Translate this page into:
Ameliorative impact of taurine on oxidative damage induced by Ipomoea carnea toxicity in wistar male rats through modulation of oxidative stress markers, apoptotic and Nrf2 pathway
⁎Corresponding author. mostafa.ataa@kfs.edu.eg (Mustafa Shukry)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
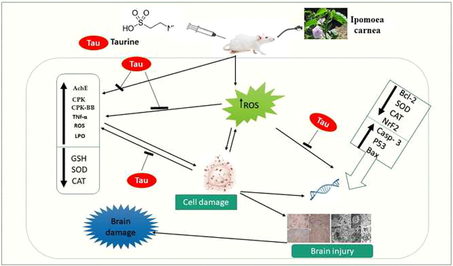
The adverse deleterious influences of Ipomoea carnea on brain tissue and Taurine's relative ability to prevent this neurotoxicity have been examined.
Methods
We utilized sixty Wistar male rats weighing 1752 g. The rats were allotted into 4 groups of fifteen each: The control group got saline i.p. daily for eight weeks; the Ipomoea carnea extract (ICE) group received ICE (15 mg/kg b.w./day) orally for eight weeks. Taurine-treated rats, in which rats were administered Taurine (200 mg·kg−1, i.p./Day) for 2 months. Taurine + ICE group in which rats were supplemented with (200 mg·kg−1, i.p. Taurine + ICE 15 mg/kg b.w./day orally) for 2 months.
Result
The findings indicated that Ipomoea persuaded an increase in lipid peroxidation markers as well as a disruption in antioxidant homeostasis, along with brain and serum cholinesterase (AChE) elevated levels, tumor necrosis factor (TNF), total creatine phosphokinase (CPK), creatine phosphokinase isoenzymes BB (CPK-BB), and lactate dehydrogenase (LDH). A considerable drop (P < 0.05) in the brain superoxide dismutase (SOD), catalase (CAT), and GSH in Ipomoea carnea. SOD, CAT, and GSH levels were enhanced in the Taurine co-treated group but did not achieve control levels. Ipomoea Furthermore, Induced apoptosis in brain tissue by Ipomoea carnea reflected in pro-apoptotic Bax, P53, and caspase 3 overexpression., while taurine upregulated the anti-apoptotic Bcl2 in addition to Histopathologic, electron microscope, and immunohistological brain examination finding support our finding concerning the protective role of taurine.
Conclusion
Our study proved the protective role of taurine as a supplement could recover Ipomoea carnea -induced oxidative changes and brain damage.
Keywords
Taurine
Ipomoea carnea
Oxidative injury
Apoptosis
Nuclear factor (erythroid-derived 2)-like 2
1 Introduction
Exposure to natural or synthetic hazardous compounds known as neurotoxins, for instance aluminum, mercury, copper, arsenic, lead, and manganese, marked by their capacity to change the normal nervous system function, which causes neural damages and neurotoxicity (Pohl et al., 2011). Neurotoxicity signs may involve brain injury, amnesia, anxiety, depression, weakness in limbs, and boring vision (Asada et al., 2010). Many neurodegenerative have implicated oxidative injury-induced degradation to neurons connected with neuronal cell deaths. The notable aspect of neurodegenerative illnesses is oxidative stress caused by neuronal death. However, it is not fully understood how oxidative injury causes neuronal death (Chen et al., 2009). Oxidative stress is a major role in neurodegenerative process modification. The hippocampus is the most sensitive brain components to oxidative injury (Araque et al., 2001). Taurine is a protective antioxidantoxidative injury that induced neuronal damage (Rivas-arancibia et al., 2001); during excitotoxicity, it serves as an antioxidant, shielding neurons against glutamate injuriousness. This defensive system is vital for the brain physiologically. Taurine syntheses at high quantities are stimulated after cell injury (Rivas-arancibia et al., 2001). The benefits of taurine, including cytoprotective properties, membrane stability, antioxidants, anti-inflammatory impacts, control of intracellular calcium and neurotransmitters, have been widely characterized (Lötsch et al., 2014). It protects body organs from toxicity; supplementing with taurine increases the pursuit of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) (GSH-Px) (Zhang et al., 2014). Ipomoea carnea (Convolvulaceae) is commonly referred to as Bush Morning Glory. It has been observed worldwide in tropical areas in goats, sheep, and cattle. Ipomoea carnea is a strong neurotoxic agent, has enormous usability as a plant pesticide, and affects animals C.N.S (Cholich et al., 2013). The purpose of this study is to explore at taurine's protective effect against oxidative injury caused by Ipomoea carnea leaves extract, as well as Taurine's intracellular protective mechanism. To achieve the objectives, measurements of acetylcholinesterase (AChE), MDA levels as a sign of lipid peroxidation; glutathione (GSH) levels, indicating cell thiol status; catalase (CAT) activity as an important component of cell antioxidant defense system, SOD and brain injury markers., (TNFα) Tumor necrosis factor, Alpha), Nuclear factor 2 (Nrf2, erythroid - derived 2) is a key transcription component regulating the transcription of antioxidant enzymes. in addition to Histopathologic , electron microscope and immunobiological brain examination.
2 Material and methods
2.1 Chemicals
Taurine has been got from Sigma-Aldrich (St. Louis, MO, USA) and primed before treatment in physiological saline. SOD Lipid Peroxidation (LPO), reduced glutathione (GSH) was derived from (Biodiagnostic, Giza, Egypt). Reagent TRIzol (Invitrogen, Life Technologies, Carlsbad, CA, USA). A cDNA kit has been purchased (Fermentas, Waltham, MA, USA). Unless otherwise noted, all chemicals were acquired from Sigma-Aldrich (St Louis, MO, USA),.
2.2 Ipomoea carnea extract preparation
The Ipomoea carnea extract was prepared according to (Schwarz et al., 2007) with some modification. Fresh leaves were mixed in a blender with ethyl alcohol 97%, homogenized in 97 % ethyl alcohol for 72 h before filtering through a Büchner funnel. Under reduced pressure, the filtrate was evaporated. Returned ethyl alcohol to leaf residue 24 h after filtration and evaporation. The 4 products were collected, and the definitive dark green extract was created. The resulting extract was diluted and filtered with filtered paper. Butanol was applied to the filtered part, separated by the decantation drums. The extract was kept at −20 °C.
2.3 Ethical declaration
The Committee accepted the experimental procedure authorizing the current investigation at the Faculty of Veterinary Medicine, University of Kafrelsheikh (KVM030/2020; May 2020).
