Translate this page into:
Amelioration of experimental hyperlipidemia in rats by Portulaca oleracea Linn from Kashmir Himalaya
⁎Corresponding authors at: Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia 11451(N.A. Siddiqui), Department of Pharmaceutical Sciences, School of Applied Sciences & Technology, University of Kashmir, Hazratbal Srinagar-190006, J&K, India(M.H. Masoodi). nsiddiqui@ksu.edu.sa (Nasir A. Siddiqui), mube5090@gmail.com (Mubashir Hussain Masoodi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Graphical Abstract
Abstract
Background
Traditionally, Portulaca oleracea Linn. treats abscesses and dysentery as well as liver disease. Additionally, recent studies have reported its effectiveness as a neuroprotective, analgesic, anti-inflammatory, bronchodilator, anti-cancer, antioxidant, and curative, in addition to its pharmacological effects.
Aim and Objective
To assess the phytochemical constituents quantitatively & qualitatively and lipid-lowering potential of different extracts of Portulaca oleracea L. from Kashmir Himalaya. Methods: Portulaca oleracea L. was extracted with chloroform, methanol, and aqueous solvents. Qualitative and quantitative phytochemical screening was carried and antihyperlipidemic activity was evaluated in experimental hyperlipidemic rats fed with cholesterol in coconut oil for 14 days.
Results
Chloroform, methanol, and aqueous extracts showed the presence of alkaloids, sapon ins, tannins, cardiac glycosides, terpenes, flavonoids, phenolic compounds, proteins, and carbohydrates. Quantitatively the dried plant powder contains alkaloids 0.72 g%, saponins 1.0 g%, phenolics 1.09 g%, tannins 0.91 g%, carbohydrates 0.53 g%, proteins 0.25 g% and lipids 0.87 g%. The aqueous extract was found to decrease the plasma total cholesterol, triglyceride, low-density lipoprotein-cholesterol, very lowdensity lipoprotein-cholesterol levels, LDL-C/HDL-C ratio and significantly elevated the high-density lipoprotein-cholesterol levels as compared to methanol and chloroform extracts against cholesterol-induced hyperlipidemic rats.
Conclusions
The results reveal that the Portulaca oleracea L. from the Kashmir region possesses alkaloids, saponins, phenolics, tannins, carbohydrates, proteins and lipids and aqueous extract of Portulaca oleracea L. at a dose of 200 mg/kg body weight possess highly significant antihyperlipidemic action than methanolic and chloroform extracts at similar doses.
Keywords
Portulaca oleracea L.
Phytochemical screening
Antihyperlipidemic
Cholesterol in oil
Fenofibrate
1 Introduction
Concentrations of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) increase, in addition to a decrease in high-density lipoprotein cholesterol (HDL-C guides to “hyperlipidemia”, which is one of the leading signs of “coronary artery disease (CAD)”. It plays a key role in the initiation and development of atherosclerotic obstruction (Goldstein et al., 1973, Harrison et al., 2003). For that reason, the most important factor in the treatment therapy for hyperlipidemia and arteriosclerosis is to attenuate the elevated blood serum/plasma levels of lipids and raise the HDL-C levels. The development of CAD and the progression of atherosclerosis are accelerated by hypercholesterolemia and hypertriglyceridemia (Lusis, A.J., 2000). High levels of LDL-C that accumulate in the subendothelial (extracellular) compartment of the arteries is another important risk factor that can lead to various unpleasant conditions such as atherosclerosis, hypertension, obesity, diabetes, functional abnormalities in certain organs, etc. (Bierman et al., 1966; Catapano et al., 2000; Jain et al., 2010).
Portulaca oleracea L. is a herbaceous weed, commonly known as Purslane. It is locally known as “Nuner” in Kashmir. P. oleracea L. is distributed all over the world, especially in temperate countries of Europe, America, Canada, New Zealand, and Australia, and abundant in India (Anonymous, 2003). It is found throughout India, at altitudes up to 170 m in the Himalayas, and is also common in countries with warm climates. The plant is an annual herbaceous succulent; The body is 15. 30 cm long, red, swollen at the joints, quite smooth. The leaves are fleshy, close to the stem, 6.25 mm long, alternate or nearly opposite. Flowers are small in number, gathered into sessile flower heads (Kirtikar and Basu, 2000). P. oleracea L. was used by different cultures for different purposes. The anti-magical uses of this plant were reported in ancient times when spread around the bed and were believed to protect against evil spirits and nightmares. (Grieve M., 1992). It is said to have been used as food (salad) for hundreds of years. (Masoodi et al., 2011). Stem and leaf juice of this herb was used to heal scorpion sting. Due to its cooling and moistening effects, this herb was used for fever patients in Jamaica. The North Americans considered this herb as a cooling diuretic and seeds of this herb as anti-helminthic. The people of Indo-China use the fresh juice of this herb for treating abscesses and uses this juice also for liver diseases and dysentery (Nadkarni K., 1976). In the Dominican Republic, P. oleracea L. is used in the treatment of internal parasites. The leaf tops of this herb are used as a main constituent in anti-haemorrhagic poultices. The herb is also listed for treating parasites, as a blood-cleanser, and for refreshing the digestive system. For burns and scalds, the seeds of this are also used. In general, the traditional medicinal uses are similar to those that are experimentally evaluated for the herb (Maheshwari and Singh, 1984). Therefore, an attempt was made to qualitatively and quantitatively study the plant constituents and anti-hyperlipidemic effects of different extracts of P. oleracea L. in hyperlipidemic rats.
