Translate this page into:
Ameliorative effects of piroxicam on perchloric acid-induced thyroid gland hormones disruption in male rats
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Background
The incidence of goitre has increased world-wide, with African countries responsible for over 28 % of the cases. The danger of perchloric acid among males working in the factories has been on the increase. Hence mitigating effects of piroxicam on perchloric acid-induced thyroid hormones disruption was studied in male Wister albino rats.
Methods
A total of 19 rats (17 males; 2 females) that weighed 145.0 ± 20.5 g were used for the.study. Four rats were used for determination of median lethal dose (LD50) of perchloric acid, whereas 15 rats divided into three groups of five each, were used for induction of thyroid hormones disruption, using 100 mg/kg of perchloric acid for a period of two weeks. Thereafter group II and III rats were treated with 2.5 and 5.0 mg/kg of piroxicam, respectively. Three millilitres of blood were collected from all the rats for determination of thyroid hormones, haematological and biochemical parameters. Brain, liver, spleen, lung, kidney and heart were harvested for allometry and histopathology.
Results
Findings have shown that LD50 of perchloric acid was 4207.5 ± 457.6 mg/kg body weight. Piroxicam significantly decreased (p < 0.05) thyroid stimulating hormone, but increased free triiodothyronine and thyroxine respectively. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and monocytes were increased significantly(p < 0.05). Urea, creatinine, haematocrit, erythrocytes, leucocytes, lymphocytes, neutrophils, platelets and eosinophils counts were decreased significantly (p < 0.05). Allometric parameters were significantly increased (p < 0.05). Histopathology revealed perivascular cuffs of brain, zenker’s necrosis with mononuclear cells infiltration of myocardium, congestion of central vein of liver, mononuclear cells infiltration of the kidney, lymphoid tissue aggregation of white pulp, congestion and thickness of the lungs were observed in the rats administered piroxicam.
Conclusion
Hence piroxicam ameliorates thyroid hormones disruption, induces liver enzymes, modulates humoral immune system, enhances renal function and decreases allometric parameters of brain, lung and liver, as evidenced by histopathological lessons.
Keywords
Piroxicam
Perchloric acid
Thyroxine
Endocrine
Goitre
Rats
1 Introduction
Male rodents are more sensitive to piroxicam and hydrocortisone as compared to female rodents (Saganuwan and Orinya, 2016). This may be due to higher ability for activation of metabolic process (NIEHS, 2000). The interest on toxicological effect of potent toxicant on living organism has increased (Forni, 2014). Hence laboratory dose is the point-of-departure for assessment of toxic chemicals. The increased attrition rate in development of a new anticancer is exasperating. Induction of thyroid disruption by fipronil was via increased elimination of thyroxine (T4) mediated by activation of hepatic enzyme. Fipronil sulfone, the metabolite of fipronil might have contributed to the disruption (Julien et al., 2009). High fat diet caused hypothyroidism mitigated by metformin(El-Sayed and Ibrahim, 2020). More so glucagon-like peptide may increase the risk of thyroid cancer. Perchloric acid could cause benign and malignant thyroid turmors and immunotoxicity. The oral median lethal dose (LD50) of perchloric acid was 1100 and 250 mg/kg in rats and dogs respectively. The subcutaneous LD50 in mice was 250 mg/kg. Chronic injection of perchloric acid of 600–1200 mg/kg was used to treat grave’s disease, in which immunoglobulin binds to thyroid stimulating hormones receptor, resulting in hypothyroidism (Baran and Gad, 2014). Hence the present study aims to assess mitigating effects of piroxicam on perchloric acid-induced thyroid gland hormones disruption in male rats.
2 Materials and methods
2.1 Animals
A total of 2 female and 17male rats weighed 145.0 ± 20.5 g were used for the study. The rats were obtained from a breeder in Makurdi Metropolis, Nigeria and housed in the Department of Veterinary Physiology and Biochemistry laboratory, Federal University of Agriculture, Makurdi, Benue State, Nigeria. Feed Growers Marsh®and water were provided ad libitum. The animals were handled according to the international guiding principle on the use of animals (NIH, 1985), and the recommendation of the Department of Veterinary Pharmacology and Toxicology, Federal University of Agriculture Makurdi Ethical Committee on the use of animals given the permit number (2021003).
2.2 Drugs
Perchloric acid manufactured in Gujarat India, with batch number (090920) and piroxicam capsule were used for the study.
2.3 Acute toxicity study
Randomized control trial was adopted for the study. Acute toxicity study of perchloric acid was carried out on rats, divided into two groups of two males and females. The first male and female each, was administered oral 5000 mg/kg body weight of perchloric acid and observed for 48 h. After death of the two rats, the 2nd male and female rats were administered 3415 mg/kg body weight of perchloric acid, and observed for 48 h and thereafter for 12 days for observation of toxicity signs (Saganuwan and Orinya, 2016).
2.4 Induction of thyroid gland-hormones disruption
Fifteen male rats were divided into three groups of five each. All the groups were treated orally, with 100 mg/kg of perchloric acid for a period of 14 days, for induction of thyroid gland hormones disruption. Pretreatment blood samples were collected before the induction.
2.5 Mitigation of thyroid gland hormones disruption
After the collection of blood samples on day 14, piroxicam was administered at dose rate of 2.5 and 5 mg/kg body weight to group 11 and 111 rats respectively, for a period of 14 days. The experiment was terminated on day 29 of the experimentation.
2.6 Collection of blood samples
One milliliter (1 ml) and 2 ml each of blood was collected into ethylene diamine tetracetic acid bottles and plain bottles for haematology and serum biochemistry respectively. Conversion of pmol to mg/dl was by multiplication of 0.0113, whereas that of mg/dl to pmol was by multiplication of 88.4 respectively.
2.7 Determination of haematological parameters
The red blood cells count (RBCs), packed cell volume, total leucocytes count, differential leucocytes count and platelets count were performed according to the established methods (Cheesbrough, 1998).