2.4 Experimental plan
Sixty Wistar male rats, 175 ± 2 g, two months age, have been held to be adapted for 14 days. The animals were housed in regular circumstances (23 ± 2 °C and moisture between 50 and 60%) with water and food ad libitum. The rats were assigned in four groups (n = 15 each group) as follows: The rats were allotted into 4 groups of fifteen each: The control group got saline i.p. daily for eight weeks; Ipomoea carnea extract (ICE) group in which rats were treated with ICE orally for eight weeks (15 mg/kg b.w./day) (Hosomi et al., 2008). Taurine-treated rats, in which rats were administered Taurine (200 mg·kg − 1, i.p./daily) for two months (Alhumaidha et al., 2016). Taurine + ICE group (Taurine, 200 mg·kg − 1, i.p. + ICE 15 mg/kg b.w. per day orally) for two months. In all groups, rats are fed a standardized regular meal (20% protein) made in a laboratory according to (Atta et al., 2017).
2.5 Sampling
Before the treatments and before the rats were slain, the initial weight of the rats was measured, and the weight gain was reported. The rats injected intravenous sodium pentobarbital (30 mg/kg) (Atta et al., 2017) and sacrificed for a appropriate sample and analysis procedures. Blood samples were taken from the tail vein using the Vacutainer PST II tube, coagulated, then centrifuged at 3000 g for 15 min. Serum samples maintained at −20 °C before further analysis. The brain sample was removed , rinced in ice-cold 1.15 % KCl before being dried and weighed on filter paper. Brain samples were homogenized in PBS. Centrifuge the homogenate at 1,800 g for 15 min at 4 °C, and The supernatant was utilized in the subsequent investigation of all brain tissue. The sample was evaluated for antioxidant status three times. Part of the hypothalamus and hippocampus was kept for histological and immunohistochemical investigation in 10% neutral formalin buffer. Another hypothalamus section was cut kept at −80 °C for molecular analysis.
2.6 Biochemical analysis
Following the manufacturer's instructions (Biodiagnostic co., Egypt), the serum cholinesterase and the spectrophotometric evaluation of creatine phosphokinase and lactate dehydrogenase activity have
2.7 Brain oxidative stress markers
Brain samples were primed for oxidative injury assessment after homogenization in PBS, and supernatants were evaluated for GSH levels (Beutler, 1963) Using a trade kit (Biodiagnostic, Giza, Egypt). Superoxide dismutase activity was analyzed following (Marklund and Marklund, 1974) according to the extent to which free radicals interact with pyrogallol's auto-oxidation. Catalase activity was determined using the standard substrate 0.2 M H2O2. It was finished by adding potassium dichromate-acetic acid in 15-second intervals for a maximum of one minute Extinction was read at 240 nm (Sinha, 1972). Malondialdehyde, an intermediate consequence of lipid peroxidation, was determined utilizing thiobarbituric acid to determine lipid peroxidation (Ohkawa et al., 1979). Brain acetylcholinesterase (AChE) was assessed colorimetrically using Sigma-Aldrich (St Louis, MO, USA). Tumour necrosis factor-alpha (TNF-α) levels were analyzed in the brain tissue homogenate utilizing a rat TNF-α ELISA kit supplied by R&D Systems (Minneapolis, MN, USA). ROS levels in the brain tissue homogenate were assessed following (Socci et al., 1999). In brief, hydrogen peroxide converts 2,7-dichlorofluorescein diacetate to fluorescent 2,7-dichlorofluorescein established 525-nm emission of wavelengths using a fluorescence plate reader 488-nm excitation. The Bradford assay was used to determine the protein content in homogenates (Bio-Rad Laboratories, Watford, UK)(Bradford, 1976)
2.8 Real-time polymerase chain reaction
RT-PCR assessed the mRNA expression of brain genes. Total RNA was isolated from about 100 mg of brain tissue measured using TRIzol (Invitrogen, Life Technologies, Carlsbad, CA, USA). A cDNA package (Fermentas, Waltham, MA, USA) was used to synthesize RNA samples with A260/A280 1.8 or above. Using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control(house keeping) gene, we employed the SYBR Green master mix and primers indicated in Table 1. Analyzed amplification data using two techniques. (Livak and Schmittgen, 2001). Bax, Bcl-2-associated X protein. Bcl-2, B-cell lymphoma 2. CASP3, caspase 3. CAT, catalase. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. GnRH1, gonadotropin-releasing hormone receptor. GSH-Px, glutathione peroxidase. HSP70, heat shock protein 70. KISS1, kisspeptin. LHR, luteinizing hormone receptor. NECTIN2, nectin cell adhesion molecule 2. SOD, superoxide dismutase. (Nrf2) Nuclear factor (erythroid-derived 2)-like 2.
Gene
Direction
Primer sequence
Accession number
CAT
Sense
TAAGACTGACCAGGGCA
NM_012520.2
Antisense
CAAACCTTGGTGAGATCGAA
CASP3
Sense
CTGGACTGCGGTATTGAGAC
NM_012922.2
Antisense
CCGGGTGCGGTAGAGTAAGC
Bax
Sense
GGCGAATTGGCGATGAACTG
NM_017059.2
Antisense
ATGGTTCTGATCAGCTCGGG
Bcl-2
Sense
GATTGTGGCCTTCTTTGAGT
NM_016993.1
Antisense
ATAGTTCCACAAAGGCATCC
SOD
Sense
AGGATTAACTGAAGGCGAGCAT
NM_017050.1
Antisense
TCTACAGTTAGCAGGCCAGCAG
Nrf2
Sense
TGTCAGCTACTCCCAGGTTG
NM_031789.2
Antisense
ATCAGGGGTGGTGAAGACTG
GAPDH
Sense
TCAAGAAGGTGGTGAAGCAG
NM_017008.4
Antisense
AGGTGGAAGAATGGGAGTTG
2.9 Histological and immunohistopathological assessment of the brain
Brain samples were immersed in a 24-hour, 10% neutral formaldehyde buffer solution (Sigma-Aldrich). Fixing tissues, including ethanol dehydration (Sigma-Aldrich), have been treated by a paraffin integration device following (Bancroft and Layton, 2012). Immunohistological examination of the brain (Malkiewicz et al., 2006). Brain samples were dried at 10% and embedded in paraffin wax. Six thick pieces were cut, mounted on glass slides, and overnight incubated. Dewaxed sections were treated with protein block for 30 min, then treated with the polyclonal anti-GFAP antibody at 4.C overnight (1:1000). Sections were then treated with biotinylated anti-rabbit IgG antibody (1:100) and avidin–biotin-peroxidase reagent at room temperature for 30 min. DAB visualized the peroxidase reaction. Samples were countered with haematoxylin. to visualize neurons, Nissl-stained slices.