2 Materials and methods
2.1 Plant material
The whole plant of P. oleracea L. was gathered in July-August 2009 from Narbal Budgam, Jammu & Kashmir, and validated by a taxonomist for future reference, a specimen of the plant was preserved in the herbarium of the Department of Botany, University of Kashmir, Hazratbal, Srinagar, under voucher no. KASH 279/10.
2.2 Chemicals
All chemicals used were of analytical reagents (AR) grade and were procured from CDH Chemicals Ltd. Mumbai and enzymatic kits used for the determination of Total cholesterol and HDL-C were obtained from Crest Biosystems, Goa, India, and for Triglycerides were obtained from Human Gesellschaft Fur Biochemica, Wiesbaden, Germany.
2.3 Extract preparation
The plant material (2.7 kg) was dried up under shade. This shade-dried (1.62 kg) herbal material was milled to a crude coarse powder. The powdered plant material (1 kg) was extracted sequentially by using organic solvents viz. chloroform and methanol in a Soxhlet apparatus till entirely exhausted. The chloroform and methanol portions were dried up under reduced pressure at 40 °C in a vacuum evaporator (IKA RV 10) to obtain the crude dried extracts of chloroform (CPo) and methanol (MPo). Another (0.5 kg) of crude coarse powder was put in a glass percolator of 10L capacity for aqueous extraction. After 24 h, infusions were passed through Whatman No.1 filter paper and the filtrate were re-extracted with equal volumes of water. After 48 hr and 72 hr, the same procedure was carried out again. The mixed supernatants were evaporated to dryness under vacuum at 50 °C using IKA RV 10 (rotary evaporator). The dried extract (APo) was weighed and poured into a labelled vial and was kept in a desiccator for future use.
2.4 Phytochemical evaluation
Qualitative phytochemical analysis was carried out on chloroform (CPo), methanol (MPo), and aqueous (APo) extracts of P. oleracea L. applying standard methods to detect the presence of phytoconstituents and quantitative phytochemical analysis was carried on whole plant powdered material as described below:
2.4.1 Qualitative screening
Qualitative phytochemical analysis was carried out on chloroform (CPo), methanol (MPo), and aqueous (APo) extracts of P. oleracea L. applying standard methods to detect the presence of phytoconstituents and quantitative phytochemical analysis was carried on whole plant powdered material as described below:
2.4.2 Quantitative screening
For quantitative estimation of various phytoconstituents in powdered plant material of P. oleracea L., the following procedures were followed.
2.4.2.1 Alkaloid content
By using a 100 mL mixture of solvent (20 % acetic acid in ethanol), 2.5 g of powdered plant material was extracted. For about 4 h the solution was covered. Filtrate was reduced to 25 mL under vacuum. Concentrated NH4OH solution was then added gradually to reach precipitation. The obtained precipitate after some time was then washed by dilute ammonium hydroxide and filtered. After filtration, the final filtrate was discarded and the remaining pellet finally remained was dried and finally weighed (Obadoni and Ochuko, 2002).
2.4.2.2 Saponin content
A 100 mL mixture of 10 g of plant powder and 20 % aqueous in ethanol was kept in a water bath shaker at a temperature of 55 °C for a period of 4 h. The same procedure was repeated and filtrate was combined and heated at 90 °C concentrated to 40 mL. The concentrate obtained was rinsed with 10 mL of diethyl ether in a separating funnel and shaken vigorously. The ether layer then obtained was rejected. The same procedure was replicated. Then n-butanol was further added to the aqueous layer. Washing of the whole mixture was carried out by using 10 mL aqueous NaCl (5 % in water). The upper layer was separated and evaporation was carried out on a heated water-bath till a semi-solid residue was formed. The obtained residue was dried to a constant weight in an oven (Obadoni and Ochuko, 2002).
