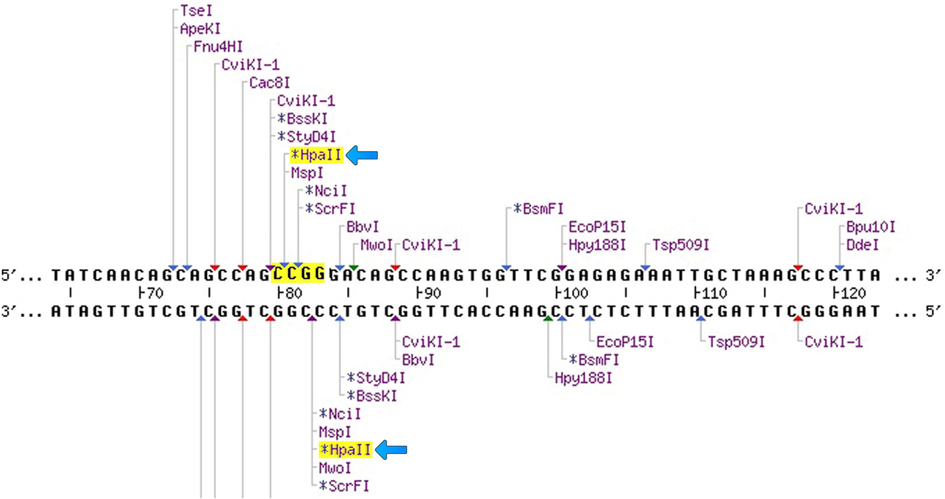
Representation of the presence of the HpaII restriction enzyme within the primer sequence used in this study.
S. No
SNPedia
Orientation
1
Gene
SLC30A8
2
Reference sequence number
rs13266634
3
Mutation type
Non-synonymous single nucleotide polymorphism
4
Amino acid substitution
R325W or Arg325Trp
5
Single nucleotide polymorphism
C-T
6
Molecular region in the SLC30A8 gene
Exon-8
7
Human chromosome region
Chromosome-8 quinine region of 24.11 (8q24.11)
8
5′-3′ Primer sequence
F: GAAGTTGGAGTCAGAGCAGTC
9
3′-5′ Primer sequence
R: TGGCCTGTCAAATTTGGGAA
10
Band Size of the PCR product
256 bp
11
Restriction enzyme
HpaII (G↓GCC)
12
Substitution of Nucleotide band size
176 bp
13
Position
117,172,544
14
Digested PCR products
CC: 176/80 bp; CT-256/176/80 bp and TT-256 bp
15
Condition
Type 2 Diabetes Mellitus
16
Organism
Homosapiens
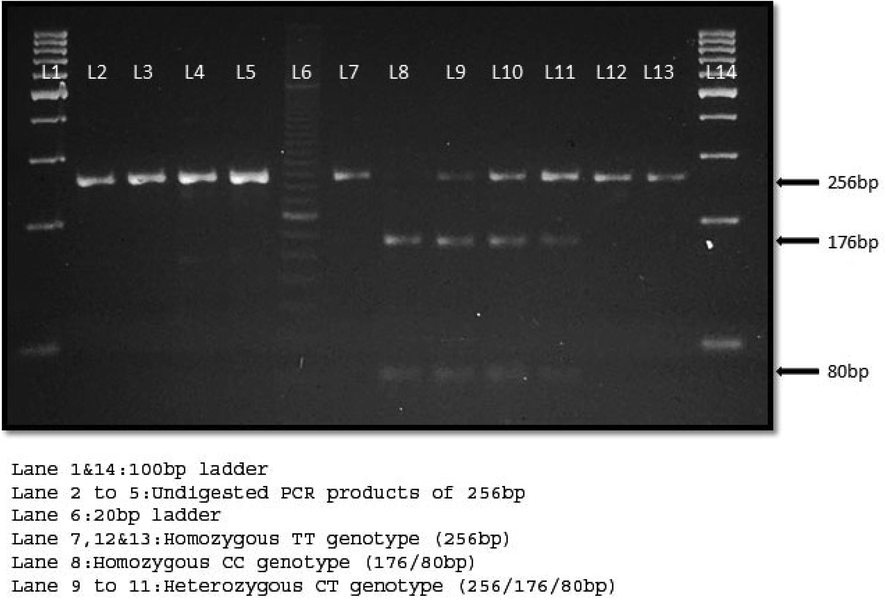
Ethidium bromide-stained 3% agarose gel electrophoresis.