2.10 Transmission Electron microscopy examination
Brain specimen was rapidly cut into small pieces about (1 mm3); then fixed into 2,5% glutaraldehyde, buffered into 0.1 M sodium cacodylate at four °C and post-fixed in 1d Osmium tetroxide then dehydrated in ascending grades of ethyl alcohol, treated with propylene oxide then embedded in EPON and sectioned by ultra-microtome. For the study of light microscopy, thick sections (1 μm thick) were mounted on glass slides and dyed with blue toluidine. Ultrathin (60–70 nm thick) has been put on copper grids and colored with uranyl acetate and citrate lead (Reynolds, 1963). Sections were inspected and photographed using the Joel electron transmission microscope (TEM)1010 (Glauret and Lewis, 1998) at the electron microscope unit, science faculty, Alexandria university.
2.11 Statistical assessment
Statistical data analysis utilizing ANOVA, Duncan multiple comparison procedures with SPSS Software version 20.0 (SPSS Inc., Chicago, IL, USA). RT-PCR data evaluated using one-way ANOVA, GraphPad Prism 5 (San Diego, CA, USA), and Tukey's multi-purpose post-hoc testing. P < 0.05 was established as statistical difference.
3 Results
3.1 Effect of Taurine and/ or Ipomoea carnea on weight gain and brain weight
No mortalities were noticed during the experiment in all treated groups. Rats treated with Ipomoea carnea indicated a substantial drop in body weight (p < 0.05) in relation to control and taurine treated groups. In Taurine + Ipomoea carnea, the body weight gain was substantially greater (p < 0.05) contrasted with Ipomoea, and the bodyweight gain substantially lower (p < 0.05) related to control and taurine groups (Table 2). Taurine co-treatment with Ipomoea carnea displayed a nonsignificant boost in the absolute, and relative brain weight of the Ipomoea carnea treated group alone. Data expressed as mean ± S.E.M. Values with different letters at the same raw significantly differed. P < 0.05. n = 15.
Control
Ipomoea carnea (ICE)
Taurine
Taurine + ICE
Initial body weight (g)
175.77 ± 3.1
176.21 ± 3.5
175.61 ± 2.7
174.71 ± 3.2
Final body weight (g)
252.71 ± 3.6a
221.82 ± 4.2c
259.2 ± 3.5a
239.6 ± 4.2b
Body weight differences (g)
76.96 ± 2.4a
45.61 ± 2.1c
83.6 ± 3.1a
64.89 ± 3.1b
Absolute brain weight (g)
2.15 ± 0.02a
1.18 ± 0.01bc
2.21 ± 0.02a
1.48 ± 0.01b
Relative brain weight (%)
0.850 ± 0.05a
0.531 ± 0.02bc
0.852 ± 0.02a
0.617 ± 0.01b
3.2 Impact of Taurine and/or Ipomoea carnea on biochemical parameters and acetylcholinesterase activity
Our data illustrated in Fig. 1 showed that Ipomoea carnea induced a significant increase (P < 0.05) in CPK, CPK-BB, and LDH concerning other treated groups as well as, there was a considerable upsurge in both serum and brain acetylcholinesterase activity (Figs. 1 and 2). The administration of Taurine stored these parameters toward the control one.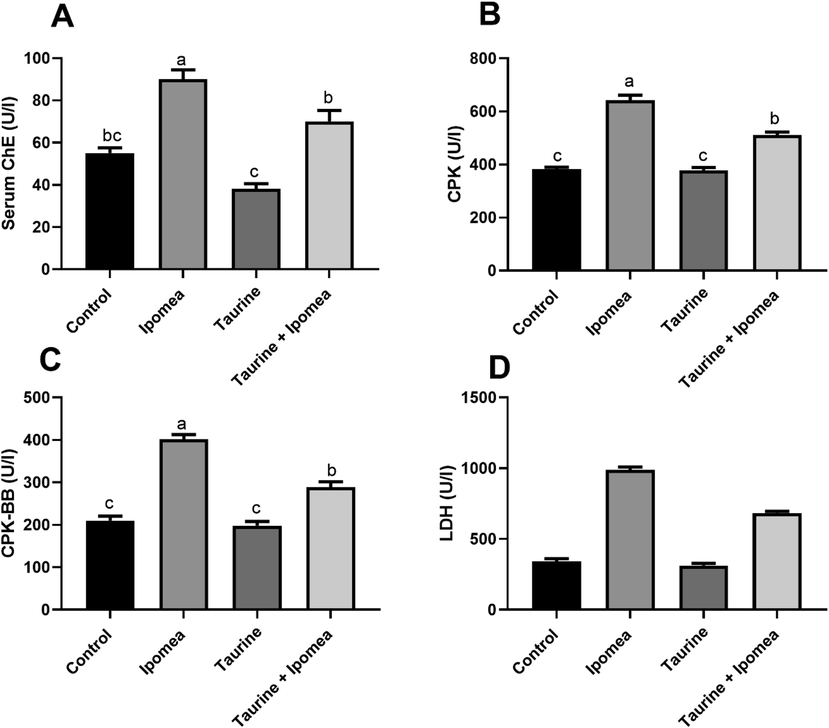
Effects of Ipomoea carnea and co-treatment with Taurine on serum acetylcholinesterase activity (A), serum CPK(B), CPK-BB (C), and LDH(D) of rats. Data expressed as mean ± S.E.M. Values column with different letters significantly differ P < 0.05. n = 15.