2.4.2.3 Tannin content
Extraction of 2 g of powdered plant material was carried out three times by using 70 % acetone. After centrifugation, the separated supernatant was collected. The residue was dissolved aliquots were taken and the volume in each test tube was made up to 3 mL of water. After vortexing the contents, 0.016 M K3Fe (CN)₆ 1 mL was added followed by 0.02 M FeCl3 1 mL in hydrochloric acid (0.1 M). After repeating the vortexing, the test tubes were kept aside undisturbed for 15 min. Then 5 mL of stabilizer (water, H3PO₄, and 1 % gum Arabic in the ratio of 3:1:1) was added and again followed by vortexing the contents. At a wavelength of 700 nm, absorbance was recorded against blank. 0.001 M Gallic acid at different concentrations was used for plotting a standard curve (Graham H., 1992).
2.4.2.4 Carbohydrate content
Powdered plant material (0.5 g) was extracted with 80 % ethanol. The procedure for carbohydrate estimation was followed as given by Krishnaveni et al., 1984 with slight modification.
2.4.2.5 Protein content
Powdered plant material (1 g) was extracted with 10 mL of water adding a few drops of Triton X-100. The process of protein estimation given by Lowry et al., 1951 was followed in this study.
2.4.2.6 Phenolic content
A sample of plant powder (0.5–1 g) was extracted with 10 mL of 80 % ethanol. The phenolic content in this study was determined as proposed by Malick and Singh, 1980.
2.4.2.7 Lipid content
A sample of plant powder (1 g) was dissolved in ether and stirred for one hour. The rest of the technique for determining the lipid content was followed as given by Ganai et al., 2005.
2.5 Animals
Albino Wistar rats of both sexes weighed 150–200 g and were employed for assessing the antihyperlipidemic activity. Under approved project proposal number. KU/Pharm/CPSCEA/2009–02. Date: 08/10/2009, rats were obtained from the Indian Institute of Integrative Medicine (IIIM) (173/CPCSEA). Hygienic optimum conditions (40–70 % RH and 12hr light/dark cycles and temperature 25 °C to 28 °C) were provided in the animal house of the Department of Zoology, University of Kashmir for rats. Standard rat feed was purchased via NITCO Pvt. Ltd., for feeding and water ad libitium to the experimental rats. The Institutional Animals Ethics Committee (IAEC) established at the Department of Pharmaceutical Sciences, University of Kashmir as per CPCSEA guidelines officially approved this study. Before starting this experimental study, acclimatization of rats was carried out for 9 days by keeping them under standard experimental conditions.
2.6 Acute oral toxicity studies
Acute oral toxicity (AOT) testing on extracts of P. oleracea Linn was carried out in agreement with the Organization for Economic Cooperation and Development (OECD) Guidelines 423 (acute toxic classic method). Animals were monitored individually a minimum of one time within the first 30 min after oral administration of CPo, MPo, and APo (2000 mg/kg), regularly during the first 24 h, with particular concentration provided during the initial 4 h and daily afterward, for entire 14 days for toxicity determination (In, O., 2001).
2.7 Induction of hyperlipidemia
Experimental hyperlipidemia was induced in the rats by oral administration of cholesterol mixed with coconut oil (5 mg/mL) and this mixture was administered orally at a dose of 25 mg/kg b.w./day to each rat for 7 days (Dhuley, et al., 1999; Ochani and D’Mello, 2009). The elevated lipid levels in rats confirmed the induction of hyperlipidemia.
2.8 Experimental design
Albino Wistar rats were randomly grouped into six groups possessed six rats in each group (n = 6) and treated once a day for 14 days as per the following protocol:
Group I (Normal) was given only vehicle (0.5 % CMC v/v),
Group II was given cholesterol (25 mg/kg b.w./day) in oil (Dhuley et al., 1999),
Group III was given fenofibrate (65 mg/kg b.w./day) plus cholesterol (25 mg/kg b.w./day) in oil (Kumar, et al., 2008),
Group IV was given CPo (200 mg/kg b.w./day), plus cholesterol (25 mg/kg b.w./day) in oil.
Group V was given MPo (200 mg/kg b.w./day), plus cholesterol (25 mg/kg b.w./day) in oil.
Group VI was administered APo (200 mg/kg b.w./day), plus cholesterol (25 mg/kg b.w./day) in oil.
On the 15th day, the blood samples were withdrawn by cardiac puncture under light ether anesthesia. At room temperature, the blood samples were allowed to clot for 30–40 min. Different biochemical parameters were evaluated by standard methods on the serum which was separated via centrifugation (3500 rpm).
2.9 Biochemical analysis
2.9.1 Lipid profile
The main biochemical markers suggested by the National Cholesterol Education Program-2002 (NCEP) for lipid assessment include total serum cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) were determined using test kits, while low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL-C) were calculated by Friedewald’s formula (Friedewald et al., 1972).
