Translate this page into:
Ameliorative effects of morin on cisplatin-induced toxicity in renal mitochondria isolated from rats
⁎Corresponding authors. Umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz), smarazi@ksu.edu.sa (Suhail Razak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cisplatin (CP) is the most effective chemotherapeutic drug used to treat various types of solid tumors. Morin is a natural flavonoid having a wide range of pharmacological properties. This investigation was aimed to explore the curative effect of morin against CP persuaded mitochondrial dysfunction in renal tissues of rats. Adult male Sprague-Dawley rats (n = 24) were randomly distributed into four groups for this experiment; control group, CP administered group, CP + morin administered group, and morin administered group. The results showed adverse effects of CP on renal biomarkers by elevating and reducing the level of urea, creatinine, and creatinine clearance sequentially. The antioxidant enzymatic and non-enzymatic activities, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione (GSH) levels were significantly decreased in renal mitochondria of CP-exposed rats. CP administration significantly elevated the ROS and TBARS levels. CP administration remarkably reduced TCA cycle enzymes activity, including succinate dehydrogenase (SDH), malate dehydrogenase (MDH), isocitrate dehydrogenase (ICDH) and alpha-ketoglutarate dehydrogenase (α-KGDH). Moreover, mitochondrial electron transport chain (ETC) enzymes, such as succinic-coenzyme Q, NADH dehydrogenase, Cytochrome c-oxidase, and coenzyme Q-cytochrome reductase activities, were also reduced after CP treatment. CP-induction significantly reduced the mitochondria membrane potential (ΔΨm). Nonetheless, morin supplementation significantly ameliorated the damaging impacts of CP in isolated mitochondria from renal tissues of rats. Thus, the present study revealed that the morin has conspicuous potential to attenuate the CP-instigated mitochondrial injuries in the renal tissues of adult male Sprague-Dawley rats.
Keywords
Cisplatin
Mitochondrial injuries
Morin
Natural flavonoid
Antioxidant
- CP
-
Cisplatin
- ROS
-
Reactive Oxygen Species
- OS
-
Oxydative stress
- TBARS
-
Thiobarbituric acid reactive substances
- NADH
-
Nicotinamide adenine dinucleotide + Hydrogen
- TCA Cycle
-
Tricarboxylic Acid Cycle
- ΔΨm
-
Mitochondrial membrane potential
- FR
-
Free radicals
Abbreviations
1 Introduction
Cisplatin (CP) is a highly effective chemo-therapeutic drug with a broad range of antineoplastic activities used to treat various cancers such as lung, testicular, bladder, ovarian, head, neck and breast (Dasari and Tchounwou, 2014). CP produces free radicals (FR), including hydroxyl radicals, hydrogen peroxide and superoxide anions in kidneys. These FR can interact with membrane lipids and cellular proteins, by injuring their structures (Ma et al., 2017). Several researchers have observed that lipid peroxidation (LPO) and FR are responsible for CP-induced nephrotoxicity. Furthermore, CP inhibits renal antioxidants activities such as CAT, GPx and SOD, by increasing the TBARS level (Yousef and Hussien, 2015).
Mitochondria are basic cellular-organelles that perform a key role in the production of energy by oxidative phosphorylation as well as it regulates various physiological process, including cell death, proliferation and calcium homeostasis (Osellame et al., 2012). Moreover, these organelles are considered one of the most dynamic ROS producing site due to the respiratory chain (Ishimoto and Inagi, 2016). It has been observed that excessive ROS production is linked to the mitochondrial dysfunction. Mitochondrial damage can induce a series of biological actions such as inflammation, cell death and oxidative stress (OS). In the past few decades, several investigators have revealed that mitochondria dysfunction due to CP is an important underlying cause of renal impairment (Yang et al., 2014).
Flavonoids are the naturally occurring polyphenolic phytochemical compounds, with their universal existence in almost all vegetables, nuts, seeds, dietary plants and fruits. Among these flavonoids, morin (2′,3,4′,5,7-pentahydroxyflavone) is a well-known flavonoid, isolated from the Maclura pomifera, Maclura tinctoria, and Psidium guajava leaves (Kuzu et al. 2018). Morin shows a broad range of curative and pharmacological properties such as anti-inflammatory, free radical scavenging, anticancer and genoprotective activity (Hussain et al., 2014). However, the potential curative effects of morin on CP induced mitochondrial toxicity has not been studied until now. Therefore, this experimental study was designed to examine the efficiency of morin against CP-induced mitochondrial toxicity in rat kidney.