3.3 Impact of Taurine and/or Ipomoea carnea on the antioxidant markers
Our result revealed that there was a noteworthy rise (P < 0.05) in brain ROS, lipid peroxidation levels, as well as TNFα in Ipomoea carnea, challenged group concerning other treated groups, in the same way, taurine co-treatment with Ipomoea carnea revealed a considerable decrease (P < 0.05) in ROS, lipid peroxidation levels concerning the Ipomoea carnea but did not reach to the control one levels as shown in Fig. 2. Fig. 3 showed a sizeable decline (P < 0.05) in the brain SOD, CAT, and GSH in Ipomoea carnea. SOD, CAT, and GSH were improved (P < 0.05) in the Taurine co-treated group but did not reach the control level.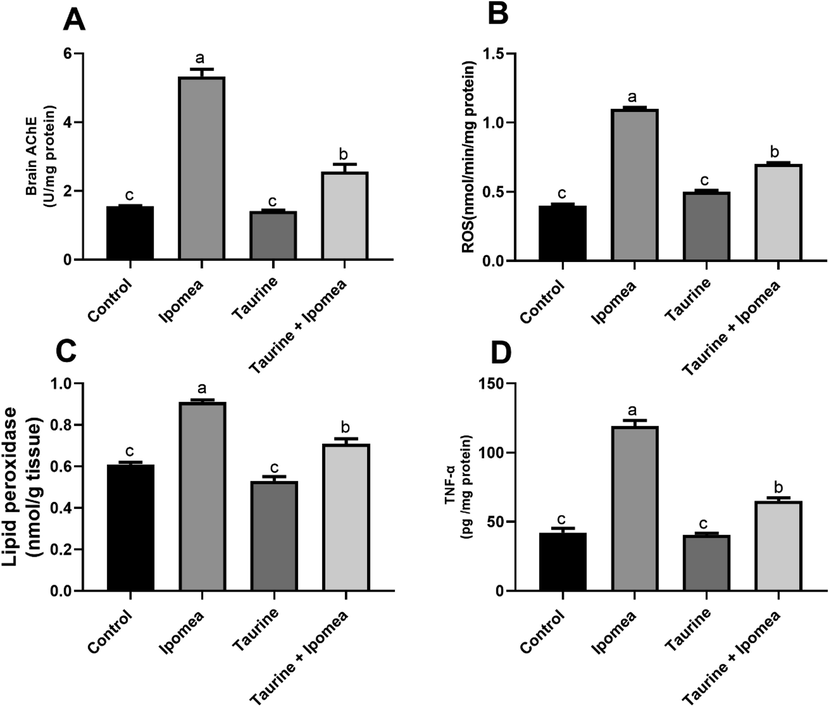
Effects of Ipomoea carnea and co-treatment with Taurine on Brain acetylcholinesterase activity (A), Brain ROS, (B), Brain lipid peroxidation (C), and Brain TNF- α (D) of rats. Data expressed as mean ± S.E.M. Values column with different letters significantly differ P < 0.05. n = 15.
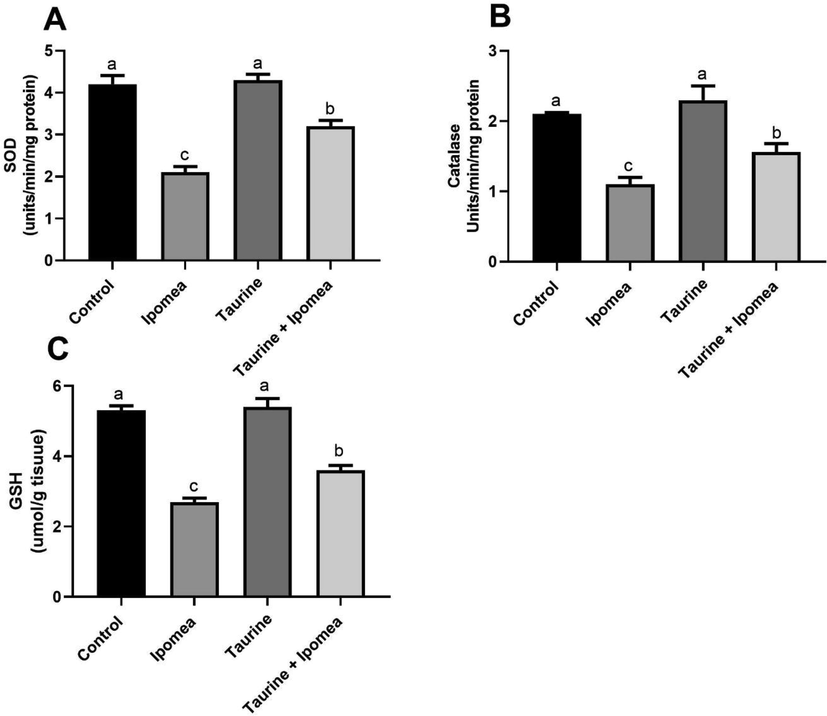
Effects of Ipomoea carnea and co-treatment with Taurine on Brain SOD (A), Brain Catalase, (B), and Brain GSH (C) of rats. Data expressed as mean ± S.E.M. Values column with different letters significantly differ P < 0.05. n = 15.
3.4 Impact of Taurine and/ or Ipomoea carnea on genes transcription levels of the antioxidants and apoptotic biomarkers
Brain tissue content of SOD and CAT were assessed using qRT-PCR, in which figure showed the effect of Ipomoea carnea on the mRNA transcription of brain SOD, CAT, and NrF2; the CAT, SOD, and NrF2 downregulation of Ipomoea carnea treated rats was quite substantial (P < 0.05) concerning other treated groups. However, these gene transcription levels significantly increased (P < 0.05) in Taurine cotreated groups related to Ipomoea carnea treated rats but did not reach the control levels as demonstrated in Fig. 4A.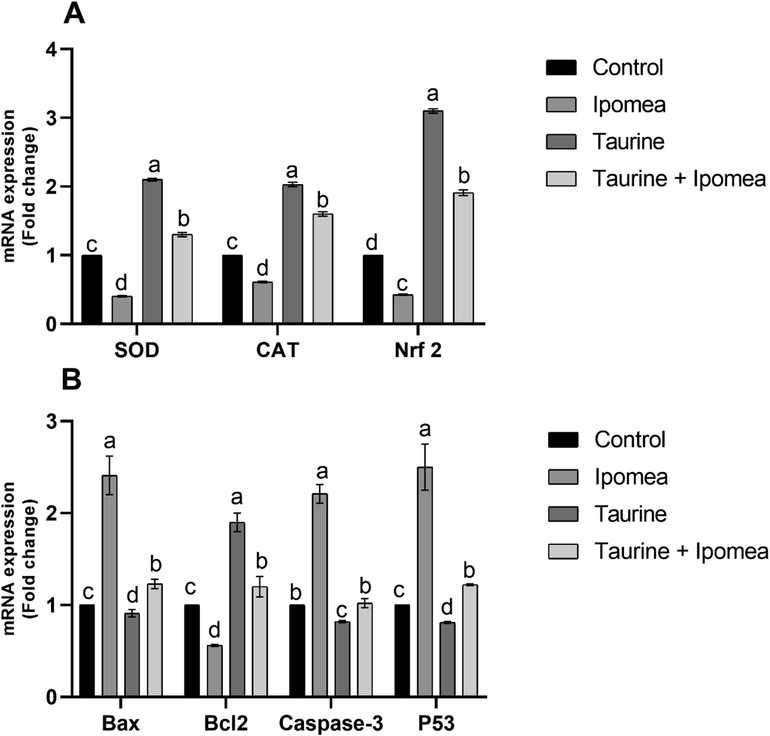
Effects of Ipomoea carnea and co-treatment with Taurine on the mRNA transcription of CAT, SOD, and NrF2 in the brain tissue of rats (A) and The apoptotic gene markers Bax, Bcl-2, Caspase 3, and P53 (B). Data expressed as mean ± S.E.M. Values column with different letters significantly differ P < 0.05. n = 15.