2.6 Validation
Sanger sequencing analysis was performed for 10% (12 samples of T2DM cases and 12 samples of healthy controls) subjects. A 256-bp primer sequence was selected for sequencing using standard di-deoxy terminal chemistry and capillary electrophoresis method(s). Bidirectional sequencing analysis for PCR was carried out using a big-dye terminator for the amplified and purified products. DNA sequencing analysis was carried out in both forward and reverse sequences (Fig. 3).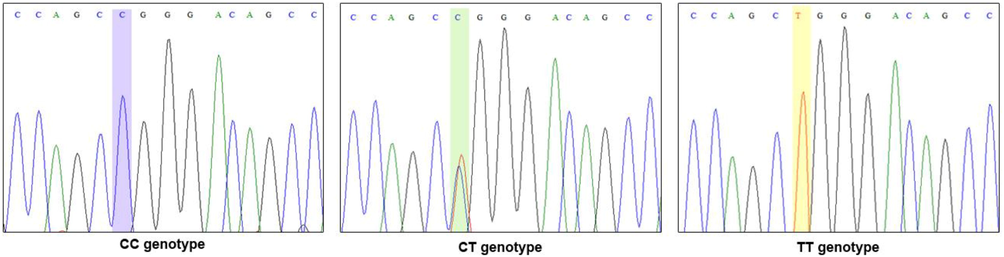
Validation analysis of the rs13266634 polymorphism through Sanger sequencing.
2.7 Statistical data analysis
The Statistical Packages for Social Sciences (SPSS) software, version 25.0, for Windows (IBM, Chicago, USA) was utilized for statistical analysis. Means (M) and standard deviations (SD) are reported as continuous variables, while categorical variables are represented as percentages (%). The intergroup significance between T2DM cases and control subjects were evaluated by Student’s t-test (continuous) and Chi-square test (categorical variables) in Table 2. Hardy-Weinberg equilibrium (HWE) was compared with one degree of freedom using the Chi-square test. Genotype and allele frequencies, including different numerous modes of inheritances (dominant, co-dominant and recessive) were performed and the results are presented in Table 3 with odds ratios (OR) and 95% confidence intervals (CI) (Das et al., 2017). The significance of one-way analysis of variance (ANOVA) and Chi-square tests were determined and the results are found in Table 4 as appropriate for T2DM cases. Binary logistic regression analysis was carried out with T2DM genotypes and lipid-profile parameters listed in Table-5. All P-values were two-sided and a p-value < 0.05 was considered as a statistically significant association between case-control groups
Baseline characteristics
T2DM cases (n = 120)
Healthy Controls (n = 120)
P-value
Age (Years)
55.80 ± 11.09
46.25 ± 7.86
<0.0001
Gender (M: F)
1.32 ± 0.46
1.46 ± 0.50
0.024
Weight (kgs)
74.61 ± 12.60
70.05 ± 9.83
0.002
Height (cms)
163.23 ± 8.91
161.98 ± 8.67
0.27
BMI (kg/m2)
28.19 ± 4.31
26.34 ± 2.94
<0.0001
Waist (cms)
90.83 ± 18.72
89.60 ± 18.22
0.60
Hip (cms)
123.94 ± 6.82
102.57 ± 20.79
<0.0001
SBP (mmHg)
124.69 ± 11.11
114.79 ± 7.90
0.001
DBP (mmHg)
78.43 ± 6.67
75.63 ± 6.10
0.001
FBG (mmol/L)
13.31 ± 5.33
5.23 ± 0.59
<0.0001
TG (mmol/L)
2.32 ± 1.88
1.65 ± 0.81
<0.0001