2 Materials and methods
2.1 Chemicals
CP and morin were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
2.2 Animals
Sexually mature Sprague-Dawley rats (180–220 g) were kept in an animal house of the University of Agriculture, Faisalabad. Rats were provided with natural light (day cycle). Temperature and humidity levels were maintained at 22 ± 1 °C and 40–60%, respectively. Standard feed and tap water were provided. Rats were handled in obedience to International Guidelines for the use and care of experimental animals.
2.3 Experimental design
CP was dissolved in NaCl solution and administrated at a dose of 10 mg/kg (bw). Morin was suspended in physiological saline and then administrated to the rats orally for 30 days. Before starting treatment, all rats were allocated into 4 equal groups, having 6 rats/group. The following administration schedule was followed: the first group was served as control. The second group received a single injection of CP (10 mg/kg) intraperitoneally on the first day of trial (Bishr et al., 2019). The third group was administered with CP (10 mg/kg) injection on the first day of the trial and morin (50 mg/kg orally) daily until the completion of the trial. The fourth group received 50 mg/kg bw of morin prepared in distilled water (Kuzu et al. 2018). Following the completion of experimental protocol, the rats were made anesthetized by using chloroform. Retro-orbital venous plexus was used for the collection of blood samples. Blood aliquot was collected in Eppendrof tube and separated the serum by centrifuging for 5 min at 12000 rpm and stored at −20° C for further study. After one month treatment rats were humanely euthanized, and kidneys were isolated, weighed, washed using normal saline, and then homogenate (10% w/v) was produced in phosphate-buffered saline (0.05 M) at pH 7. The homogenate was centrifuge for 1 h at 12000 × g at 4 ℃. The Supernatant was extracted and kept at temperature −20 ℃ till applied in additional assays.
2.4 Isolation of renal mitochondria
Mitochondria from the renal tissues were isolated by the Mingatto et al., (1996) method. The renal tissues were removed quickly and then used a medium labeled I (1 mM EDTA, 10 mM HEPES, 50 mMTris-HCl, 250 mMmannitol, 70 mM sucrose, 120 mMKCl and also pH 7.4) to homogenized it. Centrifuge the homogenate for five minutes at 755 × g, and the resultant homogenate was again centrifuged at 13300 × g for fifteen minutes. The medium labeled II (10 mM HEPES, 50 mMTris-HCl, 70 mM sucrose, 250 mMmannitol, and pH 7.4) was used to suspend the obtained pellets and then washed twice with the same buffer, via centrifuging for fifteen minutes at 13300 × g. The obtained pellets of mitochondria were resuspended in the same media and then employed for more observations.
2.5 Assessment of kidney markers
For the assessment of urea, creatinine, and creatinine clearance, the standard Randox lab kits Crumlin, Co. Antrim, UK were employed.
2.6 Assessment of biochemical markers
CAT activity was assessed by the method of (Aebi, 1984). SOD activity was evaluated by following the technique of (Sun et al., 1988). GPx was evaluated by following the process of Paglia and Valentine, (1967). GSH was assessed by spectrophotometric assay following Ellman, (1959) method. TBARS was evaluated by following the process of Ohkawa et al., (1979). The concentration of reactive oxygen species (ROS) was measured with total ROS kits (Thermo Fisher Scientific Co. Ltd., USA) in compliance with manufacturer instructions.
2.7 Evaluation of TCA cycle enzymes
The activities of ICDH, SDH, and MDH were sequentially determined according to the following methods described by (Wieland, 1965), (Slater and Bonner, 1952), (Mehler et al., 1948). Moreover, the activity of α-KGDH was evaluated via following the process of (Reed and Mukherjee, 1969).
2.8 Analysis of the activity of respiratory chain complexes in the renal mitochondria
In order to determine the mitochondrial respiratory chain complexes activity, the mitochondrial respiratory-chain assay kits (Suzhou Comin Biotechnology LTD, China) were used.
2.9 Evaluation of mitochondrial membrane potential (ΔΨm)
The ΔΨm was evaluated initially through mitochondrial absorption of Rhodamine 123, a cationic-fluorescent dye. The test tubes were lightly agitated for ten minutes at 37 0C with Rh 123 (1.5 μM) to incubate mitochondrial suspension (0.5 mg protein ml-1). At the wavelength of 490 nm and 535 nm, the luminescence fluorescence spectrophotometer (Elmer LS-50B) was employed for the evaluation of fluorescence (Baracca et al., 2003).