The apoptotic gene markers Bax, Caspase 3, and P53 offered a substantial increase(P < 0.05) in Ipomoea carnea treated group with a noteworthy lessening in Bcl2 associated to other treated rats. Our data in Fig. 4B showed that the Taurine co-treated group showed significant down-regulation of the apoptotic genes Bax, Caspase 3, and P53 with significant upregulation of Bcl2 gene transcription
3.5 Impact of Taurine and/ or Ipomoea carnea on Histopathologic changes of brain
Figure 5 showed that the Parafiffen section photomicrograph of rat brain showed control rat brain group in hippocampus region with normal neuroglial cells with rounded nuclei and prominent nucleolus, and area of proliferating hyperchromatic pyramidal cells. Ipomoea carnea treated rat showing proliferating lymphocytes with plasma cells and necrotic neuropile cells and hyperchromatic Purkinje cells, edema, and thick fibrotic sheaths area of the demyelinated sheath, as well as pyknotic neuropile cells and absence of pyramidal and neuroglia cells. Taurine-treated rats show hippocampus with its characteristic three layers polymorphic, pyramidal, and molecular, polymorphic layer appeared with normal neuroglial cells that rounded nuclei and prominent nucleolus and many myelinated sheaths. Taurine co-treated Ipomoea carnea treated showing regenerative of the hippocampus with polymorphic layer appeared with normal neuroglial cells.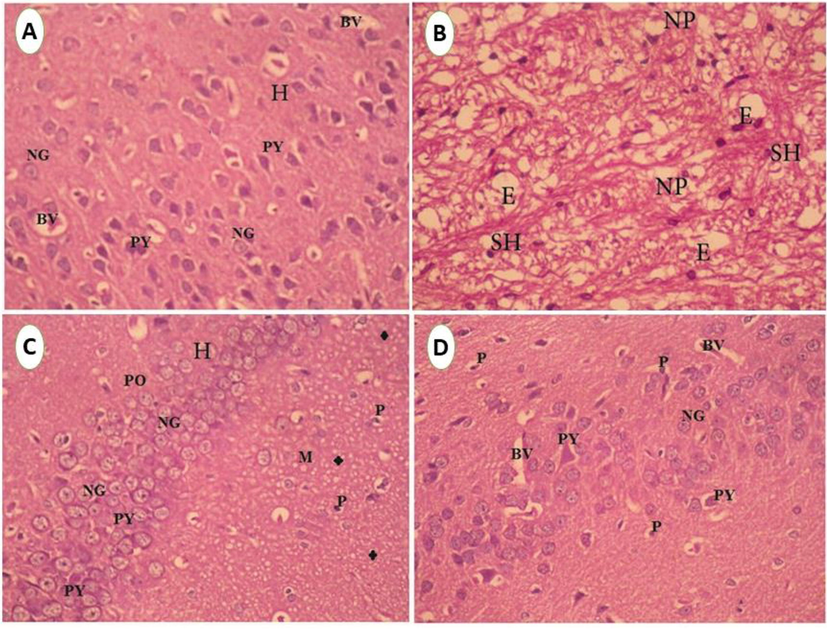
A. Paraffin section photomicrograph of control rat brain, showing hippocampus region (H) with normal neuroglia cells (NG) with rounded nuclei and prominent nucleolus and area of proliferating hyperchromatic pyramidal cells (PY). Miled dilation of blood vessels (BV) was seen, (H&E X400). B. Photomicrograph of hypothalamus region of rat histological sections treated with Ipomea carnea showing an area of the demyelinated sheath (SH) and edema (E) as well as pyknotic neuropile cells (NP) and absent of pyramidal and neuroglia cells (H&E X200). C. Paraffin section photomicrograph of rat brain treated with Taurine, show hippocampus(H) with its characteristic three layers) polymorphic (PO), pyramidal (PY) and molecular (M), polymorphic layer appeared with normal neuroglia cells (NG) which rounded nuclei and prominent nucleolus as well as many myelinated sheaths (*),(H&E X200). D. Paraffin section photomicrograph of rat brain treated with Taurine + Ipomea Carnea, showing regenerative of the hippocampus with polymorphic layer appeared with normal neuroglia cells (NG) which rounded nuclei and prominent nucleolus and normal pyramidal nuclei and few vacuolated cytoplasms of pyramidal cells (PY) spread of few pyknotic pyramidal cells (P), and mildly dilated blood vessels (BV)was seen. (H&E X200).
Figure 6 showed the electron micrograph of the control group of rat hippocampal and hypothalamus tissue showing control rat hippocampal nerve cells (light cells) arranged neatly and coated complete, containing euchromatic nuclei, lysosomes, and myelin sheath with hypothalamus showing normal myelin sheath and normal mitochondria with obvious cristae the hippocampus of treated rat with Ipomoea carnea Abnormal astrocytes with highly vacuolated cytoplasm with abnormal axons and myelin sheath, Grades of myelin sheath filled with abnormal mitochondria and nerve cell nucleus with heterochromatic nuclei surrounded by large extensive edema. Electron micrograph in Taurine treated group demonstrating normal hypothalamus nerve cell with Euchromatic nuclei, myelin sheath, axons and mitochondria Electron micrograph of rat hippocampus treated with Ipomoea carnea + Taurine revealing hippocampus granular layers with nerve cells two dark cells and astrocytes with cytoplasmic dendrites. They are normally arranged with normal mitochondria and myelin sheath.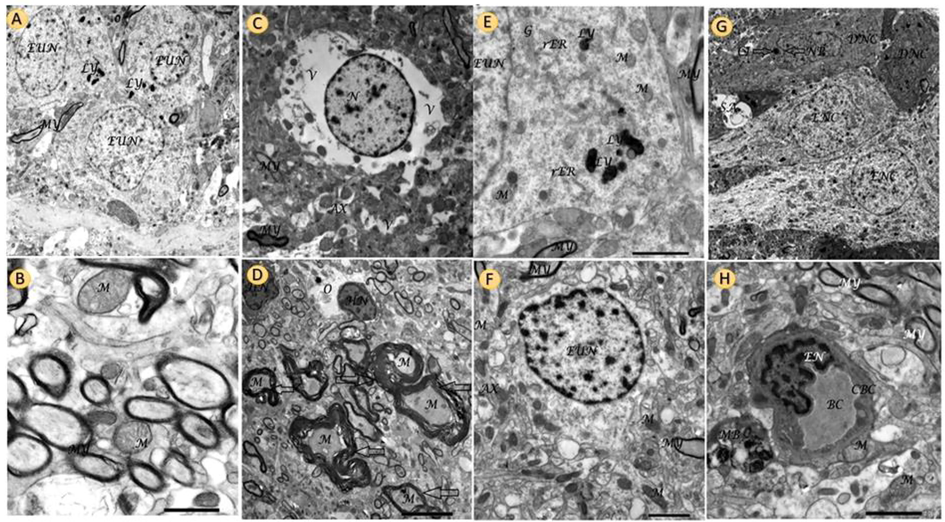