TC (mmol/L)
5.67 ± 1.23
5.20 ± 0.95
0.001
HDL-c (mmol/L)
0.88 ± 0.37
0.66 ± 0.25
<0.0001
LDL-c (mmol/L)
3.78 ± 0.80
3.82 ± 1.02
0.70
Family History
120 (100%)
00 (0%)
1.00
Mode of Inheritance
Genotype/Allele
T2DM cases (n = 120)
Controls (n = 120)
OR (95%CI)
Pvalue
Homozygous
CC genotype
55 (45.8%)
79 (65.9%)
Reference
Reference
Heterozygous
CT genotype
44 (36.7%)
28 (23.3%)
2.25 (1.25–4.05)
0.005
Homozygous variant
TT genotype
21 (17.5%)
13 (10.8%)
2.32 (1.07–5.02)
0.03
Dominant
CC vs CT + TT
65 (54.2%)
41 (34.1%)
2.27 (1.35–3.83)
0.001
Co-Dominant
CT vs CC + TT
44 (36.7%)
28 (23.3%)
1.90 (1.08–3.34)
0.02
Recessive
TT vs CC + CT
21 (17.5%)
13 (10.8%)
1.74 (0.82–3.67)
0.13
Homozygous Allele
C allele
154 (64.2%)
186 (77.5%)
Reference
Reference
Risk Allele
T allele
86 (35.8%)
54 (22.5%)
1.92 (1.28–2.87)
0.001
Baseline characteristics
CC (n = 55)
CT (n = 44)
TT (n = 21)
P-value
Age (Years)
55.95 ± 11.10
55.07 ± 11.45
56.95 ± 10.67
0.810
Gender (M: F)
1.35 ± 0.48
1.30 ± 0.46
1.29 ± 0.46
0.825
Weight (kgs)
68.64 ± 11.13
70.72 ± 8.48
72.33 ± 7.87
0.292
Height (cms)
162.55 ± 10.24
163.33 ± 7.85
164.81 ± 7.24
0.614
BMI (kg/m2)
26.18 ± 3.41
26.33 ± 2.48
26.80 ± 2.54
0.711
Waist (cms)
90.17 ± 17.83
90.53 ± 18.05
90.83 ± 18.72
0.816
Hip (cms)
23.28 ± 6.28
24.64 ± 7.89
23.94 ± 6.82
0.609
SBP (mmHg)
123.20 ± 10.71
125.43 ± 10.59
127.05 ± 13.07
0.348
DBP (mmHg)
77.95 ± 6.16
78.16 ± 6.53
80.29 ± 8.10
0.373
FBG (mmol/L)
13.66 ± 5.48
12.68 ± 5.53
13.68 ± 4.56
0.626
TG (mmol/L)
1.69 ± 0.92
1.73 ± 0.70
1.36 ± 0.66
0.200
TC (mmol/L)
4.87 ± 0.89
5.24 ± 0.76
5.29 ± 1.08
0.223
HDL-c (mmol/L)
0.65 ± 0.26
0.64 ± 0.26
0.73 ± 0.18
0.446
LDL-c (mmol/L)
3.86 ± 0.85
3.80 ± 0.74
3.52 ± 0.74
0.258
Family History
55 (100%)
44 (100%)
21 (100%)
1.00
3 Results
3.1 Baseline characteristics of participant subjects
Anthropometric, biochemical and clinical data for T2DM cases and healthy controls are shown in Table 2. The mean age of the T2DM cases (55.80 ± 11.09) and healthy controls (46.25 ± 7.86) were significantly associated (p < 0.0001). Anthropometric measurements, such as age, weight, BMI, waist, hip, SBP and DBP were strongly associated in T2DM subjects compared to healthy controls (p < 0.0001). Biochemical parameters, like FBG and lipid profiles, such as that for TC, TG and HDL-c are significantly associated (p < 0.05) and not with LDL-c (p = 0.70). All T2DM cases had a family history and all healthy controls had no family history (P = 1.00).