2.8 Determination of biochemical parameters
Serum creatinine was estimated by the Modified Jaffe’s method (Blass et al., 1974). Plasma creatinine was calculated using the formula given as follow:Scr = Pcr /1440 × 1000 ml (Saganuwan, 2018). Serum urea (Talke and Schubert, 1965), serum aspartate aminotransferase (Bergmeyer et al., 1976), and serum alanine aminotransferase (Henley, 1980) and alkaline phosphatase (Roy, 1970) were determined according to the established methods.
2.9 Determination of thyroid hormones
The quantitative determination of total thyroxine, free triiodothyronine and thyroid stimulating hormone concentration in serum was done by a Microplate Enzyme Immunoassay method (Chopra et al., 1971; Hopton and Harrap, 1986).
2.10 Histopathology
The survived animals were sacrificed using 100 mg/kg of intraperitoneal phenobarbitone. The brain, lungs, kidneys, spleen, heart, and liver were collected for allometry and thereafter fixed with 10% formalin for histopathology. The sampled organs obtained were dehydrated using 70, 95 and 100% ethanol. The embedded tissues were stained with hematoxylene and Eosin (H&E), observed under microscope (x100) and photographed using Vanox Olyonpus Microscope (Drury et al., 1976).
2.11 Statistical analyses
All data generated have been presented in mean ± standard error of mean. The data were analyzed using two-way analysis of variance and least significant difference was detected at 5% level (Daniel, 2010).
3 Results
Acute toxicity study of perchloric acid revealed median lethal dose (LD50) of 4207.5 ± 457.6 mg/kg body weight. The male and female rat administered 5000 mg/kg died within 14 days, after the administration of the chemical. Meanwhile, male and female rat administered 3415 mg/kg body weight survived the dose after 14 days. The effects of piroxicam on perchloric acid-induced thyroid hormones disruption in male rats is presented in the Table 1 . Groups treated with piroxicam showed significant decrease (p < 0.05) in thyroid stimulating hormones (TSH) and free triiodothyronine (fT3) whereas thyroxine (T4) increased significantly (p < 0.05), respectively (Table 1). Swelling of thoracic outlet and muscle of hindlimb were observed in some rats of group 2 and 3. Alanine amino transferase (AAT), aspartate amino transferase (AST) and alkaline phosphatase were significantly increased (p < 0.05) in the group administered perchloric acid only. The levels of urea, serum creatinine and plasma creatirine were significantly (p < 0.05) decreased (Table 2). Keywords: a = significantly higher along the row; b = significantly lower along the raw; c = significantly higher along the column; d = significantly lower along the column: level of significance (p < 0.05). Keywords: a = significantly higher along the row; b = significantly lower along the raw c = significantly higher along the column d = significantly lower along the column: level of significance (p < 0.05).
Dose of Piroxicam (mg/kg)
Thyroid stimulating hormones (piu/ml)
Period of treatment (days)
0
14
28
0.0
1.00 ± 0.00
1.04 ± 0.02a
0.84 ± 0.22
2.5
1.02 ± 0.01
0.51 ± 0.00bd
0.49 ± 0.01bd
5.0
1.00 ± 0.00
0.51 ± 0.01bd
0.52 ± 0.27bd
Free triiodothyronine (pg/ml)
0.0
2.67 ± 0.00
1.37 ± 0.07b
1.86 ± 0.14b
2.5
2.20 ± 0.32
1.37 ± 0.02b
2.25 ± 0.31a
5.0
2.85 ± 0.17
1.50 ± 0.19b
2.90 ± 0.17ac
Thyroxine (mg/ml)
0.0
0.78 ± 0.07
2.58 ± 0.25a
4.65 ± 0.38a
2.5
0.77 ± 0.07
2.71 ± 0.24a
4.34 ± 0.21ad
5.0
0.74 ± 0.04
3.12 ± 0.51ac
5.70 ± 0.45ac
Dose of Piroxicam (mg/kg)
Alanine aminotransterase (iu/l)
Period of treatment (days)
0
14
28
0.0
92.30 ± 7.0
131.87 ± 16.35a
122.33 ± 7.93a
2.5
70.90 ± 46.00d
47.95 ± 26.05bd
119.13 ± 11.47a
5.0
140.70 ± 27.30c
144.60 ± 35.80
124.10 ± 20.90
Asparatate aminotransferase (iu/l)
0.0
172.4 ± 62.1
283.60 ± 32.32a
215.55 ± 25.49a
2.5
132.5 ± 41.0d
216.30 ± 3.90ad
190.33 ± 37.30a
5.0
327.3 ± 94.7c
182.23 ± 60.40bd
227.20 ± 110.40b
Alkaline phosphatase
(iu/l)
0.0
8.9 ± 3.2
8.78 ± 2.38
24.54 ± 5.30a
2.5
11.7 ± 2.0
14.10 ± 3.91c
42.07 ± 7.37ac
5.0
6.8 ± 2.1
9.58 ± 4.17a
22.80 ± 0.10a
Renal parameters
Dose of Piroxicam (mg/kg)
Urea (mmol/l)
Period of treatment (days)
0
14
28
0.0
13.4 ± 0.7
6.20 ± 0.19b
10.66 ± 1.74b
2.5
13.6 ± 1.2
6.44 ± 0.21b
7.25 ± 0.95bb
5.0
14.9 ± 2.0
6.23 ± 0.16b
4.34 ± 1.33bb
Serum creatinine (umol/L)
0.0
100.0 ± 13.4
95.08 ± 4.57
71.30 ± 10.34b
2.5
105.5 ± 10.1
114.87 ± 7.65
57.67 ± 7.57b
5.0
92.8 ± 5.7
98.40 ± 10.78
69.00 ± 5.25b
Plasma
creatinine (umol/L)
0.0
144.0 ± 19.3
136.92 ± 6.58
102.67 ± 14.89b
2.5
157.9 ± 14.5
165.41 ± 11.02
83.04 ± 10.90bd
5.0
133.6 ± 8.2
141.70 ± 15.52
99.36 ± 7.56b
Haematocrit, erythrocytes and leucocytes counts were significantly (p < 0.05) decreased in perchloric acid and piroxicam treated groups, whereas monocytes counts were significantly increased (p < 0.05) in the groups treated with piroxicam respectively. Lymophocytes and neutrophils were significantly (p < 0.05) increased and decreased in the perchloric acid treated group respectively. Platelets and eosinophils were significantly decreased in all the groups (p < 0.05) except basophils, which decreased in 2.5 mg/kg piroxicam treated group (Table 3). The weight, width and length of heart were significantly (p < 0.05) increased. The significant increase in the weight and decrease (p < 0.05) in the length of right and left lobes of the liver were observed in the group administered piroxicam. However, the width of the right and left lobes of the liver was significantly (p < 0.05) decreased in the group administered piroxicam. Nevertheless the length and width of right and left kidneys, weight, length and width of spleen as well as length and width of right lung were significantly increased (p < 0.05) in the group administered piroxicam, whereas length of the lung was significantly decreased (p < 0.05). Meanwhile allometric ratio of the brain was significantly increased (p < 0.05) in the group administered piroxicam. Allometric ratios of the liver, heart, kidney, lung and spleen were increased significantly p < 0.05) in the group administered 5 mg/kg of piroxicam (Table 4). Keywords: a = significantly higher along the row; b = significantly lower along the raw c = significantly higher along the column d = significantly lower along the column: level of significance (p < 0.05). Keywords: a = significantly higher along the row; b = significantly lower along the raw; c = significantly higher along the column; d = significantly lower along the column; level of significance (p < 0.05).