Electron micrograph of control group of rat hippocampal and hypothalamus tissue showing control rat hippocampal nerve cells (light cells) arranged neatly and coated complete, containing euchromatic nuclei, lysosomes, and myelin sheath,(A). And control hypothalamus showing normal myelin sheath and normal mitochondria with obvious cristae (B). Electron micrograph of the hippocampus of treated rat with Ipomea carnea Abnormal astrocytes with highly vacuolated cytoplasm (V) with abnormal axons (AX) and myelin sheath (MY), Scale bar 2.0 µm, X3000 (C). Electron micrograph of treated rat hypothalamus with Ipomea carnea showed grades of the myelin sheath (MY) filled with abnormal mitochondria (M) and nerve cell nucleus (N) with heterochromatic nuclei (HN) surrounded by large area (extensive edema). Scale bar 5.0 nm, X1500) (D). Ultrastructural image of neurofibrils (NF) of the hippocampus in Taurine group revealing part of the nucleus and cytoplasm of hippocampal nerve cell contain mitochondria (M), rough endoplasmic reticulum (rER) and lysosomes (LY), Golgi apparatus (G). Scale bar 2.0 µm, X4000. Electron micrograph of rat hippocampus treated with Ipomea carnea + Taurine revealing hippocampus granular layers with nerve cells two dark cells (DNC), and two light cells (LNC) (astrocytes) with cytoplasmic dendrites (extensions) (CEX). They arranged neatly and coated completely, one of the dark cells its nucleus with nuclear pocket (NP) with cytoplasmic inclusion (CI) and synaptic alteration (SA). (Scale bar 5.0 nm, X1000) (G). Transmission electron micrograph in the hypothalamus of rat Taurine + Ipomea carnea group illustrating blood capillary (BC) lined by endothelial cell (EN) surrounded by a capillary basement membrane (CBM), myelin sheath and axon (MY) multivesicular bodies (MB), lysosomes (LY), and normal mitochondria (M), scale bar 5.0 µm, X4000) (H).
Immunohistological examination Fig. 7 revealed that control and taurine treated groups showing faint brown color rays of immunostaining GFAP protein in microglial cells in rat brain and absent in the area of the proliferating neuropile cells, the blue color of the negative immunostaining of nuclei. The number of astrocytes of GFAP increased significantly from the control group shows chronic tissue injury, seen at the Parafiffen section photomicrograph of rat brain treated by Ipomoea carnea. Mild astrocytes of GFAP proteins in a myelinated sheath around the proliferating negative staining neuropile cell at hippocampus area of rat treated with taurine + Ipomoea carnea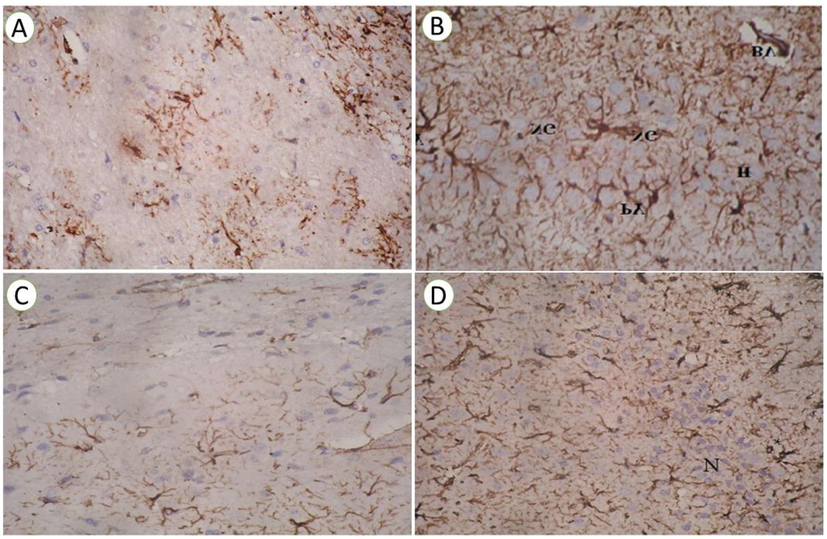
(A) Paraffin section photomicrographs of rat brain section in control group showing faint brown color rays of immunostaining GFAP protein astrocytes in microglia cells rat brain and absent in the area of the proliferating neuropile cells, the blue color of the negative immunostaining of nuclei (cytoplasmic reaction), (x 200). (B) Paraffin section photomicrograph of rat brain treated by Ipomea carnea of GFAP protein stained by DAB revealed a significant increase in several GFAP astrocytes, dark brown color of immunostaining antibody in neuroglial cells (NG) and pyramidal one(PY) as a cytoplasmic reaction in hippocampus layers (H), blood vessels (BV) with a negative blue color of the cell nuclei (x 400). (C) Paraffin section photomicrographs of rat brain section in Taurine treated group showing faint brown color rays of immunostaining GFAP protein astrocytes in microglia cells rat brain and absent in the area of the proliferating neuropile cells, the blue color of the negative immunostaining of nuclei (cytoplasmic reaction), (x 200). (D) Paraffin section photomicrographs are depicting the Mild brown color of immunostaining GFAP astrocytes in the myelinated sheath (*) around the proliferating negative staining neuropile (N) cell at hippocampus area of rat treated with taurine + ipomea carnea ( × 200).
4 Discussion
Due to excessive expenditure of oxygen, lipid content, and low levels of mitotic and antioxidant, the brain is particularly susceptible to oxidative injury, more than any other tissue (Said and Abd Rabo, 2017). Our data showed that the challenge to Ipomoea carnea has severely altered both the weight of the body and brain weight, showing potential harm to the body and brain relative to other treated groups. The reduction of brain weight after Ipomoea carnea may result from spongiosis in the neurons, leading to delayed animal growth (Julka et al., 1996). In the same line, (Hueza et al., 2007) reported a decrease in body-weight of Pups of mothers treated with Ipomoea Carnea due to this to the swainsonine (the toxic principles of Ipomoea carnea). The deleterious effect of Ipomoea carnea in rats body weight was ameliorated by taurine co-administration; our obtained result was inconsistent with (Hwang and Wang, 2001) in which they showed that Taurine administration recovered the body weight decreased induced by cadmium owing this effect to the trophic effect of taurine.