3.2 HWE and genotyping analysis
Genotype frequency distributions of the rs13266634 polymorphism in the SLC30A8 gene within the control group obeyed the HWE (p > 0.05). Allele and genotype frequencies between T2DM patients and control groups in the rs13266634 polymorphism of the SLC30A8 gene (Fig. 4) and their genetic mode of inheritances with the risk of T2DM are shown in Table 3. The frequencies of CC, CT and TT genotypes were 45.8%, 36.7% and 17.5% in T2DM patients, respectively, and 65.9%, 23.3% and 10.8% in healthy controls, respectively. Statistical analysis between T2DM cases and healthy control subjects exhibited signiifcant association with alleles (T vs. C: OR-1.92 (95% CI: 1.28–2.87; p = 0.001), genotypes (CC vs CT + TT: OR-2.27 (95%CI: 1.35–3.83; p = 0.001; CT vs. CC + TT: OR-1.90 (95% CI: 1.08–3.34); p = 0.02) and different inheritance patterns, such as heterozygous (CT vs. CC: OR-2.25 (95% CI: 1.25–4.05); p = 0.005) and homozygous variants (TT vs. CC: OR-2.32 (95%CI: 1.07–5.02); p = 0.03). However, the recessive mode of inheritance (TT vs. CC + CT: OR-1.74 (95% CI: 0.82–3.67); p = 0.13) was not statistically associated with this study.
Genotype frequencies between T2DM cases and Healthy controls in rs13266634 polymorphism.
3.3 Analysis of variance
One-way ANOVA was performed in T2DM cases and on rs13266634 polymorphism genotypes. Anthropometric measurements, biochemical parameters and clinical data were categorized as CC, CT and TT genotypes of the rs13266634 polymorphism in the SLC30A8 gene. The genetic risk associated with the involved underlying characteristics and genotypes was calculated and is seen in Table 4. Age (56.9 ± 10.6), weight (72.3 ± 7.8), height (164.8 ± 7.2), BMI (26.8 ± 2.54), waist (90.8 ± 18.7), SBP (127.05 ± 13.07), DBP (80.29 ± 8.1), FBG (13.6 ± 4.5), TC (5.29 ± 1.0) and HDL-c (0.73 ± 0.1) have been found to be high in TT genotypes. Hip (24.6 ± 7.8) and TG (1.7 ± 0.7) were high in CT genotypes while gender (1.3 ± 0.4) and LDL-c (3.8 ± 0.8) were high in CC genotypes. Statistical ANOVA for genotypes CC, CT and TT did not exhibit any statistical association (p > 0.05).
3.4 Binary logistic regression analysis
Binary logistic regression analysis was performed with CC, CT and TT genotypes on lipid-profile parameters, such as TC, TG, HDL-c and LDL-c. Fig. 5 describes the relationship with lipid profile and confirms the negative association with CC, CT and TT genotypes. No significant correlation was documented between the three genotypes and lipid-profile parameters (p > 0.05).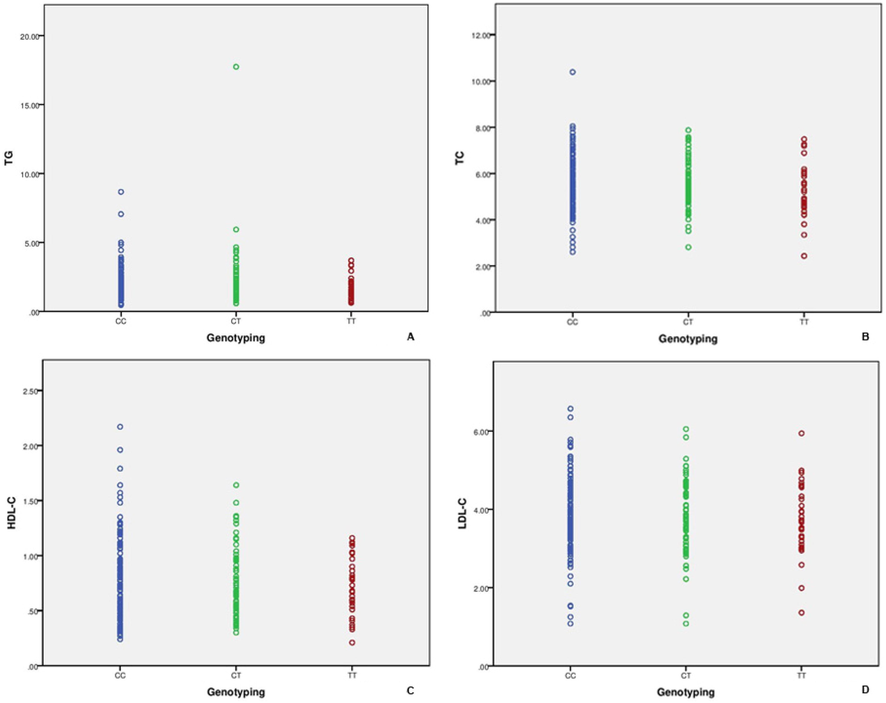
Binary logistic regression analysis performed between lipid profile and genotyping.