Dose of Piroxicam (mg/kg)
Hematocrit (%)
Hematocrit (%)
Period of treatment (days)
0
14
28
0.0
44.20 ± 3.37
38.80 ± 0.73b
40.75 ± 0.85bd
2.5
46.80 ± 1.53
42.25 ± 1.03bc
36.00 ± 1.00bd
5.0
41.80 ± 4.09
38.25 ± 0.75b
43.50 ± 0.41bc
Emthrocytes counts (x1012/L)
0.0
4.91 ± 0.37
4.31 ± 0.08
4.52 ± 0.09
2.5
5.20 ± 0.17
4.69 ± 0.12b
4.00 ± 0.11b
5.0
5.00 ± 0.45
4.25 ± 0.08b
4.83 ± 0.04b
Leucocytes counts (x109/L)
0.0
8.34 ± 1.18
7.96 ± 1.00
4.30 ± 1.16b
2.5
5.02 ± 0.72
4.37 ± 1.30
3.57 ± 0.55b
5.0
4.64 ± 1.29
5.88 ± 1.64
7.55 ± 2.05
Monocytes (%)
0.0
7.60 ± 0.51
7.60 ± 1.21
7.50 ± 0.96
2.5
6.60 ± 0.68
10.25 ± 0.25ac
9.33 ± 1.33a
5.0
5.00 ± 1.67
7.25 ± 0.76
9.50 ± 0.50a
Lymphocytes (%)
0.0
47.20 ± 4.62
57.20 ± 5.75
70.25 ± 2.06a
2.5
55.20 ± 2.96
57.00 ± 3.49
48.00 ± 13.08bd
5.0
56.60 ± 5.49
48.75 ± 3.59b
61.00 ± 2.00d
Neutrophils (%)
0.0
44.40 ± 35.24
40.60 ± 5.35b
22.25 ± 1.93b
2.5
36.60 ± 2.46
32.75 ± 3.52a
42.67 ± 12.03c
5.0
36.40 ± 3.84
75.00 ± 3.28ac
29.00 ± 2.00bc
Platelets (x1012/L)
0.0
398.00 ± 35.24
159.00 ± 16.31b
337.25 ± 82.12
2.5
605.80 ± 103.67
177.50 ± 16.14b
341.67 ± 27.29b
5.0
432.20 ± 54.74
135.00 ± 9.79b
350.00 ± 195.00b
Eosinophils (%)
0.0
1.00 ± 0.55
0.60 ± 0.24b
0.00 ± 0.00b
2.5
1.20 ± 0.20
0.00 ± 0.00bd
0.00 ± 0.00b
5.0
2.00 ± 0.71
0.25 ± 0.25bd
0.50 ± 0.00b
Basophils (%)
0.0
0.00 ± 0.00
0.00 ± 0.00
0.00 ± 0.00
2.5
0.40 ± 0.24
0.00 ± 0.00
0.00 ± 0.00c
5.0
0.00 ± 0.00
0.00 ± 0.00
0.00 ± 0.00
Parameters
Treatment
Groups (mg/kg)
0
2.5
5.0
Body weight (8)
150.5 ± 30.9
112.0 ± 5.06
143.5 ± 11.5
Brain (g)
1.6 ± 0.0
1.6 ± 0.1
1.4 ± 0.05
Brain length (cm)
1.9 ± 0.0
2.2 ± 0.1a
1.7 ± 0.1b
Brain width (cm)
1.5 ± 0.1
1.3 ± 0.1b
1.3 ± 0.1b
Heart (g)
0.41 ± 0.07
0.42 ± 0.06
0.63 ± 0.02a
Length (cm)
1.28 ± 0.14
1.30 ± 0.00
1.60 ± 0.20a
Width (cm)
0.65 ± 0.06
0.70 ± 0.10
1.00 ± 0.00a
Liver right lobe (g)
2.48 ± 0.38
2.68 ± 1.0 0a
2.47 ± 0.47
Left lobe (g)
1.63 ± 0.52
1.76 ± 0.60a
2.50 ± 0.08
Left lobe length (cm)
2.87 ± 0.03
2.65 ± 0.45b
3.25 ± 0.35
Right lobe length (cm)
3.07 ± 0.23
2.70 ± 0.00b
3.00 ± 0.00a
Width of right lobe (cm)
1.70 ± 0.15
1.85 ± 0.45a
1.40 ± 0.10b
Width of Left lobe (cm)
1.50 ± 0.06
1.55 ± 0.60
1.85 ± 0.15b
Left Kidney (g)
0.49 ± 0.04
0.45 ± 0.05
0.52 ± 0.02
Right kidney (g)
0.46 ± 0.06