Our data revealed significant increases in LDH, CPK, and CPK-BB in the Ipomoea carnea treated group. In Ipomoea carnea treated rat, increased serum activity by LDH, CPK-BB and CPK correlated with brain damage. This rise could be coupled to the interaction of free radicals with the cell, leading to cell damage. Our research showed that the brain is more prone to the dangerous effect of Ipomoea carnea as reported by (Hueza and Górniak, 2011). They reported that Ipomoea carnea has a toxic component: an indolizidine alkaloid swainsonine. In the same way, our result revealed that there was a substantial upsurge in both serum and brain acetylcholinesterase activity together with a noteworthy rise in lipid peroxidation levels that may be the cause of increasing the activity of serum and brain acetylcholinesterase due to disruption of cell membrane increased lipid peroxidation (Kaizer et al., 2005). Furthermore, calystegines inhibit glucosidase, which influences oligosaccharides’ metabolism. Possibly this can enter the brain at large levels and build up in cellular organelles with a low pH (Asano et al., 2000); these supported our finding in which the Ipomoea carnea go across the blood–brain barrier and trigger oxidative damage. Our result revealed a considerable rise in brain ROS along with TNFα in Ipomoea carnea treated rats; these findings were inconsistence with (Hu et al., 2015). They confirmed that Swainsonine exposure significantly stimulates pro-inflammatory cytokines TNF-α secretion.
Taurine is an important amino acid most commonly found in the brain and heart of mammalian species (Beyranvand et al., 2011). It has various cytoprotective properties such as neurotransmitter and neuromodulator, neuroprotective, antioxidant, and anti-inflammatory (El Idrissi, 2008). Our result revealed that Taurine co-treatment with Ipomoea carnea could make a recovery to the CPK, CPK-BB, and LDH parameters but not reach the control levels , our results were coherent with (Alhumaidha et al., 2016) they stated that taurine administration leads to decreases in CPK, CPK-BB, and LDH markers, Taurine, by its membrane-stabilizing effect, is responsible for limiting the leakage of biochemical indicators (Timbrell et al., 1995). Taurine co-treatment with Ipomoea carnea prevents the augmentation of acetylcholinesterase activity. This result was in line with (Rosemberg et al., 2010). They reported that taurine inhibits acetylcholinesterase induced by ethanol challenge, and (Niu et al., 2018) reported that taurine administration significantly decreased acetylcholinesterase and tumor necrosis factor–α.
Additionally, our findings revealed that a major decline occurred (P < 0.05) in the brain SOD, CAT, and GSH in Ipomoea carnea, while other treated groups were a major rise (P < 0.05) in the Taurine co-administered group but not reach to the control level. These were accompanied by a significant improvement of the gene transcription levels of SOD and CAT; our result was under (Niu et al., 2018) they revealed that Taurine raises antioxidant levels by increasing Electron transmission chain activity (Shimada et al., 2015).
The current study attributes the neurotoxicity of Ipomoea to the oxidative injury caused by free radical creation. The taurine's protecting action may be due to antioxidant enzymes and lipid peroxidation inhibition (Oliveira et al., 2010). Taurine osmoregulatory function and its antioxidant activity are due to scavenging ROS, alleviating lipid peroxidation (Schaffer et al., 2000). Taurine reduces lipid peroxidation through radical scavenging or by binding Fe2 + like chelator (Erdem et al., 2000).
Nuclear Factor 2 (Nrf2) is a crucial transcription factor for antioxidant regulations (de Vries et al., 2008). In the present work, the consequences of our result recorded that Ipomoea carnea treatment diminishes Nrf2 expressions. However, Taurine strengthens Nrf2 that controls the antioxidative system (de Vries et al., 2008). Therefore, Nrf2 is projected to protect the neurodegeneration caused by Ipomoea carnea as it essential tissue repair (ADTG Wagener et al., 2010). We assume that taurine upregulation -instigated Nrf2 is essential to improved neurodegeneration
Apoptosis cell death was triggered by oxidative stress. Our work showed that, increase the apoptotic gene markers Bax, Caspase 3, and P53 and decreased the Bcl2 expression. The taurine co-treated group showed a substantial down-regulation of the apoptotic genes Bax, Caspase 3, and P53 with significant upregulation of Bcl2 gene transcription; these results were inconsistence with (Niu et al., 2018) they found that Taurine administration decreased the p53, caspase-3, and Bax expression as well as, TNF-α, IL-6, IL-1α, and IL-1β in the neuronal grievance. Our obtained result revealed the promoting effect of taurine administration against oxidative injury, inflammation, and apoptosis.
Our result concerning the histopathological analysis showed that the control hippocampus region with normal neuroglial cells with rounded nuclei and proliferating hyperchromatic pyramidal cells is and Taurine treated rats show normal hippocampus with its characteristic three layers. However, Ipomoea carnea administration area of proliferating lymphocytes with plasma cells and hyperchromatic Purkinje cells, edema , was similar to several previously reported publications (Salinas et al., 2019),. This also supported the significant toxicity of Ipomoea carnea for Wistar male rats (Amna et al., 2011). Taurine is needed to keep cellular and subcellular membranes stable and organized by preventing detrimental or oxidative reactions and directly affecting the expansion of brain cells (Pasantes-Morales and Hernández-Benítez, 2010). Taurine and Ipomoea carnea treated rats showed regenerative hippocampus with polymorphic layer appeared with normal neuroglial cells. These data are supported by (Pasantes-Morales and Hernández-Benítez, 2010) they recorded the cellular protection effect of Taurine. The results of the transmission electron microscope support our histological findings; control and taurine groups showed hippocampal nerve cells arranged normally and the hypothalamus showing normal myelin sheath and normal mitochondria with obvious cristae.
In contrast, an Electron micrograph of hippocampus treated rat with Ipomoea carnea demonstrating damaged astrocyte cell and nucleus, damaged lysosomes and abnormal myelin sheath. Electron micrograph of hypothalamus treated rat with Ipomoea carnea revealing grades of myelin alternations, indicate local disarrangement of myelin sheath. These alterations were recovered by taurine co-treatment.
Immunohistological examination revealed that the hippocampus area of Ipomoea carnea significantly increased GFAP astrocytes than other treated groups; this was in harmony with (Cholich et al., 2013) neuropathological variations in guinea pigs treated with Ipomea carnea. Our findings revealed that taurine co-treatment declined the GFAP upregulation. This result was confirmed by demonstrated by (ZHANG et al., 2007); they found that Taurine decreased GFAP control in cells resulting from high glucose. It indicated that a decrease in injury response might be a protective effect of Taurine, indirectly inhibiting colloid cell activity. Or its protective action was accomplished through the transmembrane active transferring of the TauT cell membrane to preserve intracellular high taurine concentration (Huxtable, 1992).