4 Discussion
Diabetes is a polygenic metabolic condition that impairs an extremely complex interaction of genetic and environmental factors. Diabetes can lead to chronic complications following a deficiency in protein, fat and carbohydrate metabolism. The Kingdom of Saudi Arabia is one of the emerging countries in the Middle East region, where diabetes is recognized as a primary clinical and public health conundrum. Previous studies have revealed that 23.7% of Saudis are affected with diabetes, with a higher concentration in males compared to females (Alhomayani et al., 2020). However, based on an IDF report, the estimated cost of diabetes patients is expected to reach 592 million by 2035 (Wang et al., 2018). The economic and health burden of diabetes and its accompanied complications is substantial. Subsequently, the progression of diabetes is growing specifically in the era of obesity and diabetes in both children and young adults (Guan et al., 2020). Inherited factors play a leading role for human diseases along with the genes that remain poorly defined. Mutations or SNPs in any gene are unique. Mutations of any gene in pronounced form of diabetes can lead to the disease’s formation. In the case of T2DM, numerous genes are associated with the development of the disease in humans. The advancement of T2DM disease can be attributed to the enhanced effects of combined or individual genetic factors with environmental exposure (Xiao et al., 2016). Enormous functional and non-functional SNPs implicated in the genetic basis of the pathogenesis of T2DM are massively applied in genome-wide linkage analysis, the candidate gene approach and GWAS (McCarthy and Zeggini, 2009).
The results of the current study demonstrate the genetic association between allele (p = 0.001), genotype (CT = 0.005 and TT = 0.03), as well as dominant (p = 0.001) and co-dominant (p = 002) mode of inheritances between T2DM cases and controls with the rs13266634 polymorphism in the SLC30A8 gene. ANOVA analysis did not demonstrate the correlation between the rs13266634 genotypes or the anthropmetric and biochemical parameters involved in this study (p > 0.05). Multiple logistic regression analysis also failed to show the statistical association in T2DM cases (p > 0.05). One of the nominal reasons could be the limited sample size involved in this study. However, Al-Aama et al. (2019) conducted a pilot study in 46 T2DM subjects diagnosed in the Saudi population of the Jeddah region, along with 42 controls analyzed for rs13266634 and other PCR-specific SNPs, and they were followed by SNaPshot analysis that confirmed the negative association. The current study findings were not in agreement and one of the reasons for this is the large (n = 120) sample size of this work.
The rs13266634 polymorphism is carried across the global population and confirms the strong potential risk factors, statistical association (Soltanian et al., 2020, Yazdi et al., 2020) and non-significant associations (Kulkarni et al., 2016, Khan et al., 2015b) based upon ethnicities. The CT genotypes in our study group was found to be 36.7% in T2DM cases, which was similar in other ethnic groups, such as 38.2% and 38% (Cauchi et al., 2008a, 2008b). TT genotypes were documented to be 17.5% in our study and close frequencies were confirmed in the Chinese population (Han et al., 2010). Limited meta-analysis studies have been carried out with T2DM disease focusing on the rs13266634 polymorphism in the SLC30A8 gene. Cheng et al. (Cheng et al., 2015) carried out meta-analysis studies between the rs13266634 polymorphism with T2DM and confirmed the probable important genetic factor in Asians and Europeans, yet failed to show the association in African ethnicity. An updated meta-analysis study by Fan et al. (Fan et al., 2016) confirmed the significant association of T2DM with the rs13266634 polymorphism in the SLC30A8 gene in Asian, European and African ethnic groups. Dong et al. (2018) also performed a meta-analysis study selecting T2DM patients afflicted with the rs13266634 polymorphism only in the Chinese population and as a potential risk factor.