0.48 ± 0.06
0.53 ± 0.02
Length of right kidney(cm)
1.60 ± 0.06
1.40 ± 0.15
1.75 ± 0.05 a
Length of left kidney (cm)
1.57 ± 0.07
1.47 ± 0.24
1.75 ± 0.25 a
Width of right kidney (cm)
0.60 ± 0.06
0.77 ± 0.22
0.65 ± 0.05
Width of left kidney (cm)
0.50 ± 0.06
0.70 ± 0.10a
0.65 ± 0.15 a
Spleen (g)
0.40 ± 0.06
0.39 ± 0.10
0.73 ± 0.08 a
Length of spleen (cm)
3.15 ± 023
2.63 ± 0.09b
3.55 ± 0.35a
Width of spleen (cm)
0.40 ± 0.04
0.90 ± 0.35
0.65 ± 0.25a
Right lung (g)
0.55 ± 0.09
0.67 ± 0.18
0.68 ± 0.03
Allometric ratio
0.004 ± 0.003
0.006 ± 0.036a
0.005 ± 0.003a
Left lung (g)
0.53 ± 0.05
0.51 ± 0.12
0.51 ± 0.01
Allometric ratio
0.004 ± 0.002
0.005 ± 0.024a
0.004 ± 0.001
Length of right lung (cm)
2.22 ± 0.36
2.07 ± 0.23
2.45 ± 0.05a
Width of right lung (cm)
1.03 ± 0.08
1.00 ± 021d
1.05 ± 0.05
Length of left lung (cm)
2.18 ± 0.27
1.83 ± 0.23
2.05 ± 0.05b
Width of left lung (cm)
1.00 ± 0.10
1.10 ± 0.12d
1.35 ± 0.05ad
3.1 Histopathology
Fig. 1 shows perivascular cuffs and normal neurological cells of the male rats administered perchloric acid and perchloric acid/piroxicam respectively. Fig. 2 shows zenker’s necrosis of myocardial cells of the rats administered, perchloric acid only.Congestion of the central vein and mononuclear cells infiltrations were observed in the group administered, perchloric acid and piroxicam (Fig. 3). Normal medullary rays, normal glomeruli surrounded by Bowman’s capsule and distal convoluted tubules with mononuclear cells infiltration were observed in the group administered perchloric acid and piroxicam, respectively (Fig. 4). White pulp with aggregation of lymphoid tissue and red pulp with aggregation of both white and red pulps, were observed in all the three groups (Fig. 5). Wide alveolar space was observed in the group administered piroxicam. Congestion and thickness of alveolar sac were observed in the group administered perchloric acid, whereas thick narrow air sacs with congested artery and large alveolar sac with thick wall comprising pneumocytes were observed in the group administered piroxicam, respectively (Fig. 6).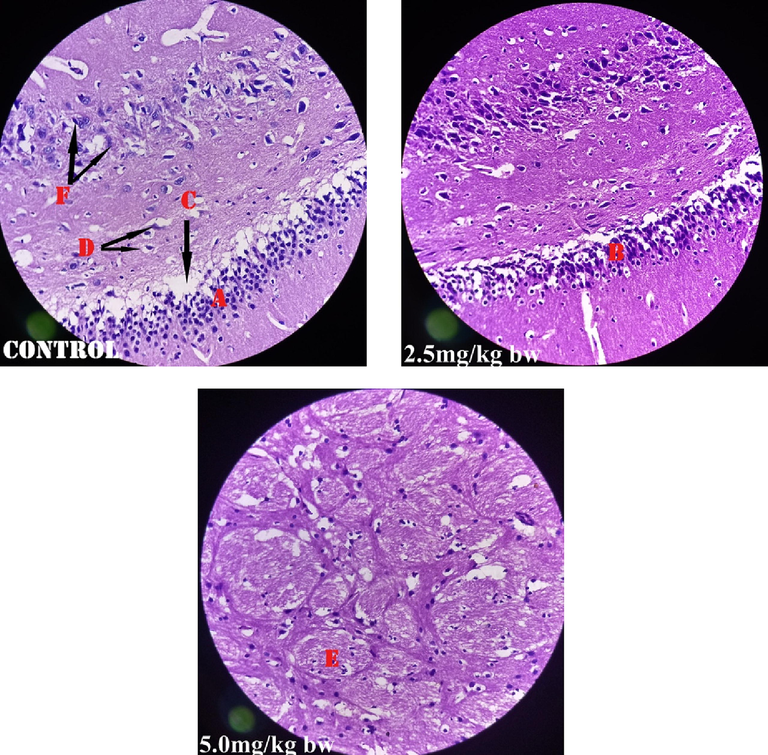
Shows perivascular cuffs (A&B), under perivascular space (C), glial cells (D), peripheral nerve (E) and mononuclear cells (F).