2.10 Statistical analysis
The entire results are displayed as Mean ± Standard error in all figures. Statistical analysis was performed in Minitab software using one way analysis of variance which followed by Tukey’s test. The p < 0.05 was set as the significance level.
3 Results
3.1 Effects of morin on kidney markers
CP exposure resulted in a substantial (p < 0.05) rise in urea as well as level of creatinine, nevertheless, a substantial (p < 0.05) decrease in the creatinine clearance was detected in CP-treated group. However, morin cotreatment with CP substantially (p < 0.05) declined the levels of creatinine and urea, while enhanced the creatinine clearance in comparison to CP-administered group. Only morin-treated rats showed a normal level of markers which were comparable to the control group (Fig. 1).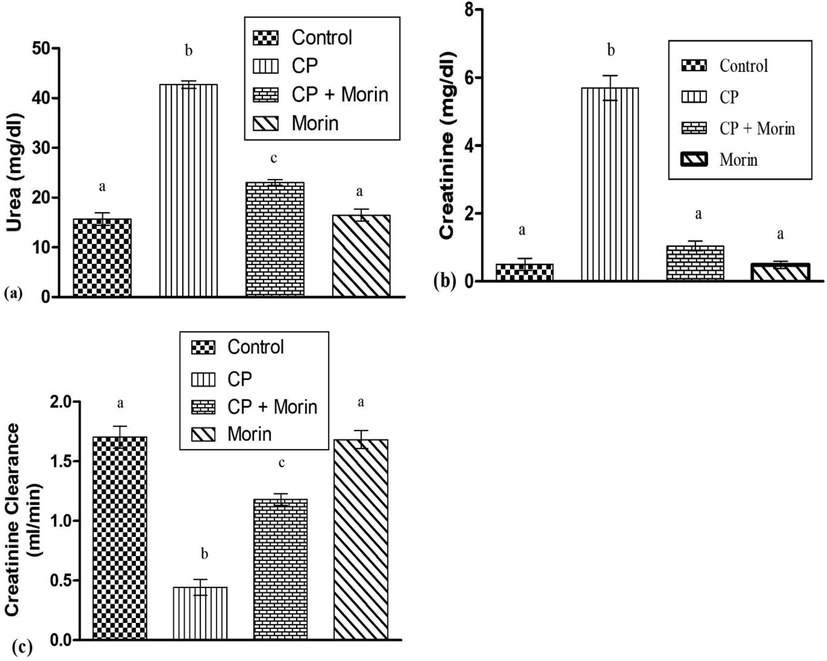
Effect of Morin on serum (a) urea, (b) creatinine and (c) creatinine clearance in the kidneys of CP-intoxicated rats. Bars sharing different superscripts are significantly (p < 0.05) different from each other.
3.2 Effects of morin on antioxidative enzymes, TBARS and ROS in CP-exposed rats
CP exposure substantially (p < 0.05) reduced the activities of antioxidant enzymes such as CAT, SOD, GSH, and GPx while considerably (p < 0.05) increased the concentration of TBARS and ROS. However, the antioxidant status was substantially (p < 0.05) increased along with substantial (p < 0.05) reduction in TBARS and ROS levels after the co-administration with morin, compared to CP administered rats. In the morin alone treated group, antioxidants, ROS, and TBARS values remained near to the control group (Fig. 2).
Effect of Morin on the mitochondrial antioxidant enzymes; (a) CAT, (b) SOD, (c) GPx, (d) GSH, (e) ROS and (f) TBARS in CP-treated rats. Bars sharing different superscripts are significantly (p < 0.05) different from each other.
3.3 Effects of morin on TCA cycle enzymes
Figure 3 shows that the CP-induction substantially (p < 0.05) decreased the activities of TCA cycle enzymes such as ICDH, MDH, SDH, α-KGDH in CP-treated rats as matched with the control rats. However, in the cotreated (CP + Morin) rat's morin substantially (p < 0.05) restored the activities of the TCA enzyme as contrasted with CP administered group. Only morin administration rats displayed non-significant changes in the activities of the TCA cycle enzyme when perform a comparison with control rats.