In an Asian Indian population, Khan et al. (2019), Khan et al. (2015b), Khan et al. (2015a) performed a case-control study with three different forms of diabetes, including T2DM, post-transplant diabetes mellitus (PTDM) and GDM with the rs13266634 polymorphism, and their findings indicated there to be a non-significant association (p = 0.74) with T2DM, a nominal association (p = 0.01) with PTDM and a strong significant association (p = 0.0003) with GDM. One of the parallel relationships between T2DM, PTDM and GDM is shared by the similar pathophysiology and once the diabetic disease develops in any individual, it will last forever throughout their lives. Moreover, one of the anti-parallel relationships between these three forms of diabetes is T2DM develops based on the age of onset, PTDM develops after renal transplantation via immunosuppressive drugs and GDM develops only in women during the middle trimester of pregnancy based on the lack of glucose intolerance. One of the multifactorial T2DM disease etiological factors emanates from defects in β-cell dysfunction. The SLC30A8 gene is connected and expressed in pancreatic β-cells involved in the development of T2DM-specific diabetes by disrupting the function of β-cells and transporting zinc from the cytoplasm to insulin-secretary vesicles (Khan et al., 2019; Fan et al., 2016). Fig. 6 describes the relationship between diabetes with the SLC30A8 gene as based on the aid of glucose metabolism, whereby glucose enters into β-cells and is converted into glucose-6-phosphate dehydrogenase, so its stimulus and its secretion is based on β-cell coupling. Further, glucose causes the closure of ATP-sensitive K+ (KATP) channels through mitochondrial ATP production and an increased ATP-ADP ratio, eliciting action potentials that are associated with the opening of Ca+2 voltage-gated channels. The rise in (Ca+2)I within β-cells and insulin production is more or less constant, regardless of blood glucose levels. To-be-released Ca2+ is stored within vacuoles via exocytosis, which is primarily caused by food, mainly nutrition containing absorbable glucose. Blood glucose levels rise after digestion, the main trigger. Finally, insulin secretion is inhibited by the SLC30A8 gene (Khan et al., 2015a, Khan et al., 2019, Khan et al., 2015b).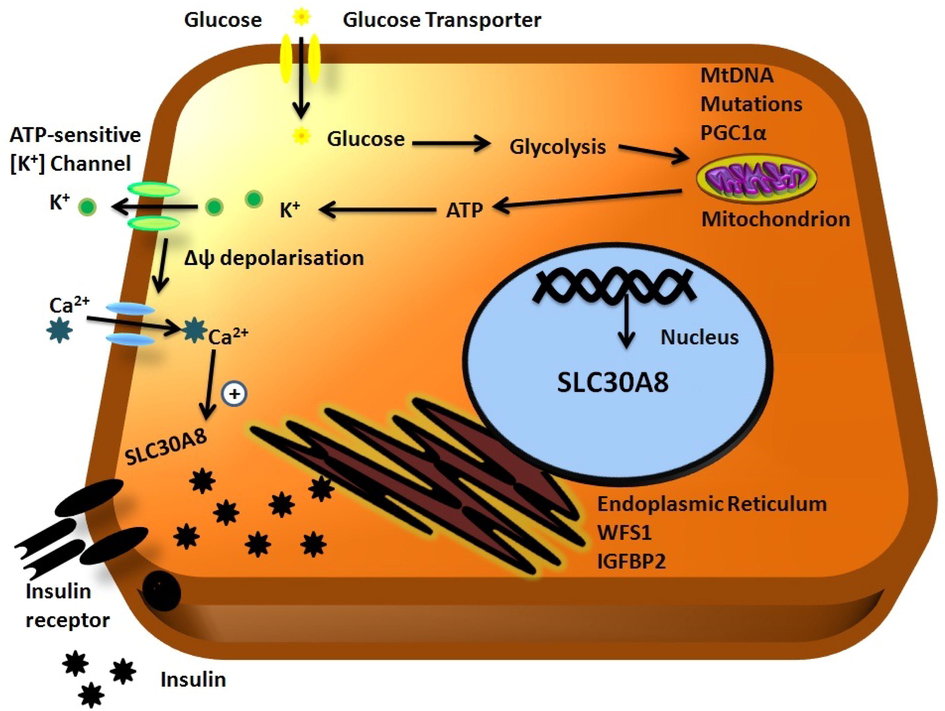
Genetic relationship between the SLC30A8 gene and diabetes.