2.9.2 Tissue markers of oxidative stress
Lipid peroxidation of plasma and liver tissues was measured by estimating TBARS (Thiobarbituric acid reactive substances) by following methods depicted by Ohkawa et al., 1979, while as Ellman method was used for estimation of glutathione (GSH) (Ellman, G.L., 1959), Marklund method for evaluation of superoxide dismutase (SOD) (Marklund and Marklund, 1974). The method depicted by Green et al., 1982 was used for the estimation of nitrate/nitrites (NO) levels.
2.9.3 Histopathological evaluation
The tissue samples of the liver were conserved in a solution of 5 % phosphate-buffered neutral formalin, dehydrated in graded (30–100 %) ethanol, and fixed in paraffin for histopathological purposes. 3-μm thickness sections were cut down and then stained with haematoxylin and eosin and then histopathological alteration in the normal architecture of the liver was observed at a magnification of 40X under a light microscope and photomicrographs of all samples were recorded.
2.10 Statistical testing
Biochemical marker data obtained in the experimental study were described as mean ± S.E, where n = 6. For determining the statistical significance one-way analysis of variance (ANOVA) and Dunnett’s test was employed. p-values of less than 0.05 were considered statistically significant (Dunnett, C.W., 1964).
3 Results
3.1 Percentage yield of extracts
From 100 g of dry plant material, the yield of extract obtained by using different solvents was calculated for each extract (Fig. 1). Methanol solvent has given the highest yield (8.2 %) of extract as compared to chloroform (2.9 %) and aqueous solvents (5.5 %).
Percentage yield of all the three extracts *Each individual value is the average of three measurements ± standard deviation.
3.2 Qualitative and quantitative phytochemical screening results
Qualitative phytochemical screening of CPo, MPo, and APo extracts revealed positive results for the presence of tannins, alkaloids, saponins, cardiac glycosides, terpenes, flavonoids, phenolics, carbohydrates, proteins, fats & oils (Table 1). Quantitative phytochemical screening of whole plant powdered material revealed that the amount of alkaloids was 0.72 g%, saponins 1.0 g%, phenolics 1.09 g%, tannins 0.91 g%, carbohydrates 0.53 g%, proteins 0.25 g% and lipids 0.87 g% (Fig. 2). (+ Present ; - Absent).
Phytoconstituents
Result
CPo
MPo
APo
Tannins
Alkaloids
Saponins
Glycosides
Terpenes
Flavonoids
Phenolics
Carbohydrates
Proteins
Fats & oils+
+
-
+
+-
-
-
+
-+
+
-
+
++
+
+
+
-+
-
+
+
+
++
+
+
-
Percentage yield of Phytoconstituents in Portulaca oleracea L.
3.3 Acute toxicity study
CPo, MPo, and APo at a dose of 2000 mg/kg in rats after oral administration did not produce any signs of toxicity and no fatality was observed up to 14 days. The result of this toxicity test revealed that up to an oral dose of 2000 mg/kg b.w., all three extracts viz. CPo, MPo, and APo were found to be nontoxic (Table 2). ‘O’ indicates no mortality.
Extract
Dose (mg/kg b.w.)
Observation
Safe dose (mg/kg b.w.)
CPo
2000
OOOOO
> 2000
MPo
2000
OOOOO
> 2000
APo
2000
OOOOO
> 2000
3.4 Effect on lipid profile
The effect of different extracts of P. oleracea L. on serum lipid profile in control and experimental hyperlipidemic rats is shown in Table 3 and in Fig. 3 (A to F). The results obtained revealed that feeding the rats with cholesterol in oil caused a substantial enhancement in TC, TG, LDL, and VLDL lipoprotein levels (p < 0.01) as assessed in comparison to rats who were on a normal diet. When cholesterol in oil was co-administered with P. oleracea L. extracts, the raised levels of TC, TG, and LDL-C showed significant (p < 0.01) decline. The APo extract (200 mg/kg b.w.) was observed to cause a significant decrease in total cholesterol levels (Fig. 3A), triglyceride levels (Fig. 3B), LDL-C levels (Fig. 3C), VLDL-C levels (Fig. 3E) of rats than chloroform and methanol (200 mg/kg b.w.) extracts against cholesterol-induced hyperlipidemia in comparison to standard fenofibrate. There was a remarkable rise in plasma HDL-C (p < 0.01) in APo extract (200 mg/kg b.w) treated rats than chloroform and methanol extracts treated groups in cholesterol in oil-fed rats (Fig. 3E), thus indicating the efficacy of APo extract (200 mg/kg b.w) in averting the rise seen in various components of lipid profile under experimentally caused hyperlipidemia than CPo and MPo extracts. The APo extract (200 mg/kg b.w) showed a more pronounced effect on the LDL-C/HDL-C ratio than CPo and MPo extracts (Fig. 3F, Table 3). A number of suggestions exist with respect to the statement that “HDL-C is inversely connected to total body cholesterol and a drop-in plasma HDL-C concentration may quicken the progress of atherosclerosis leading to ischemic heart diseases by weakening the clearing of cholesterol from the arterial wall”. CPo = Chloroform extract of P. oleracea; MPo = Methanol extract of P. oleracea; APo = Aqueous extract of P. oleracea; HDL-C, High denisty lipoprotein cholesterol; LDL-C, Low denisty lipoprotein cholesterol; VLDL, Very low denisty lipoprotein cholesterol, p.o., per oral. Also were ↑ indicates increase and ↓ indicates decrease in respective serum levels. **p < 0.01; *p < 0.05 vs cholesterol in oil; p > 0.05; ns, non-significant.