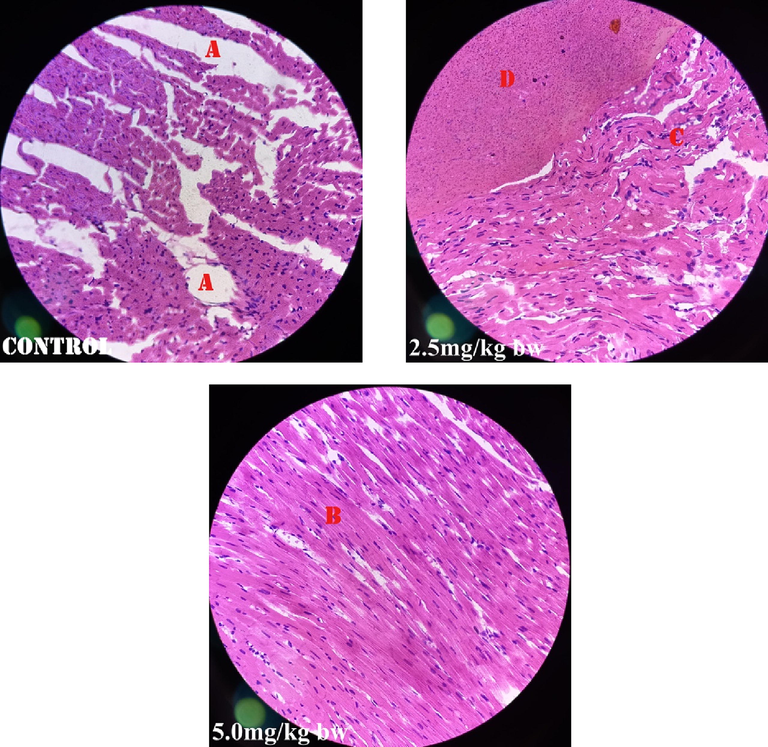
Shows Zenker’s necrosis (A), normal myocardium (B&D), and Zenker’s necrosis with mild mononuclear cellular infiltration (C).
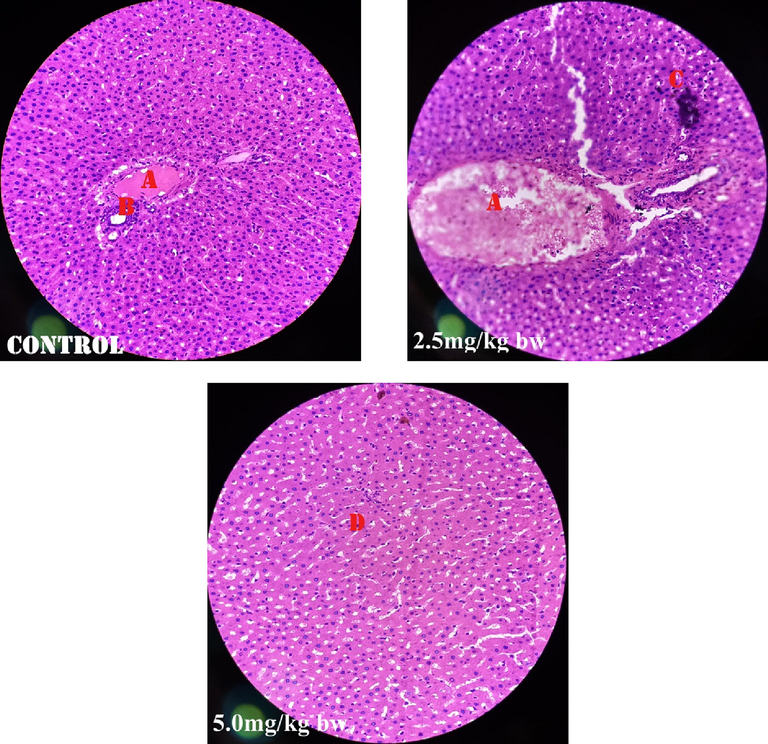
Shows congestion of central vein (A), normal portal tract (B), mononuclear cell infiltration (C), whereas (D) is normal hepatocytes.
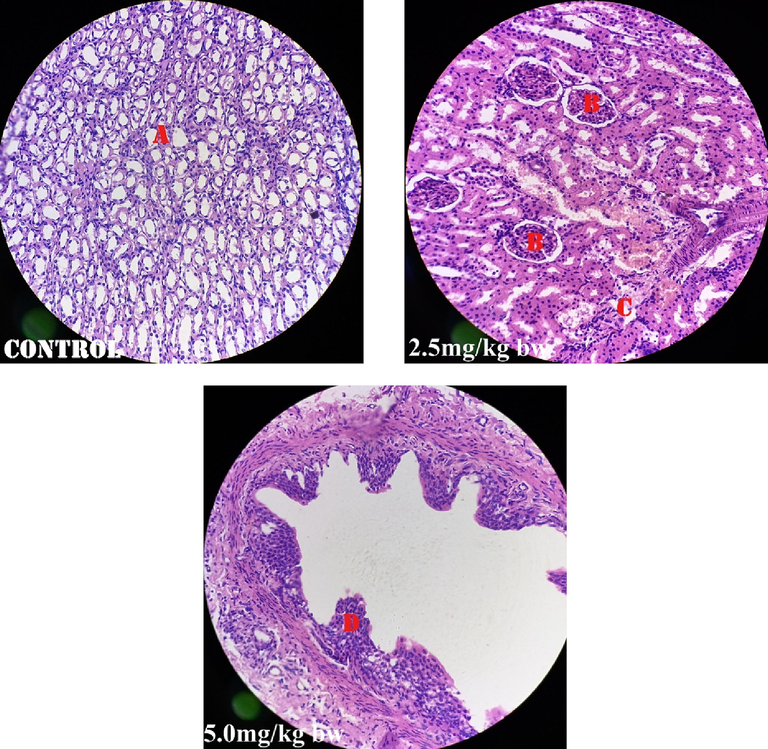
Shows normal medullary rays (A), glomeruli (B), parenchymal tubule (C), and distal convulated tubule with mononuclear cells infiltration (D).