A family history of Diabetes is related to a number of metabolic disorders, which is a major risk factor for T2DM, which has a powerful genetic link, and if both parents have diabetes, individuals with a family history are at greater risk of developing the disease with a two-fold risk (Consortium, 2013). In this study, all T2DM patients had a family history of diabetes, which indicated that participants had a risk of manifesting T2DM and inheriting the disease within the family pedigree. The current study had certain strengths, such as: (i) opting for Saudi subjects as an ethnic group; and (ii) all subjects had a family history of diabetes. The limitations of this study were: (i) performing only single SNP analysis along with the limited sample size; (ii) not conducting protein measurement; and (iii) not establishing the lifestyle and physical activities of T2DM patients.
5 Conclusion
In conclusion, the present study’s results confirmed the genetic relationship with the rs13266634 polymorphism in the SLC30A8 gene in T2DM cases in the Saudi population. However, this study was not in agreement with the prior study implemented in the Hejaz region featuring a low sample size. Future studies with a large sample size with an additional SNPs in the SLC30A8 gene should be carried out. Additionally, this study recommends that the meta-analysis should be updated for rs13266634 in the SLC30A8 gene as case-control studies have been documented in global T2DM ethnicities.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research group project No. RGP-244.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rapid detection of type II diabetes mellitus in Saudi patients via simultaneous screening of multiple SNPs. Biotechnol. Biotechnol. Equip.. 2019;33:1319-1326.
- [Google Scholar]
- Vitamin D supplementation as an adjuvant therapy for patients with T2DM: an 18-month prospective interventional study. Cardiovasc. Diabetol.. 2012;11:85.
- [Google Scholar]
- Alhomayani, F.K.H., Alotibi, Y.Z.M., Nasseralharbi, A.A., Alsuwat, H.A.M., Altowairqi, M.H.A., Alotaibi, H.A.A., 2020. Knowledge and attitude toward diabetes mellitus complications in Saudi Arabia; a systematic review.
- Non-alcoholic fatty liver disease treatment in patients with type 2 diabetes mellitus; new kids on the block. Curr. Vasc. Pharmacol.. 2020;18:172-181.
- [Google Scholar]
- Barriers and facilitators to successful management of type 2 diabetes mellitus in Latin America and the Caribbean: A systematic review. PLoS ONE. 2020;15:e0237542
- [Google Scholar]
- Cauchi, S., Meyre, D., Durand, E., Proença, C., Marre, M., Hadjadj, S., Choquet, H., De Graeve, F., Gaget, S., Allegaert, F., 2008a. Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PloS one, 3, e2031.
- The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med. Genet.. 2008;9:45.
- [Google Scholar]
- Association between SLC30A8 rs13266634 polymorphism and type 2 diabetes risk: a meta-analysis. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res.. 2015;21:2178.
- [Google Scholar]
- Consortium, I., 2013. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia, 56, 60–69.
- Role of TLR4 (C1196T) and CD14 (C-260T) polymorphisms in development of ischemic stroke, its subtypes and hemorrhagic stroke. J. Mol. Neurosci.. 2017;63(3–4):300-307.
- [Google Scholar]
- Dong, F., Zhang, B.-H., Zheng, S.-L., Huang, X.-X., Du, X.-B., Zhu, K.-H., Chen, X.-J., Wu, J., Liu, D.-D., Wen, Z.-H., 2018. Association between SLC30A8 rs13266634 polymorphism and risk of T2DM and IGR in Chinese population: a systematic review and meta-analysis. Front. Endocrinol. 9, 564.