Treatment Groups (n = 6)
Dose (p.o mg/kg)
Total cholesterol (mg/dL)
Triglycerides (mg/dL)
HDL-C (mg/dL)
LDL-C (mg/dL)
VLDL-C (mg/dL)
LDL-C/HDL-C ratio
Normal
–
64.53 ± 1.11
66.65 ± 1.07
34.39 ± 1.15
16.81 ± 0.5
13.33 ± 0.35
0.49
Toxic
25
98.73 ± 1.1
135.73 ± 1.1
40.31 ± 1.12
46.27 ± 1.12
27.15 ± 0.13
1.14
Fenofibrate
65
61.31 ± 1.1*↓
64.79 ± 0.1**↓
26.53 ± 0.21**↑
21.82 ± 0.5**↓
12.96 ± 0.15**↓
0.82
CPo
200
85.61 ± 1.42*↓
99.35 ± 1.03**↓
31.23 ± 1.12**↑
34.51 ± 0.55 ns↓
19.87 ± 1**↓
1.11
MPo
200
84.25 ± 0.43**↓
96.43 ± 1.23*↓
35.24 ± 1.13 ns↑
29.72 ± 0.5**↓
19.29 ± 1.25*↓
0.84
APo
200
69.43 ± 0.53**↓
84.61 ± 1.35*↓
39.65 ± 0.5**↑
12.86 ± 1**↓
16.92 ± 0.5**↓
0.32
3.5 Effect on histopathology of liver in cholesterol fed rats
The liver of normal (control) rat after staining showed normal architecture (Fig. 4A), while the toxic (toxic control) group (Fig. 4B) fed with cholesterol in oil (25 mg/kg) showed accumulation of LD (lipid droplet), congestion in CV (central vein) and centrifugal necrosis affecting a large area, also showing fatty infiltration and granular degeneration. The liver morphology of rats treated with the standard drug Fenofibrate (Fig. 4C) exhibited negligible cytoplasmic fatty infiltration and granular degeneration. The liver treated with CPo exhibited improvement in hepatic architecture (Fig. 4D), MPo treated liver showed improvement in liver architecture by mild to moderate cytoplasmic fatty infiltration and granular degeneration (Fig. 4E). APo treated liver showed improvement in liver architecture and a significant decrease in the appearance of LD and low fatty infiltration and granular degeneration (Fig. 4F).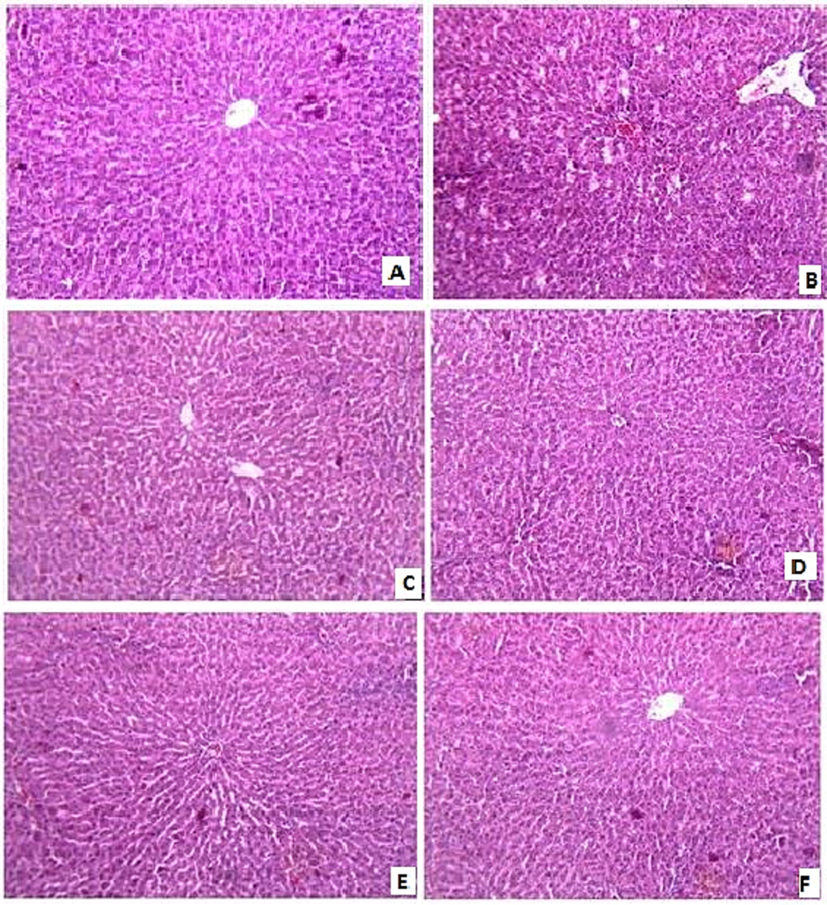
Effect of P. oleracea (Po) extracts on histopathology of liver in cholesterol fed rats (A) Normal (control) rat liver with glomerulus stained by Haematoxylin Eosin, (B) Liver of toxic (toxic control) rat group fed with cholesterol in coconut oil (25 mg/kg) showing accumulation of LD (lipid droplet), congestion in CV (central vein) and centrizonal necrosis effecting large area, (C) restoration of liver morphology of rats treated with standard drug Fenofibrate, (D) rat liver treated with CPo and MPo (E) showed improvement in liver architecture, (F) APo treated liver showed improvement in hepatic architecture and significant decrease in the appearance of LD.
3.6 Effect on oxidative stress marker levels