Effect of Morin on the TCA cycle enzymes activities; (a) ICDH, (b) α-KGDH, (c) SDH (d) MDH in the renal mitochondria of CP-administered rats. Bars sharing different superscripts are significantly (p < 0.05) different from each other.
3.4 Effect of morin on mitochondrial respiratory chain complexes (I-IV)
As stated in Fig. 4, CP-induction substantially (p < 0.05) reduced the mitochondrial complex (I-IV) activities in the CP intoxicated rats as compared with control rats. On the other hand, morin co-treatment with CP substantially (p < 0.05) elevated the activities of the kidney's mitochondrial complexes as contrasted with the CP-exposed rats. Morin only treated rats exhibited routine activities of mitochondrial complexes as compared to control rats.
Effect of Morin on mitochondrial ETC complexes activities (a) Complex-I (b) Complex-II (c) Complex-III (d) Complex-IV of CP-treated rats. Bars sharing different superscripts are significantly (p < 0.05) different from each other.
3.5 Effect of morin on mitochondrial membrane potential (ΔΨm)
As shown in Fig. 5, the rats treated with CP displayed a substantial depolarization of ΔΨm in comparison to the control group. Nevertheless, in the co-treated (CP + Morin) group, morin substantially (p < 0.05) reversed the loss in ΔΨm as compared to CP treated group. Morin alone treatment displayed normal ΔΨm as seen in control rats.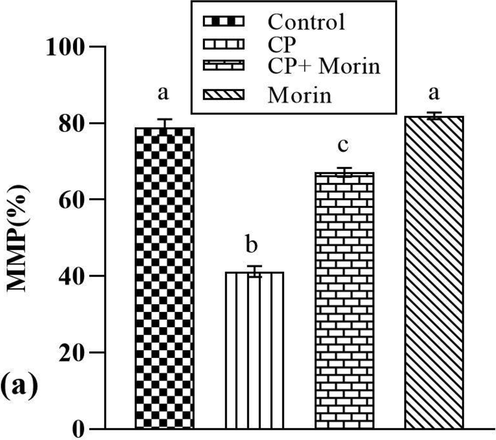
Effect of Morin on mitochondrial membrane potential (MMP) of CP-treated rats. Bars sharing different superscripts are significantly (p < 0.05) different from each other.
4 Discussion
Mitochondria are the well-known powerhouse of the eukaryotic cells. Besides ATP production, these organelles participate in different cellular functions such as ROS production, calcium homeostasis and biosynthesis of steroid hormones (Amaral et al., 2013). The mitochondrial damage directly disturbs ATP production (Zanellati et al., 2015). Excessive production of mitochondrial ROS suppresses the antioxidant enzyme activities, resulting in cell damage (Srivastava et al., 2014). The use of flavonoids is common in treating different diseases nowadays. Morin is a potent bioactive-compound present in various herbs and fruits. Previous studies have revealed that morin displays anti-carcinogenic, antioxidant, anti-diabetic, neuroprotective, and anti-inflammatory properties (Kuzu et al., 2018). Therefore, current experiment was planned to ascertain the protective effects of morin against CP-instigated mitochondrial damage in rats kidneys.
Our results revealed that serum creatinine and urea levels were increased, however, creatinine clearance was decreased after CP administration. Plasma levels of urea and creatinine reflects the glomerular filtration rate (GFR), however, creatinine clearance is a beneficial index of GFR (Higgins, 2016). CP induced nephrotoxicity showed a reduction in renal functions due to the elevation of urea and creatinine while decline in creatinine clearance (Ahmad et al., 2019: Yang et al., 2020). Increased creatinine level and reduced level of creatinine clearance are the markers for acute oxidative damage in the renal tissues (Lopez-Giacoman and Madero, 2015). However, the supplementation with morin exhibited a decline in creatinine and urea levels while augmented creatinine clearance. It may be due to the stimulating effect of morin on the GFR.