- SLC30A8 gene polymorphism (rs13266634 C/T) and type 2 diabetes mellitus in south Iranian population. Mol. Biol. Rep.. 2014;41:2709-2715.
- [Google Scholar]
- Fan, M., Li, W., Wang, L., Gu, S., Dong, S., Chen, M., Yin, H., Zheng, J., Wu, X., Jin, J., 2016. Association of SLC30A8 gene polymorphism with type 2 diabetes, evidence from 46 studies: a meta-analysis. Springer.
- Methylenetetrahydrofolate reductase genetic polymorphism and the risk of diabetic nephropathy in type 2 diabetic patients. Medicine. 2020;99
- [Google Scholar]
- Fan, M., Li, W., Wang, L., Gu, S., Dong, S., Chen, M., Yin, H., Zheng, J., Wu, X., Jin, J., 2016. Association of SLC30A8 gene polymorphism with type 2 diabetes, evidence from 46 studies: a meta-analysis. Springer.
- Validation of the association of TCF7L2 and SLC30A8 gene polymorphisms with post-transplant diabetes mellitus in Asian Indian population. Intractable rare Dis. Res.. 2015;4:87-92.
- [Google Scholar]
- Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab. Syndrome. 2019;13:688-694.
- [Google Scholar]
- Type 2 diabetes mellitus and the association of candidate genes in Asian Indian population from Hyderabad, India. J. Clin. Diagn. Res.. 2015;9:Gc01-5.
- [Google Scholar]
- kulkarni, H., mamtani, M., peralta, J.M., DIEGO, V., dyer, T.D., goring, H., Almasy, L., mahaney, M.C., Williams-Blangero, S., Duggirala, R., 2016. Lack of association between SLC30A8 variants and type 2 diabetes in Mexican American families. J. Diabetes Res.
- Genome-wide association studies in type 2 diabetes. Curr. Diab. Rep.. 2009;9:164-171.
- [Google Scholar]
- Contribution of common variants of ENPP1, IGF2BP2, KCNJ11, MLXIPL, PPARγ, SLC30A8 and TCF7L2 to the risk of type 2 diabetes in Lebanese and Tunisian Arabs. Diabetes Metabol.. 2012;38(5):444-449.
- [CrossRef] [Google Scholar]
- GLP-1 receptor agonists in type 1 diabetes: a MAG1C bullet? The Lancet Diabetes Endocrinol.. 2020;8:262-264.
- [Google Scholar]
- Laboratory parameters of hemostasis, adhesion molecules, and inflammation in type 2 diabetes mellitus: Correlation with glycemic control. Int. J. Environ. Res. Public Health. 2020;17:300.
- [Google Scholar]
- The Interaction between gene profile and obesity in type 2 diabetes: a review. Obesity Med.. 2020;100197
- [Google Scholar]
- A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881-885.
- [Google Scholar]
- A Bayesian analysis for investigating the association between rs13266634 polymorphism in SLC30A8 gene and type 2 diabetes. J. Diabetes Metabol. Disorders. 2020;19:337-342.
- [Google Scholar]
- Type 2 diabetes-associated genetic polymorphisms as potential disease predictors. Diabetes Metabol. Syndrome Obesity: Targets Ther.. 2019;12:2689.
- [Google Scholar]
- Gene polymorphism association with type 2 diabetes and related gene-gene and gene-environment interactions in a Uyghur population. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res.. 2016;22:474.
- [Google Scholar]
- SLC30A8, CDKAL1, TCF7L2, KCNQ1 and IGF2BP2 are associated with type 2 diabetes mellitus in Iranian patients. Diabetes Metabolic Syndrome Obesity: Targets Ther.. 2020;13:897.
- [Google Scholar]
- Increased serum level and impaired response to glucose fluctuation of asprosin is associated with type 2 diabetes mellitus. J. Diabetes Investig.. 2020;11:349-355.
- [Google Scholar]