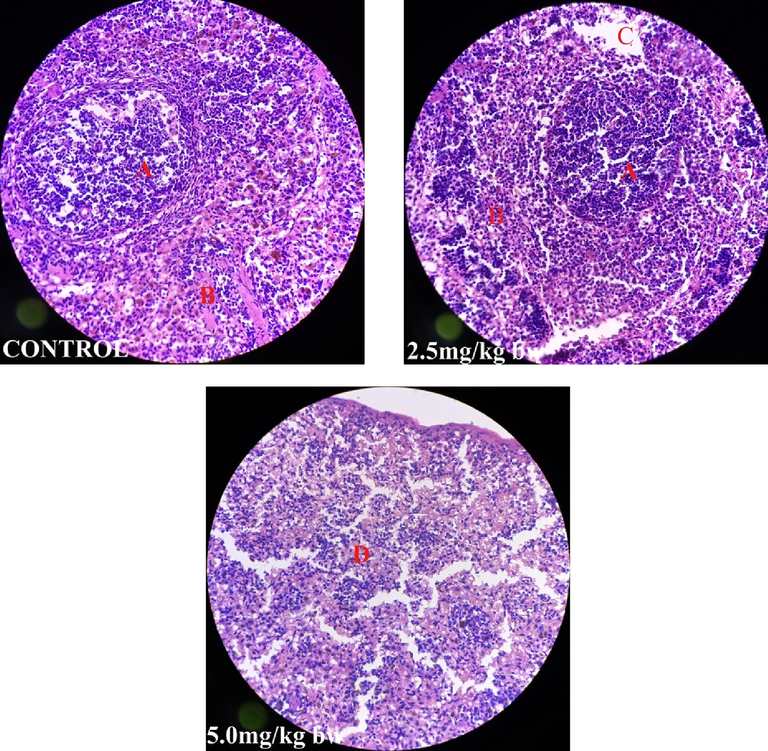
Shows white pulp with aggregation of lymphoid tissue (A), red pulp with red blood cells and white blood cells (B), degeneration of red pulp (C) and wide spread combined degeneration of white pulp and red pulp (D).
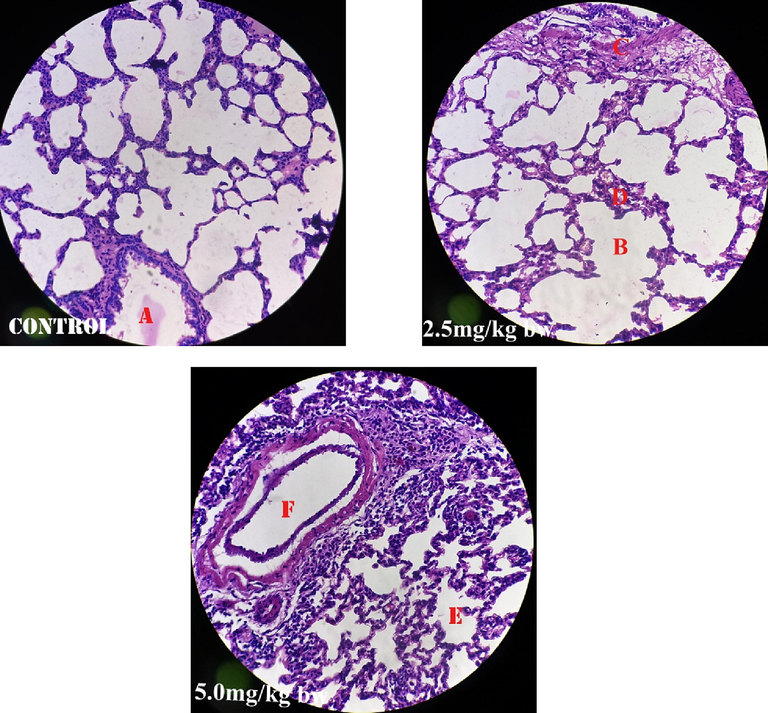
Shows congested alveolar sac (A), wide alveolar space (B), congested smooth muscle (C), thick pneumocytes (D), thick air sac (E) and congested artery (F).
Haemorrhages in the lung, spleen, kidney and heart, paleness of small and large intestine, shrinking of the stomach and diarrhoea where observed in the group administered 5 mg/kg body weight of piroxicam plus perchloric acid 100 mg/kg. Haemorhages were observed in the brain of the group administered 2.5 mg/kg of piroxicam plus perchloric acid. The animals were emaciated and the average weight reduced from 163.3 ± 10.5 g to 135.5 ± 11.8 g translating to 17 % of total body weight loss.
4 Discussion
The calculated LD50 of 4207.5 ± 457.6 mg/kg body weight of perchloric acid shows that the chemical is not safe. The finding agrees with the report of Saganuwan and Orinya (2016) indicating that toxic agent with LD50<5000 mg/kg is not safe. Hypnosis, calmness and dullness observed in the present study show that perchloric acid may depress central nervous system.Meanwhile, the increased T4 observed in all the groups on day 14 and 28 showed that both perchloric acid and piroxicam could cause increased thyroxine secrection. Perchlorate blocks the transport of iodide from entry into thyroid gland leading to iodine deficiency, which stimulates production of thyroid stimulating hormones (TSH) by the pituitary gland, invariably causing goitre. Perchlorate (HCLO4) absorbed through gastrointestinal tract was excreted in the urine within 48 h, with elimination half-life of 6.99 h. Goitrogens cause physiologic perturbations of TSH, which can lead to tumor associated with hormonal imbalance (Capen, 1997).
Decrease in triiodothyronine (T3) and thyroxine (T4) associated with increased TSH are the biomarkers of exposure to perchlorate. Perchlorate is highly persisted in surface water and ground water. Perchloric acid can react with aldehydes, alcohols and ketones, suggesting possible reaction with piroxicam (Baran and Gad, 2005). Iodine deficiency is a risk factor for thyroid cancer and anaplastic thyroid cancer characterized by chronic TSH stimulation and microcarcinoma with lower iodine intake (Zimmermann and Galetti,2015).