Activity of antioxidant enzymes was measured to estimate the oxidant/antioxidant stability in the kidney mitochondria. Outcomes of the study demonstrated that CP exposure decreased the activities of mitochondrial antioxidant defense such as CAT, SOD, GPx, and GSH contents; while, raised the concentration of ROS and TBARS levels. OS is an imbalance between the antioxidant defense system and ROS, which attacks protein, lipids and bio-membrane system, resulting in oxidative impairment (Deng et al., 2019). GSH is a key scavenger of ROS while SOD, GPx and CAT are the major enzymes of defense system that perform vital role in improving cellular functions (Kurutas, 2015). SOD converts O2 into hydrogen peroxide, then CAT and GPx convert hydrogen peroxide into water (Nieskens et al., 2018). It has been narrated that CP-treatment reduces the antioxidant enzymes activities which results in the elevation of LPO (Yousef et al., 2015). TBARS are the important biomarkers of LPO and previous observation reveals that CP significantly increases the level of TBARS (Yousef et al., 2009). Nonetheless, the morin treatment reduced the induction of ROS and TBARS via recovering antioxidant enzymes (CAT, SOD, GSH, and GPx). Our results in line with (Çelik et al., 2020), who proved that morin helps in reducing FR and participate in increasing the activities of antioxidant enzymes.
Our observation revealed that CP reduced TCA-cycle enzymes such as MDH, SDH, ICDH, and α-KGDH. It has been well recognized that enzymes (α-KGDH, SDH, ICDH and MDH) present in mitochondria, catalyze the oxidation of various substrates, which yields reducing equivalent. The reducing equivalents are further directed towards the respiratory chain where they yield ATP through oxidative phosphorylation; therefore, they can afford the requirement of energy for various cellular functions (Chandramohan et al., 2015). CP induced ROS might be a one of the major factors in reducing the activities of TCA cycle enzymes. Inhibition of TCA cycle enzymes may disturb the mitochondrial substrate's oxidation, which end up in reduced transferring rate of reducing equivalents to the molecular oxygen (Capetanaki, 2002). However, in the present study, when rats were co-treated with morin, the activities of enzymes were reversed. Decreased ROS production might be the reason for the restoration of these enzyme activities.
We observed that administration of CP reduced the activities of mitochondrial complex (I-IV) of ETC. Mitochondrial respiratory chain complexes are the most important enzymes during the mitochondrial oxidative phosphorylation and are also considered as the important condition to maintain mitochondrial activities (Rawat et al., 2019). The respiratory chain on the inner membrane of mitochondria is the key site for the intracellular ROS generation. Elevated oxidative stress in a cell may disrupt the mitochondrial ETC, which leads toward mitochondrial dysfunction (Wang et al., 2019). The previous investigation shows that ROS elevation can contribute to stoichiometric variations in mitochondrial ETC complexes (Mapuskar et al., 2017). The results of our research are in line with the study conducted by (Pan et al., 2015), who documented that the treatment of CP substantially decreases the activities of complex I-IV. However, co-treatment with morin substantially recovered the activities of ETC complexes to the normal, which may be attributed to ROS reducing potential of morin.
The present investigation revealed that CP induces significant depolarization of ΔΨm. ΔΨm plays an important role in maintaining mitochondrial homeostasis and provides driving energy for ATP production in mitochondria (Zorova et al., 2018). For mitochondrial movement, ΔΨm plays a pivotal role and it has been reported that the inhibition of ETC decreases proton efflux across the inner membrane of mitochondria which is generally linked with the depolarization of ΔΨm (Forkink et al., 2014). The present study revealed that CP treatment induced the loss of ΔΨm, which was effectively reversed by oral administration of morin.
5 Conclusion
Current observations emphasized that CP treatment led to mitochondrial dysfunction in renal tissues of adult male Sprague-Dawley rats. Outcomes of the present study showed that morin treatment potentially alleviated the CP-instigated adverse consequences on mitochondrial antioxidant enzymes, ETC complexes, membrane potential, urea, creatinine, and creatinine clearance. Morin regulated the renal mitochondrial functions via reducing overproduction of ROS, TBARS levels and also restored the activities of ETC complexes or TCA cycle enzymes. Conclusively morin ameliorated mitochondrial dysfunctions in renal tissues of the rats might be due to its FR scavenging property.
6 Ethical approval and consent for the participation
Animals were treated in accordance with the international guidelines for the use and care for the laboratory animals (CEE Council 86/609) approved by European Union of animal experimentation.
Funding
We are obliged to the Deanship of the Scientific-Research at King Saud University for financing this study via Research Group Project No# RGP-193.
Acknowledgement
Authors are thankful to the Deanship of Scientific-Research at King Saud University for financing this study via Research Group Project No# RGP-193.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tangeretin protects renal tubular epithelial cells against experimental cisplatin toxicity. Iran. J. Basic Med. Sci.. 2019;22:179.
- [Google Scholar]
- Baracca, A., Sgarbi, G., Solaini, G., Lenaz, G., 2003. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim. Biophys. Acta (BBA)-bioenergetics, 1606, 137-146.
- Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/ p-ERK. Can. J. Physiol. Pharmacol.. 2019;97(1):55-64.
- [Google Scholar]
- Desmin cytoskeleton: a potential regulator of muscle mitochondrial behavior and function. Trends Cardiovas. Med.. 2002;12(8):339-348.
- [Google Scholar]
- Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of oxidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology. 2020;76:126-137.
- [Google Scholar]
- Chandramohan, G., Al-Numair, K.S., Veeramani, C., Alsaif, M.A., Almajwal, A.M., 2015. Protective effect of kaempferol, a flavonoid compound, on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Prog. Nutr. 17, 238-244.
- Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol.. 2014;740:364-378.
- [Google Scholar]
- Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere. 2019;217:140-149.
- [Google Scholar]
- Forkink, M., Manjeri, G.R., Liemburg-Apers, D.C., Nibbeling, E., Blanchard, M., Wojtala, A., Smeitink, J.A., Wieckowski, M.R., Willems, P.H., Koopman, W.J., 2014. Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochim. Biophys. Acta (BBA)-bioenergetics, 1837, 1247-1256.
- Urea and creatinine concentration, the urea: creatinine ratio. Acute Care Test Hand 2016:1-8.
- [Google Scholar]
- Isolation and bioactivities of the flavonoids morin and morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—a wild Arabian medicinal plant. Molecules. 2014;19(11):17763-17772.
- [Google Scholar]
- Mitochondria: a therapeutic target in acute kidney injury. Nephrol. Dial. Transplant.. 2016;31(7):1062-1069.
- [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J.. 2015;15:1-22.
- [Google Scholar]
- Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed. Pharmacother.. 2018;106:443-453.
- [Google Scholar]
- Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol.. 2015;4:57.
- [Google Scholar]
- Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett.. 2017;17(2):928-937.
- [Google Scholar]
- Mitochondrial superoxide increases age-associated susceptibility of human dermal fibroblasts to radiation and chemotherapy. Cancer Res.. 2017;77(18):5054-5067.
- [Google Scholar]
- The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J. Biol. Chem.. 1948;174(3):961-977.
- [Google Scholar]
- In vitrointeraction of nonsteroidal anti-inflammatory drugs on oxidative phosphorylation of rat kidney mitochondria: respiration and ATP synthesis. Arch. Biochem. Biophys.. 1996;334(2):303-308.
- [Google Scholar]
- Expression of organic anion transporter 1 or 3 in human kidney proximal tubule cells reduces cisplatin sensitivity. Drug Metab. Dispos.. 2018;46(5):592-599.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab.. 2012;26(6):711-723.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70:158-169.
- [Google Scholar]
- Mitochondrial modulation by Epigallocatechin 3-Gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-kB in mice. PLoS One. 2015;10(4):e0124775.
- [CrossRef] [Google Scholar]
- Aggregation of respiratory complex subunits marks the onset of proteotoxicity in proteasome inhibited cells. J. Mol. Biol.. 2019;431(5):996-1015.
- [Google Scholar]
- [12] α-ketoglutarate dehydrogenase complex from Escherichia coli. Meth. Enzymol.. 1969;13:55-61.
- [Google Scholar]
- Slater, E.C., Bonner, W.D., 1952. The effect of fluoride on succinic oxidase system. Biochem. J., 5, 185.
- Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis.. 2014;5(8):e1394.
- [Google Scholar]
- Sun, Y.I., Oberley, L.W., Li, Y., 1988. A simple method for clinical assay of superoxide dismutase. Clin. Chem., 34, 497-500.
- Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere. 2019;218:577-588.
- [Google Scholar]
- Methods of Enzymatic Analysis. New York and London: Academic Press; 1965. p. :211-214.
- Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol.. 2014;88(6):1249-1256.
- [Google Scholar]
- Ursodeoxycholic acid protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction through acting on ALDH1L2. Free Radic. Biol. Med.. 2020;152:821-837.
- [Google Scholar]
- Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem. Toxicol.. 2015;78:17-25.
- [Google Scholar]
- Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem. Toxicol.. 2009;47(6):1176-1183.
- [Google Scholar]
- Mitochondrial dysfunction in Parkinson disease: evidence in mutant PARK2 fibroblasts. Front. Genet.. 2015;6:78.
- [Google Scholar]