Based largely on different enzymatic modules, like superoxide dismutase (SOD), reduced glutathione (GSH) etc cells set-up an antioxidant protective mechanism to protect themselves from ROS-produced harm to cells (Deponte, 2013). The alteration of both enzymatic as well as non-enzymatic antioxidative system like SOD and reduced GSH gets modified in hyperlipidemic condition which results in ROS facilitated impairment (Table 4) (Devi, 2004). As revealed in Table 4, the significant (p < 0.05) increase in oxidative stress marker levels (SOD, GSH, TBARS, and NO) of serum and liver of cholesterol fed rats in comparison to that of NC group. In previous many studies it was revealed that high levels of serum cholesterol reduce the antioxidant resistance which in turn decreases the potential of SOD and GSH in rats (Fki, 2005). CPo: P. oleracea chloroform extract; MPo: P. oleracea methanol extract; APo: P. oleracea aqueous extract; NC: Normal control; Toxic: Cholesterol-fed; standard: Fenofibrate; n = 6, mean ± SEM.
Parameters
NC
Toxic
Standard
CPo (200 mg/kg)
MPo (200 mg/kg)
Apo (200 mg/kg)
Liver
SOD (%)GSH
(µg/mg)TBARS
(µM/mg)NO
(µM/mg)86.35 ± 1.2
350 ± 0.10
76.4 ± 5.3
21.47 ± 2.355.14 ± 1.1
258 ± 6.5
187.2 ± 7.1
47 ± 3.2679.26 ± 1.03
344.32 ± 4.5
147.57 ± 8.1
23.64 ± 2.4358.22 ± 3.21
282 ± 8.99
122.21 ± 5.7
21.09 ± 2.1161.87 ± 5.52
266.2 ± 2.05
112.65 ± 5.07
21 ± 1.5462.63 ± 1.87
273.76 ± 3.81
101 ± 2.19
18.3 ± 3.5
Serum
SOD (%)GSH
(µg/mg)TBARS
(µM/mg)NO
(µM/mg)32.1 ± 3.7
132 ± 5.3
29.2 ± 1.4
26.63 ± 1.519.49 ± 2
201 ± 7.3
46.92 ± 3.6
56.56 ± 1.222.43 ± 1.7
104 ± 4.2
37.37 ± 4.2
23.82 ± 1.5119.36 ± 2.9
161.72 ± 4.21
33.01 ± 1.1
35.18 ± 3.5220.02 ± 1.81
142.11 ± 8.12
29.94 ± 2.87
36.09 ± 3.3121.92 ± 3.12
133.09 ± 7.22
26.32 ± 1.92
32.99 ± 3.21
In this study, the effect of P. oleracea (Po) extracts on oxidative stress markers like superoxide dismutase (SOD), reduced glutathione (GSH), thiobarbituric acid reactive substance (TBARS), and nitric oxide (NO) in the liver and serum of rats is depicted in (Table 4) Fig. 5A to 5D and 6A to 6D, respectively. The results showed the SOD, TBARS, and NO levels in the liver were improved significantly by APo than CPo and MPo (Fig. 5A, Fig. 5C, and Fig. 5D) while the CPo showed a more significant improvement in liver GSH levels (Fig. 5B). In serum the effect of P. oleracea (Po) extracts on oxidative stress markers were observed and the results revealed that the APo significantly improved the SOD, GSH, TBARS and NO levels than CPo and MPo, (Fig. 6A to Fig. 6D).