5 Conclusion
This is the first study to show Ipomoea carnea is a potent oxidative damage agent and brain damage in adult rats through reduced neuronal apoptotic gene expressions. Interestingly, the co-treated taurine with Ipomoea carnea poisoned rats seems effective for reducing Ipomoea carnea’s hazardous effects via inhibiting oxidative stress indicators and changes in the activity of acetylcholinesterase as well as inhibiting the apoptosis, inflammatory cytokines, decreasing ROS boosting with significant activation of brain SOD, CAT, and GSH and modification of Nrf2 that controls the transcription of antioxidant responsive genes in addition, our histopathological and immunohistochemical investigation in the extant study The biochemical and molecular studies revealed that taurine had protective effect from Ipomoea carnea caused brain toxicity.
6 Ethical animal research
All experimental procedures were performed according to the rules of the Animal Care and Ethics Committee of the Faculty of Science, Kafrelsheik University, Egypt.
CRediT authorship contribution statement
Asmaa R. Abou El-khair: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Nora Ghanem: Conceptualization, Formal analysis, Project administration, Supervision. Samaa bakr: Conceptualization, Investigation, Supervision. Mohamed Mohamed Soliman: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing. Mohamed M. Soliman: Conceptualization, Formal analysis, Funding acquisition, Supervision. Mayada R. Farag: Writing – original draft, Writing – review & editing. Mohamed E. Abd El-Hack: Conceptualization. Mustafa Shukry: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Acknowledgement
We thank and appreciate the financial help provided by Taif University, Saudi Arabia through its researchers supporting project (TURSP-2020-09). Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ADTG Wagener, F., Scharstuhl, A., M Tyrrell, R., W Von den Hoff, J., Jozkowicz, A., Dulak, J., GM Russel, F., Marie Kuijpers-Jagtman, A., 2010. The heme-heme oxygenase system in wound healing; implications for scar formation. Curr. Drug Targets 11, 1571-1585.
- Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Can. J. Physiol. Pharmacol.. 2016;94(2):131-139.
- [Google Scholar]
- Toxic effect of Ipomoea carnea leaves on wistar rats. J. Pharmacol. Toxicol.. 2011;6(1):18-23.
- [Google Scholar]
- Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol.. 2001;63(1):795-813.
- [Google Scholar]
- Accumulation and mobility of zinc in soil amended with different levels of pig-manure compost. J. Environ. Sci. Health Part B. 2010;45(4):285-292.
- [Google Scholar]
- Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry. 2000;11:1645-1680.
- [Google Scholar]
- Thymoquinone defeats diabetes-induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int. J. Mol. Sci.. 2017;18(5):919.
- [CrossRef] [Google Scholar]
- Bancroft, J.D., Layton, C., 2012. The hematoxylins and eosin. Bancroft’s theory and practice of histological techniques, 173-186.
- Improved method for the determination of blood glutathione. J. Lab. Clin. Med.. 1963;61:882-888.
- [Google Scholar]
- Effect of taurine supplementation on exercise capacity of patients with heart failure. J. Cardiol.. 2011;57(3):333-337.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72(1-2):248-254.
- [Google Scholar]
- Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochemi. Cell Biol.. 2009;41(6):1284-1295.
- [Google Scholar]
- Experimental intoxication of guinea pigs with Ipomoea carnea: Behavioural and neuropathological alterations. Toxicon. 2013;76:28-36.
- [Google Scholar]
- Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med.. 2008;45(10):1375-1383.
- [Google Scholar]
- Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids. 2008;34(2):321-328.
- [Google Scholar]
- Erdem, A., Gündogan, N.Ü., Usubütün, A., Kılınç, K., Erdem, Ş.R., Kara, A., Bozkurt, A., 2000. The protective effect of taurine against gentamicin‐induced acute tubular necrosis in rats. Nephrology Dialysis Transplantation 15, 1175-1182.
- Biophysical, specimen preparation for transmission electron microscopy. Vol Vol. 17. London: Parhand Press; 1998.
- Embryotoxic effects of prenatal treatment with Ipomoea carnea aqueous fraction in rats. Braz. j. vet. res. anim. sci. 2008;45:67-75.
- [Google Scholar]
- The immunomodulatory effects of Ipomoea carnea in rats vary depending on life stage. Hum. Exp. Toxicol.. 2011;30(10):1690-1700.
- [Google Scholar]
- Assessment of the perinatal effects of maternal ingestion of Ipomoea carnea in rats. Exp. Toxicol. Pathol.. 2007;58(6):439-446.
- [Google Scholar]
- Distribution of aluminum in different brain regions and body organs of rat. Biol. Trace Elem. Res.. 1996;52(2):181-192.
- [Google Scholar]
- Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J. Inorg. Biochem.. 2005;99(9):1865-1870.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402-408.
- [Google Scholar]
- Congenital taurine deficiency in mice is associated with reduced sensitivity to nociceptive chemical stimulation. Neuroscience. 2014;259:63-70.
- [Google Scholar]
- Cypermethrin alters glial fibrillary acidic protein levels in the rat brain. Environ. Toxicol. Pharmacol.. 2006;21(1):51-55.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep.. 2018;18:4516-4522.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep.. 2010;62(1):185-193.
- [Google Scholar]
- Taurine and brain development: trophic or cytoprotective actions? Neurochem. Res.. 2010;35(12):1939-1943.
- [Google Scholar]
- The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol.. 1963;17:208-212.
- [Google Scholar]
- Taurine increases rat survival and reduces striatal damage caused by 3-nitropropionic acid. Int. J. Neurosci.. 2001;108(1-2):55-67.
- [Google Scholar]
- Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience. 2010;171(3):683-692.
- [Google Scholar]
- Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh. Hig. Rada Toksikol.. 2017;68:27-36.
- [Google Scholar]
- Neurological syndrome in goats associated with Ipomoea trifida and Ipomoea carnea containing calystegines. Toxicon. 2019;157:8-11.
- [Google Scholar]
- Interaction between the actions of taurine and angiotensin II. Amino Acids. 2000;18(4):305-318.
- [Google Scholar]
- Rats offspring exposed to Ipomoea carnea and handling during gestation: neurochemical evaluation. Braz. Arch. Biol. Technol.. 2007;50(3):425-433.
- [Google Scholar]
- Role of ROS production and turnover in the antioxidant activity of taurine. Taurine. 2015;9:581-596.
- [Google Scholar]
- Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp. Neurol.. 1999;155(1):109-117.
- [Google Scholar]
- The in vivo and in vitro protective properties of taurine. General Pharmacol.: Vascular Syst.. 1995;26(3):453-462.
- [Google Scholar]
- Effect of taurine on GFAP and TauT expressions in rat retinal Müller cells in high glucose culture. J. Med. Colleges PLA. 2007;22(3):137-142.
- [Google Scholar]
- Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep.. 2014;10:2255-2262.
- [Google Scholar]