History of thyroid cancer in a family, diet and chemotherapy are predictors of thyroid cancer. Hence theoretic model of antineoplastic could be challenging in cancer chemotherapy (Saganuwan, 2019). Chemically induced cancer in lab animals portends potential hazards in human. TSH-producing pituitary cells undergo hyperplasia and neoplasia (Hill et al., 1998). Iodine 131I administered to patients suffering from thyroid toxicosis may cause failure of thyroid gland. Hepatocellular enzymes induction could cause hypertrophy of thyrotrophs in rats. Polychromated biphenyl (PCB) and prenenoline-160α carbonitrile (PCN) reduce thyroid hormone levels in rats. The mechanism of action of PCB is via induction of microsomal enzymes. Hyperplasia of parathyroid gland could be caused by X-irradiation on rats (Lindsay et al., 1961). However131I caused transitional changes to thyroid gland with associated iron T4 (Rudquist et al., 2017).Thyrotoxicosis is associated with follicular cell hypertrophy, follicular cell hyperplasia, follicular cell adenoma, follicular cell carcinoma, C-cell hyperplasia, C-cell adenoma, C-cell carcinoma, hyperplasia, adenoma and carcinoma (Botts et al., 1991). Thyroid carcinogenesis is via inhibition of thyroid peroxide and 5-minodeodinate (Hurley et al., 1998). Pyrethrins and sodium phentobarbital caused thyroid hypofunction via induction of cytochrome ,CYP2B enzyme in rat liver (Frinch et al., 2006).Perfluorohexane sulfurates decreased thyroid hormones level and brain development is sensitive to T4 (Ramhoj et al., 2020). Progression of thyroid tumor to anaplasic thyroid carcinoma is via p53 in the mouse model of papillary thyroid cancer (Mc Fadden et al., 2014). Thyroid hormones are essential for growth, development of muscular, skeletal and neurological systems, erythropeisis and thermogenesis. About 99% of thyroid hormones are stored in protein bond-active form. Free T4 and T3 are basically active and stimulate feedback mechanism on the hypothalamus and pituitary gland (Thorson, 2014). Disruption of circadian rhythms leads to obesity, diabetes and cardiovascular disease (Bae and Androulakis, 2018).The decrease in renal parameters observed in the present study agrees with the report of Saganuwan (2018), indicating that creatinine could be used for identification of renotoxic agent.
The higher therapeutic effects produced by 2.5 mg/kg of piroxicam as compared to 5 mg/kg shows that piroxicam has hormetic dose response. Saganuwan and Orinya (2016) had earlier reported hormetic dose–response of piroxicam, which is toxico-neurological in rats. The stimulating effect was attributed to the parent drug, piroxicam (Saganuwan, 2017a), whereas depressant effect was attributed to piroxicam metabolites (Saganuwan, 2017a; Saganuwan, 2017b). The hormetic dose response can be due to tautomeric behaviour of piroxicam, that makes the drug isomers to be in equilibrium, and changed from acidic to basic or inert form (Saganuwan, 2016). Hormesis is a reversal between high and low doses of physical stressors, phytochemicals, chemicals or biological molecules, and the overall effects could be beneficial or hazardous (Jodynis-Liebert and Kujawska, 2020). Hormetic dose–response is exhibited by endocrine disruptors. The response could be low-moderate, low–high, low or high, represented by Arndt-Schulz curve and generalized adaptive curve. Therefore, the statistical methods used for hormesis are Cedergreen-Ritz-Strenbig model, Brain-Consens model, biologistic model and An-Johnson-Lovett model (Nweke et al., 2021) . Hormesis is constrained by plasticity, though evolutionary, frequent independent, remote-conditioning, risky, synergistic, resilient and requires assessment. The 2.5 mg/kg which produced better effect against disruption of thyroid hormones by perchloric acid is dose–response-time dependent. This type of response requires dose selection, repeated dosing, different inputs, rates and route of drug administration (Gabrielsson et al., 2019). Unfortunately, 25% of medical errors are due to drugs and 14% are attributed to poor drug calculations. The increased basophills, platelets, and eosinopils showed that piroxicam could be used in the treatment of histamine, serotonin and heparin induced reaction, whereas neutropenia observed is suggestive of inflammatory response and lymphocytosis observed may be due to stimulation of thymus. Lymphocytes are produced from B-lymphocytes (Watkins and Levy, 1988). IgA in the body fluid activates the complement system, 1gE acts on mast cells and basophills which play roles in inflammation and allergy, 1gD is a membrane receptor and 1gC is found on almost every cell (Percus, 2010).
Perivascular cuffs observed in the group of rats administered 100 mg/kg of perchloric acid and 2.5 mg/kg of piroxicam, show that both agents can cause acute and chronic perivascular cuffs, ameliorated by 5.0 mg/kg of piroxicam. The acute cuffs consist of lymphoid cells, and a few macrophages usually observed in nondemyelinated white matter and in the margin of plaque. The chronic cuffs consist of macrophages, plasma cells and plasmatoid cells seen in the plaques. Macrophages phagocytize myelins and axon (Tanaka et al., 1975), suggesting that piroxicam is a strong immunocytogenic agent. The observed Zenker’s necrosis in group administered 100 mg/kg of perchloric acid suggests that the chemical could cause waxy degeneration of heart striated muscle. The degeneration was ameliorated by 2.5 and 5.0 mg/kg of piroxicam. Zenker’s necrosis is caused by infiltration of albuminous material from outside heart cells (Wells, 1908). Since lactic acid may cause Zenker’s necrosis, perchloric acid may be the incriminating agent in the present study. Congestion of central veins caused by perchloric acid (100 mg/kg), and in addition to 2.5 mg/kg of piroxicam ameliorated by 5.0 mg/kg of piroxicam, shows that both perchloric acid and piroxicam have negative and positive effects on liver. Normal kidney architecture suggests beneficial effect of the two agents on the kidney. The observed dense white pulp and red pulp in the group administered perchloric acid (100 mg/kg) and in addition to 2.5 mg/kg of piroxicam show that the two agents could stimulate cellular immune system as evidenced by perivascular cuffing of the leucocytes observed in the brain. The mild degeneration of white and red pulps by piroxicam suggests suppressive potential of lymphoid tissues and erythrocytes.
5 Conclusion
Piroxicam mitigated disruption of thyroid gland hormones at 2.5 and 5 mg/kg body weight. Thyroxine, fT3 and thyroid stimulating hormone, creatinine, urea, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, mononuclear and polymorphonuclear cells and organ allometry are reliable parameters for identification of thyroid hormones disruptors. Hence piroxicam has neuro-endocrine-immunomodulatory potential and could be used in the management of goitre and treatment of autoimmune diseases.