4 Discussion
In the present investigation, P. oleracea L. was studied to screen for its phytochemical character and hypolipidemic activity. Traditionally P. oleracea L. is a famous medicinal plant with diverse biological activities (Masoodi et al., 2011). Many findings show that saponins and steroids from plants have hypolipidemic activity (Sidhu and Oakenfull, 1986; Inoue et al., 1999). Flavonoids and other polyphenols are also thought to have cholesterol-lowering and lipid-lowering effects (Chan et al., 1999; Guimarães et al., 2000). Phenols are very important plant constituents. Flavonoids and polyphenols besides other bioactive phytoconstituents found in P. oleracea L. extracts could therefore be considered favourable in increasing high-density lipoprotein-cholesterol and decreasing triglycerides, low-density lipoprotein-cholesterol, and very low-density lipoprotein-cholesterol in rats. Synthetic hypolipidemic drugs have a number of adverse effects like cutaneous flushing, headaches, pruritis, dermatitis, hyperuricemia, gout, gastritis GI bleeding, etc. (Handelsman et al., 2011). Most of the South-Asian countries including India are blessed with huge medicinal flora. Replacement of synthetic drugs due to higher cost and severe side effects, with naturally derived plant based for various ailments including hyperlipidemia is the need of the hour (Eghdamian and Ghose, 1998).
The results obtained from the current study revealed that the aqueous extract of P. oleracea L. at doses (200 mg/kg b.w./day; p.o) significantly (p < 0.05) lessened both the TC and LDL-cholesterol when given initially to the hyperlipidemic rats causes a sharper and more significant reduction in the serum TC (69.43 mg/dL) and LDL cholesterol (12.86 mg/dL) and TG (84.61 mg/dL) level and increases in HDL-C (39.65 mg/dL) than 200 mg/kg b.w./day; p.o) of chloroform and methanol extracts. Fenofibrate (65 mg/kg; p.o) was also included in the study to understand the extent to which the activity of different P. oleracea L. extracts were equivalent to that of a standard drug. Several studies reveal that increased HDL-C and decreased TC, LDL-C, and TG are associated with reduced risk of ischemic heart disease (Goldstein et al., 1973; Harrison et al., 2003). Most lipid-lowering drugs significantly reduce TC and HDL cholesterol levels (Wilson, P.W., 1990). The rise in HDL level and decline in LDL level is one of the advantages in the treatment of high cholesterol, where low HDL cholesterol is a prevalent lipoprotein abnormality (Gupta et al., 1994). Also, the aqueous extract of P. oleracea L. causes sharp decreases in LDL-C/HDL-C ratio (Table 3) compared to chloroform and methanol extracts at a dose of 200 mg/kg body weight. Recently, some studies have also shown that triglyceride levels directly or indirectly lead to coronary heart disease (Bainton et al., 1992). The improvement in oxidative stress markers like SOD, GSH, TBARS, and NO in the liver and serum of rats also supports its antihyperlipidemic potency (Table 4).
The exact way in which P. oleracea L. extracts reduce serum cholesterol is not clearly understood, but it may imply that they act by influencing endogenous cholesterol biosynthesis in the liver and increasing the excretion of cholesterol products through the liver. Various plant constituents such as phenolic compounds, saponins, flavonoids, etc. are present in high concentrations in the aqueous extract compared to chloroform and methanol extracts. From the results, it is concluded that aqueous extract possesses active phytoconstituents in high quantity and hence possesses potent antihyperlipidemic than other extracts and this may impact the cardioprotection by anti-atherosclerotic effect.
5 Conclusion
The data supporting the antihyperlipidemic effect of the aqueous extract of P. oleracea L. indicates the presence of some phytoconstituents that act as anti-lipid entities. Moreover, the quantitative phytochemical study revealed the presence of phenolics (1.09 %) as most leading category of active ingredients present in P. oleracea L. and the previous studies already proved the anti-lipid effect of phenolic compounds. Additional studies aimed at isolating, identifying, and characterizing the operating principle(s) are underway to support the current results.
Acknowledgement
The authors of this study are highly obliged to the “ Researchers Supporting Project” number (RSPD2023R981), King Saud University, Riyadh, Saudi Arabia, for supporting this study and funding the research work. The authors are also thankful to Dean, School of Applied Science and Technology and Head Department of Pharmaceutical Sciences, University of Kashmir for useful suggestions and support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anonymous, 2003. A dictionary of Raw materials and Industrial Products, Raw Materials. The Wealth of India. New Delhi, CSIR, PID. 8: 219-220.