Acknowledgements
I sincerely thank Mrs. Miriam Johnson Oluchi and Daniel Achanya of the Department of Veterinary Pharmacology and Toxicology, Federal University of Agriculture, Makurdi for their assistance in various capacities.
Author’s contributions
SAS designed and carried out the study, did the statistical analysis, wrote and proofread the manuscript.
Funding
The author funded the research using his monthly emoluments.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mathematical analysis of circadian disruption and metabolic re-entrainment of hepatic gluconeogenesis: the intertwining entraining roles of light and feeding. Am. J. Physiol. Endocrinol. Metab.. 2018;314:531-542.
- [Google Scholar]
- Perchloric acid. In: Wexler P., ed. Encyclopedia of Toxicology. Imprint of Elsevier: Academic Press; 2014. p. :78.
- [Google Scholar]
- Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin. Chim. Acta. 1976;70(2):19-29.
- [Google Scholar]
- Proliferative lessons of the thyroid and parathyroid glands. In: Guides for Toxicology, pathology. Washington DC: STP/ARP/AFIP; 1991. p. :1-12.
- [Google Scholar]
- Mechanostic data and risk assessment of selected toxic end points of the thyroid plant. Toxicol. Pathol.. 1997;251:39-48.
- [Google Scholar]
- District Laboratory practice in tropical countries (2nd ed.). Cambridge University Press; 1998.
- Biostatistics: Basic Concepts and Methodology for the Health Sciences. Wiley and Sons Inc; 2010. p. :783.
- Carleton’s Histopathology Techniques (4th ed). London: Oxford University Press; 1976. p. :21-70.
- Effect of high fat diet-induced obesity on thyroid gland structure in female rats and the possible ameliorating effect of metformin therapy. Folia Morphol.. 2020;79(3):476-488.
- [Google Scholar]
- Dose-response-time data analysis:an underexploited trinity. Pharmacol. Rev.. 2019;71(1):89-122.
- [Google Scholar]
- Risk assessment of thyroid follicular cell tumors. Environ. Health Perspect.. 1998;106:447-457.
- [Google Scholar]
- Immunoradiometric assay of thyrotropin as a “first-line” thyroid-function test in the routine laboratory. Clin. Chem.. 1986;32(4):691-693.
- [Google Scholar]
- Mode of carcinogenic action by pesticides inducing thyroid follicular cell tumors in rodents. Envir. Healt. Perspect.. 1998;106(8):437-445.
- [Google Scholar]
- Biphasic dose-response induced by phytochemicals: experimental evidence. J. Clin. Med.. 2020;9(3):718.
- [Google Scholar]
- Fipronil-induced disruption of thyroid function is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxiocol.. 2009;255(1–2):38-44.
- [Google Scholar]
- Induction of neoplasms in the thyroid gland of the rat by X-irradiation of the gland. Cancer Res.. 1961;21:9-16.
- [Google Scholar]
- P53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. PNAS. 2014;111(16):1600-1609.
- [Google Scholar]
- NIEHS, 2000. National Toxicology Program. Notice of Peer Review Meeting on the Revised Up-and-Down Procedure (UDP) as an Alternative Test Method for Assessing Acute Oral Toxicity. Request for Comments, 65 FR 35109 June 1, 2000.
- NIH,1985. Respect for Life. National Institute of Environmental Health Science. httsp://www.nichs.nih.gov/a/factoshects/wri/studybgnhtm p85-93.
- Statistical modeling of effective doses in hormetic dose-response relationship by reparametrization of a biologistic model for inverted U-shaped curves. Afr. J. Biotechnol.. 2021;20(11):451-464.
- [Google Scholar]
- Mathematic methods in immunology. In: Currant. Vol 23. Lecture Notes, AMS; 2010. p. :111.
- [Google Scholar]
- Evaluating thyroid hormone disruption: investigations of long term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHXS). ID: Sc Rep; 2020. p. :102672.
- Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin. Chem.. 1970;16(5):431-436.
- [Google Scholar]
- Transcriptional response to 131I exposure of rat thyroids glands. Plus One. 2017;12(2):1-12.
- [Google Scholar]
- Physiochemical and structure-activity properties of piroxicam – a mini review. Comp. Clin. Pathol.. 2016;25(5):941-945.
- [Google Scholar]
- A review of mathematical concepts for calculations of cancer parameters. Cancer Ther. Oncol. Int. J.. 2019;12(5):001-008.
- [Google Scholar]
- Toxico-neurocological effects of piroxicam in monogastric animals. J. Exp. Neurosci.. 2016;10(1):121-128.
- [Google Scholar]
- Piroxicam: source for synthesis of central nervous system (cns) acting drugs. Centr. Nerv. Syst. Agent. Med. Chem.. 2017;17(2):135-140.
- [Google Scholar]
- Saganuwan ,S.A., 2017b.In vivo piroxicam metabolites: possible source for synthesis of central nervous system (cns) acting depressants. Centr. Nerv. Syst. Agent. Med. Chem. 17(3), 172-177.
- use of body surface area for determination of age, body weight, urine creatinine, plasma creatinine, serum creatirine, urine volume, and creatinine clearance: the reliable canonical method of assessing renotoxicity in animals.Comp. Clin Pathol. 2018.The;27:1531-1536.
- [Google Scholar]
- Enzymatic urea determination. in the blood and serum in the Warburg optical test. Klin Wochenschr. 1965;43:174-175.
- [Google Scholar]
- Ultrastructural studies of perivascular cuffing cells in multiple sclerosis brain. Am. J. Pathol.. 1975;81(3):467-478.
- [Google Scholar]
- Thyroid diseases in rodent species. Vet. Clin. North Am. Exot. Anim. Pract.. 2014;17(1):51-67.
- [Google Scholar]
- Guide to Immediate Anaesthetic Reactions. Publishers Essay UK: Butterworths & Co.; 1988. p. :128.
- Wells, H.G. The pathogenesis of waxy degeneration of striated muscles (Zenker’s degeneration) 1908: 1-9.
- Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res.. 2015;8(8):26146517.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102661.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