- Plasma triglyceride and high density lipoprotein cholesterol as predictors of ischaemic heart disease in British men: the Caerphilly and Speedwell Collaborative Heart Disease Studies. Br. Heart J.. 1992;68(1):60-66.
- [CrossRef] [Google Scholar]
- Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr. Opin. Cardiol.. 2000;15(5):355-363.
- [Google Scholar]
- Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J. Nutr.. 1999;129(6):1094-1101.
- [Google Scholar]
- Glutathione catalysis and the reaction mechanisms of glutathione- dependent enzymes. Biochim. Biophys. Acta. 2013;1830(5):3217-3266.
- [CrossRef] [Google Scholar]
- Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol.. 2004;90(1):63-68.
- [CrossRef] [Google Scholar]
- Hypolipidaemic and antioxidant activity of diallyl disulphide in rats. Pharm. Pharmacol. Commun.. 1999;5(12):689-696.
- [Google Scholar]
- New tables for multiple comparisons with a control. Biometrics. 1964;20(3):482-491.
- [Google Scholar]
- Mode of action and adverse effects of lipid lowering drugs. Drugs Today. 1998;34(11):943-947.
- [Google Scholar]
- Hypocholesterolemic effects of phenolic-rich extracts of Chemlali olive cultivar in rats fed a cholesterol-rich diet. Bioorg. Med. Chem.. 2005;13(18):5362-5370.
- [CrossRef] [Google Scholar]
- Friedewald, W.T., L.evy, R.I., Fredrickson, D.S.,1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18: 499-502.
- Experimental Biochemistry. J&K: Valley Publications; 2005.
- Hyperlipidemia in coronary heart disease II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Invest.. 1973;52(7):1544-1568.
- [CrossRef] [Google Scholar]
- Modified Prussian Blue assay for total phenols. J. Agric. Food Chem.. 1992;40:801-805.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem.. 1982;126(1):131-138.
- [Google Scholar]
- Grieve, M., 1992. A Modern Herbal the Medicinal Culinary Cosmetic and Economic Properties Cultivation and Folk-Lore of Herbs, Grasses Fungi, Shrubs & Trees with All Their Modern Uses. Hafner Publishing.
- Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz. J. Med. Biol. Res.. 2000;33(9):1027-1036.
- [Google Scholar]
- Lipoprotein lipids and the prevalence of hyperlipidaemia in rural India. J. Cardiovasc. Risk. 1994;1(2):179-184.
- [Google Scholar]
- American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr. Pract.. 2011;17(2):287-302.
- [Google Scholar]
- Role of oxidative stress atherosclerosis. Am. J. Cardiol.. 2003;91(3A):7A-11A.
- [CrossRef] [Google Scholar]
- In, O., 2001. Acute oral toxicity-Acute oral toxic class method. Guideline 423, adopted 23/06/1996, Eleventh Addendum to the OECD guidelines for the testing of chemicals.
- Lipoprotein lipase activation by red ginseng saponins in hyperlipidemia model animals. Phytomedicine. 1999;6(4):257-265.
- [CrossRef] [Google Scholar]
- Current drug targets for antihyperlipidemic therapy. Mini Rev. Med. Chem.. 2010;10(3):232-262.
- [Google Scholar]
- Kirtikar, K. R. and Basu, B. D., 2000. Kirtikar and Basu's Illustrated Indian Medicinal Plants: Their Usage in Ayurveda and Unani Medicines, Sri Satguru Publications, a division of Indian books centre.
- Antihyperlipidemic activity of Camellia sinensis leaves in Triton WR-1339 induced albino rats. Pharmacogn. Magaz.. 2008;4(13):60.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Contribution to the ethnobotany of Bhoxa tribe of Bijnor and Pauri Garhwal districts. UP J. Econ. Taxon. Bot.. 1984;5(2):251-259.
- [Google Scholar]
- In: Plant enzymology and histo enzymology. New Delhi: Kalyani Publishers; 1980.
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- Nadkarni, K., 1976. Indian materia medica: With ayurvedic, unani-tibbi, siddha, allopathic, homeopathic, naturopathic and home remedies, appendices and indexes-Vol. 2, Ramdas Bhatkal, Popular Prakashan Private Ltd.
- Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Global J. Pure Appl. Sci.. 2002;8(2):203-208.
- [Google Scholar]
- Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa Linn. leaves and calyces extracts in rats. Indian J. Exp. Biol.. 2009;47(4):276-282.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- A mechanism for the hypocholesterolaemic activity of saponins. Br. J. Nutr.. 1986;55(3):643-649.
- [Google Scholar]
- High-density lipoprotein, low-density lipoprotein and coronary artery disease. Am. J. Cardiol.. 1990;66(6):A7-A10.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103048.
Appendix A
Supplementary material
The following are the Supplementary data to this article:










