Translate this page into:
Ameliorating Effect of Grape (Vitis vinifera L.) Seed and Peel Extracts with Selenium on Ochratoxin-A Exposed Renal Dysfunction in Male Wistar Rats
⁎Corresponding author at: Nutrition and Food Science Department, Faculty of Home Economics, Helwan University, Egypt. tasnem_sobhy@yahoo.com (Tasneem Sobhy Fahmy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Aim
Drug-induced nephrotoxicity is extensively documented as the initial stage of kidney disease that particularly results in acute renal injury and chronic renal dysfunction. The single and combined effect of grape peel extract (GPE), grape seed extracts (GSE) and selenium (Sel) were investigated in current study for controlling of renal injury induced by Ochratoxin-A (OTA) in male rats.
Methods
Forty nine male Wistar rats weighing 200 ± 10 g were injected with OTA (0.5 mg/kg bw) to induce nephrotoxicity, then treated with singleand combined applications of GSE, GPE and Sel. In addition, the OTA induced nephrotoxicity rats were screened for various biological and physiological parameters like food ingestion, body weight gain, liver weight, serum kidney biomarkers, serum lipid fractions, enzymatic and non-enzymatic lipid peroxidation indicators and histopathological studies.
Results
Our findings suggested that the combination of GSE 2 % and Sel (50–50) treatment reduced renal dysfunction in OTA-induced rats by significantly lowering lipid peroxidation indicators, lipid and kidneys profiles and enhancing enzymatic and non-enzymatic antioxidants biomarkers followed by GSE 2 % then Selenium (0.4 mg/kg) in OTA. Histological analysis of the kidney revealed more severe renal tubule degeneration in OTA-induced rats, While GSE and GPE combonation showed only moderate glomerular tuft enlargement and minimal renal tubule deterioration. Furthermore, the combination of GSE 2 %+Sel (50–50) revealed normal histological structure.
Conclusions
Grape seed extracts along with selenium mightbe used as dietary supplements to reduce renal toxicity induced by chemicals or compounds that cause nephrotoxicity.
Keywords
Grape seed extract (GSE)
Grape peel extract (GPE)
Selenium (Sel)
Ochratoxin-A (OTA)
Nephrotoxicity
Data availability
Data will be made available on request.
1 Introduction
The kidney is a crucial organ for maintaining blood vessel homeostasis and regulating the extracellular environment, including detoxifying and eliminating harmful compounds and medicines (Kim and Moon, 2012). Indeed, renal failure is a potentially fatal disorder that causes multiple organ failure and mortality. Furthermore, since renal failure is irreversible, the gradual loss of kidney function leads to the accumulation of urea, creatinine, and nitrogen in the blood (De Zeeuw et al., 2005). Thus; dialysis or kidney transplantation may be indispensable to avoid waste products building up in our bodies.
Drug-induced nephrotoxicity is increasingly recognized as a cause of kidney illness, particularly acute kidney damage and chronic kidney disease. Ochratoxin-A (OTA) is a fungal toxin thathas been proposedto be responsible for endemic nephropathy and urothelial tumors in humans through food. Indeed, it has potential risks, such as teratogenicity and carcinogenicity (Fuchs and Peraica 2005; Patil et al., 2006). As a result, the kidney is the target organ impacted by OTA in both people and animals (Damiano et al., 2020). However, because to its great thermal stability, eliminating OTA from the food chain will be difficult, and after being taken, OTA promotes lipid peroxidation and decreases glutathione, catalase, and superoxide dismutase in the kidneys and liver of rats. These effects may be related to oxidative pathways as well as pathological abnormalities associated with its toxicity, such as liver and kidney damage (Schaaf et al., 2002). Numerous researches have been carried out with the goal of lowering reactive oxygen species (ROS) generation, oxidative stress, or focusing on the nuclear factor E2-related factor 2 (Nrf2) pathway to lessen the impact of OTA toxicity. Malondialdehyde (MDA) is a common kind of free radical produced by lipid peroxidation. This extremely hazardous aldehyde is utilized as a biomarker to gauge how much oxidative stress is affecting vital body organs like the kidneys and liver (Alshammari et al., 2023). Numerous studies either make use of natural substances like flavonoids or nanoparticle technologies (Abdel-Wahhab et al., 2017; Yang et al., 2019; Ren et al., 2019). Nevertheless, the majority of these studies were dose dependent, making their application as dietary additives difficult.
Grape (Vitisvinifera, Vitaceae) seed extract hasa polyphenolic combination that contains antioxidant and anti-inflammatory activities (Turki et al., 2016). According to Khanal et al. (2009), grape seed extract, sold commercially as IH636, exhibited multi-organ protective abilities against medication and chemical-induced toxicity and exhibited long-term safety. The components resveratrol, quercetin, catechins, flavonoids, and unsaturated fatty acids from grape seed oil are the potential sources for its beneficial effect (Wang et al. 2016), also, administration of grape juice along with the high cholesterol diet for 12 weeks to the rats, improved the lipid profile, kidney functionswhich might be due to its high contents of fibers and proteins, thus associated with with the kidneys showing downregulation of Txnip (for the generation of ROS) and upregulation of antioxidants (Dhcr24, Gstk1, Prdx2, Sod2, Gpx1, and Gpx4) (Ali et al., 2019).
As far as we know, no published research article assessed the nephroprotective and curative benefits of grape GSE and GPE, with or without selenium (an antioxidant co-factor), against OTA-induced renal failure. Therefore, these OTA-induced nephrotoxic rats were examined for a number of biological parameters, including dietary intake, weight gain, animal liver weight, the bloodstream lipid division, including Very Low Density Lipoprotein (VLDL-C),Low Density Lipoprotein (LDL-C),High Density Lipoprotein (HDL-C), Total Cholesterol (TC) and Triglycerides (TG), blood serum kidney diagnostic markers (Urea Nitrogen,Uric acid, Albuminand Creatinine), non-enzymatic (MDA, GSH& Vitamin E) and enzymatic (GPx,SOD and CAT) lipid peroxidation indicators and histopathological effects.
2 Materials and methods
2.1 Chemicals
Unless stated otherwise, all analytical-grade chemicals and reagent kits used for analysis were purchased from Sigma Chemicals Co., USA.
2.2 Preparation of plant material extracts
Freshly picked matured red grapes (Vitisvinifera L.) were acquired from a neighbouring farm in Cairo, Egypt, and were separated, rinsed and removed from the stems. To retain the moisture level at 6.2 % w/w and prevent lipid breakdown, the grape skins and seeds were meticulously removed before being dried in an oven at 70 °C for seven hours. After blending the partially dried grape seeds and skins in an electric blender, a 0.5 mm sieve was used to get the powdered samples. The filtered powder was then concentrated and extracted with 95 % ethanol for 24 h at 60 °C using a high-capacity evaporator (EYELA Rotary vacuum evaporator N-11; Tokyo Ridadidai Co., Ltd., Japan) (Teresa et al., 2008).
2.3 Basal diet preparation
The basal diet, which contains 20 % casein, 10 % percent sucrose, 4.7 percent oil from corn, 2 % choline chloride, 1 % vitamin blend, 3.5 percent salt mixture, 5 % fibers, and the remaining corn starch, was produced using methodology AIN-93 (Reeves et al., 1993).
2.4 Experimental animals
Forty nine adult male Sprague Dawley albino mice with an average weight of (200 ± 10 g) were sourced from the Experimental Animal House in Helwan, Egypt under the ethical approval protocol number ARC-FU-41–24. The rats were maintained in stainless steel wire mesh bottomed cages with temperature and humidity controls and a 12-hour light/dark cycle. To assist them adjust to the lab environment, all rats were given free access to a basic diet and water for the first seven days.Rats were randomly divided into seven groups (each group = 7 rats) shortly after acclimatization. To cause nephrotoxicity, rats were gastrogaved with OTA at a dose of 0.5 mg/kg body weight per day (Arbillaga et al., 2008). The rats were fed for 6 days and the orders of groups are explained below:
Group 1 (Normal control) was given a basal diet with no other treatment.
Group 2: OTAgastro-gavaged rats fed withbasal diet (Positive control).
Group 3: OTAgastro-gavaged ratsfed with basal diet and Grape seed extract (GSE- 2 %).
Group 4:OTAgastro-gavaged ratsfed with basal diet andGrape peel extract (GPE-2 %).
Group 5:OTAgastro-gavaged rats fed with2% GSE+0.4 mg Sel/kg/bw(50 %+50 %).
Group 6: OTAgastro-gavaged ratsgroups fed 2 % GPE and 0.4 mg Sel /kg/bw(50 %+50 %).
Group 7: OTAgastro-gavaged ratsfed with basal dietand Selenium (0.4 mg/Kg bw) alone.
Daily records of dietary intake and body weight change were recorded using the method described by El-Shafie et al. (2015). Once completion of the trail, all rats wasexposed toaesthetic ether,the rats’ blood was separately collected, and thenthe kidneys were removed, rinsed in ice-cold saline to be clean from any blood. The kidney tissues were divided into pieces and ground into a 20 % homogenate (w/v) in a cold buffer (pH 7.0). The homogenates were cold spun at 0 °C for ten minutes at a speed of 1000 rpm. The amount of various biomarkers present in the control and treatment OTA-induced animals was estimated by separating the supernatant.
2.5 Estimation of lipid fractions and kidneysbiomarkers activities
Blood samples were given enough time to coagulate before the serum was extracted for 15 min at a speed of 2500 rpm. Employing the enzymatic techniques outlined by Allain et al. (1974), Wahlefeld, (1974), and Albers et al. (1983), several biochemical parameters, including serum total cholesterol (TC), triglycerides (TG), and high density lipoprotein (HDL-c), were assessed. Using Fridewald's equation, the concentrations of low density lipoprotein (LDL-c) and very low density lipoprotein(VLDL-c) were estimated as follows: LDL-c = TC − (HDL-c) − (VLDL-c); and VLDL-c = triglycerides / 5 (Fridewald et al., 1972). Additionally, the methods used by Husdan and Rapoport (1968), Doumas et al. (1971), Fossati et al. (1980), Patton and Crouch (1977), were used to estimate the amounts of serum urea nitrogen, uric acid, creatinine, and albumin.
2.6 Lipid peroxidation, enzymatic and non-enzymatic antioxidants biomarkers determinations
According to Rudnicki et al. (2007), kidney homogenates were used to determine the tissue lipid peroxide (MDA) that developed in terms of thiobarbituric acid reactive substances (TBARS). Non-enzymatic antioxidants biomarkers (GSH and Vitamin E) were also determined as a reflection for its tissue availability in relation to treatments. Baker et al.'s method (1980) was used to measure vitamin E levels, while Ellman's methods (1959) were utilised to quantify reduced glutathione (GSH). Additionally, according to Spitz and Oberley (1989), Sinha (1972), and Paglia and Valentaine (1979), the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in kidney tissues were all colorimetrically assessed using the appropriate kits.
2.7 Histomorphological examinations
After being immolated, kidney samples from the treatment and control rats were extracted and immediately submerged in 10 % neutral buffered formalin. The conserved specimens underwent trimming, rinsing, and dehydration in increasing concentrations of alcohol, clearing in xylene, embedding in paraffin, sectioning at 4–6 µm thickness, staining with hematoxylin and eosin, and microscopically examining them for anomalies in accordance with Carleton's 1979 guidelines.
2.8 Statistical analysis
The data obtained in the results of the study was evaluated using one-way analysis of variance (ANOVA) in SPSS version 11 (SPSS, Chicago, IL) followed by Duncan's Multiple Range Test (DMRT). The P<0.05 was selected as the upper limit of statistical significance.
3 Results
3.1 Biological effect of GPE,GSEand selenium(single and combined treatments) in OTA induced nephrotoxicity rats
The determination of the biological effect of GPE and GSE with/without selenium on Food ingestion, increase in body and liver weight in OTA induced nephrotoxicity are reported in Table 1. The result indicated a decrease in Food intake and body weight gain and an increase in liver weight in OTA induced rats compared to normal (untreated) rats. The treatments of GPE and GSE with/without selenium had slight effects on food intake;increase in body and liver weight ofOTA induced nephrotoxicity rats.However, the OTA induced nephrotoxicity rats treated with GSE+Sel (50–50) showed a maximum increase of feed intake and body weight gain of 19.35 g/day and 32.32 %, respectively. Also, the same treatment decreases liver weight by up to 4.022 %. Values are means ± S.D n = 7 rats/group. Values with the same superscript do not significantly differ from one another at p < 0.05 (DMRT).
GROUPS
PARAMETERS
Food intake (g/day)
Body weight gain (%)
Liver weight/body weight (%)
Normal Control
20.22
34.97 ± 2.56a
3.933 ± 1.024d
Intoxicated Control
17.23
18. 72 ± 3.12d
5.125 ± 0.410a
OTA+GSE 2 %
17.96
27.45 ± 2.02c
4.223 ± 0.125b
OTA+GPE 2 %
17.42
26.39 ± 2.07c
4.421 ± 0.225cd
OTA+GSE+Sel (50–50)
19.35
32.32 ± 1.66b
4.022 ± 0.257cd
OTA+GPE+Sel (50–50)
18.95
30.46 ± 1.97b
4.082 ± 0.232c
OTA+Sel (0.4 mg/kg)
18,63
29.57 ± 1.83bc
4.803 ± 0.114c
3.2 Impact of GPE and GSEwith/without selenium on the different lipid parameters in OTA induced nephrotoxicity rats
The influence of grape seed and peel extract with/without selenium on TG, HDL-c, LDL-c VLDL-c and TC on the OTA induced nephrotoxicity rats are presented in Table 2. Compared to the normal control, the OTA induced nephrotoxicityrats indicated high levels of TG, LDL-c, VLDL-c and TC by producing 77.43, 83.74, 15.48 and 130.72 mg/dl respectively. Also, a significant drop in HDL level of 31.52 mg/dl was detected in OTA induced ratsin comparison to the normal control. However, in the treated group, a maximum reduction of TG, LDL-c,VLDL-c and TC of 49.27, 26.62, 9.854 and 87.28 mg/dl were observed in the GSE+Sel (50–50) treated OTA induced rats. In addition, the same treatment showed a significant rise in HDL levels of 50.81 mg/dl. This effect of treatment GSE+Sel (50–50) was on par with the normal (untreated) control. Further, the treatment of GPE+Sel (50–50) also showed a significant drop in TG, LDL-c, VLDL-c and TC levels (54.32, 31.48, 10.86 and 91.31 mg/dl) and a significant rise in HDL (48.92 mg/dl) compared to intoxicated control. Values are means ± S.D n = 7 rats/group. Values with the same superscript do not significantly differ from one another at p < 0.05 (DMRT).
GROUPS
PARAMETERS
Total cholesterol
Triglycerides
HDL
LDL
VLDL
mg/dl
mg/dl
mg/dl
mg/dl
mg/dl
Normal Control
086.47 ± 4.28d
48.42 ± 3.9d
51.93 ± 4.06a
24.856 ± 1.8d
09.684 ± 0.78d
Intoxicated Control
130.72 ± 4.54a
77.43 ± 3.5a
31.52 ± 4.37d
83.714 ± 1.9a
15.486 ± 0.71a
OTA+GSE 2 %
102.33 ± 3.77ab
60.47 ± 2.9bc
43.73 ± 3.14b
46.506 ± 1.7c
12.094 ± 0.61c
OTA+GPE 2 %
112.42 ± 4.83b
66.31 ± 1.7bc
41.46 ± 2.18b
47.610 ± 1.8c
13.262 ± 0.79bc
OTA+GSE+Sel (50–50)
087.28 ± 3.97d
49.27 ± 2.7d
50.81 ± 3.14a
26.616 ± 1.6d
09.854 ± 0.43d
OTA+GPE+Sel (50–50)
091.31 ± 3.89cd
54.32 ± 3.2c
48.92 ± 2.18a
31.483 ± 1.9cd
10.864 ± 0.64b
OTA+Sel (0.4 mg/kg)
118.29 ± 4.30ab
70.25 ± 2.7ab
37.42 ± 2.39c
66.820 ± 1.6b
14.05 ± 0.55a
3.3 Effect of GPE and GSEwith/without selenium on serum kidney functions and albumin in OTA-induced nephrotoxicityrats
The effect of GSE and GPE with/without selenium onserum urea nitrogen, uric acid, albumin and creatinine of OTA induced nephrotoxicity ratswere presented in Table 3. It was observed that the oral administration of GSE and GPE with/without selenium generally lowers the Urea Nitrogen, Uric acid and Creatinine serum levels and increases the albumin level compared to intoxicated control. However, a maximum reduction of all treatments were Sel (0.4 mg/kg) with 1.77 and 3.57 respectively for uric acid and albumin,while GSE 2 % reached 23.53 for urea nitrogen and 0.59 for creatinine determinations. Values are means ± S.D n = 7 rats/group. Values with the same superscript do not significantly differ from one another at p < 0.05 (DMRT).
GROUPS
PARAMETERS
Uric acid (mg/dl)
Urea Nitrogen (mg/dl)
Creatinine (mg/dl)
Albumin (mg/dl)
Normal Control
2.02 ± 0.12b
21.66 ± 0.84c
0.58 ± 0.02c
3.82 ± 0.29a
Intoxicated Control
2.25 ± 0.10a
48.56 ± 1.60a
1.04 ± 0.06a
2.56 ± 0.43c
OTA+GSE 2 %
1.95 ± 0.14b
23.53 ± 0.67c
0.59 ± 0.03c
2.93 ± 0.34b
OTA+GPE 2 %
1.97 ± 0.15b
24.76 ± 0.88c
0.67 ± 0.02b
3.11 ± 0.31bc
OTA+GSE+Sel (50–50)
1.87 ± 0.11b
26.88 ± 0.47bc
0.61 ± 0.04c
2.49 ± 0.28c
OTA+GPE+Sel (50–50)
1.99 ± 0.09b
29.53 ± 0.67b
0.67 ± 0.04b
2.78 ± 0.24c
OTA+Sel (0.4 mg/kg)
1.77 ± 0.16b
28.88 ± 0.47b
0.61 ± 0.07c
3.57 ± 0.30b
26.88, 1.87and 0.61 mg/dl in urea nitrogen, uric acid and creatinine level was achieved in the GSE+Sel (50–50) treated OTA induced rats. However, all the other treatments also, recorded a considerable decrease in the urea nitrogen, uric acid, and creatinine levels and showed more variations in the albumin level.
3.4 Effect of GPE and GSEwith/without selenium on the on the level of oxidative stress indices in OTA treated rats
The modifications in serum lipid biomarkers, including MDA, GSH, SOD, GPx, and CAT, and a non-enzymatic antioxidant vitamin E are depicted in Figs. 1a–c and 2a–c. Fig. 1a illustrates the modification in the oxidative stress marker MDA. We observed that the OTA intoxicated nephrotoxicity control rats showed remarkably increased MDA level (0.773 nmol/mg) protein in relation to normal control rats (0.253 nmol/mg protein). The treatment of OTA-induced nephrotoxicity rats fed with GSE+Sel (50–50) has a significant effect on MDA by reducing it to 0.269 nmol/mg and this reduction level was comparable to normal control. Moreover, the rats fed with GPE+Sel (50–50) recorded 0.293 nmol/mg of MDA, which was slightly similar to normal rats. Nevertheless, the nephrotoxicity rats treated with selenium alone increases the MDA level by showing 0.537n mol/mg compared to intoxicated control.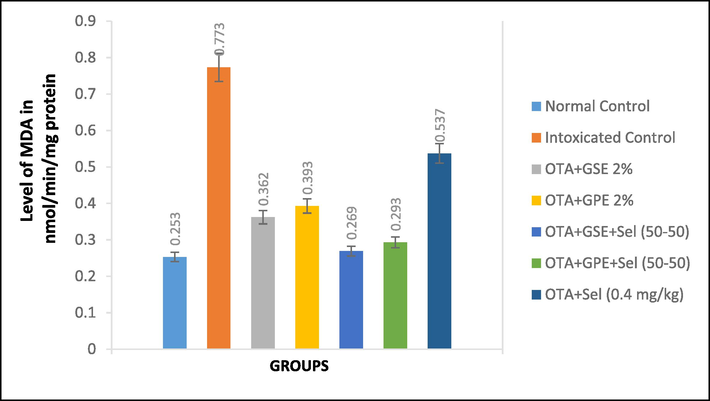
Effect of GSE, GPE and/or selenium addition on MDA in OTA-induced nephrotoxic rats.
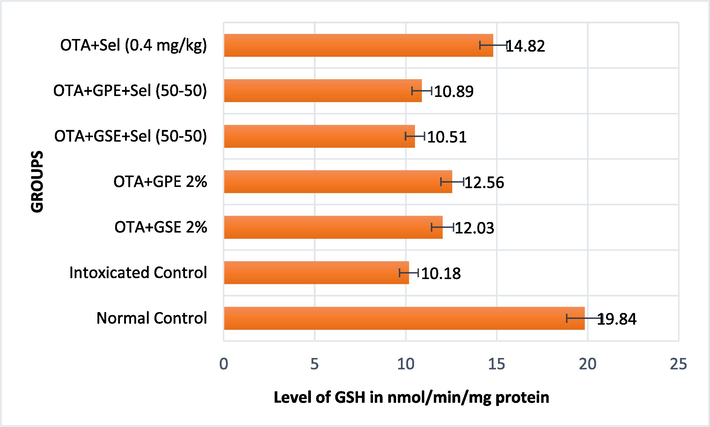
Effect of GSE, GPE and/or selenium addition on GSH in OTA-induced nephrotoxic rats.
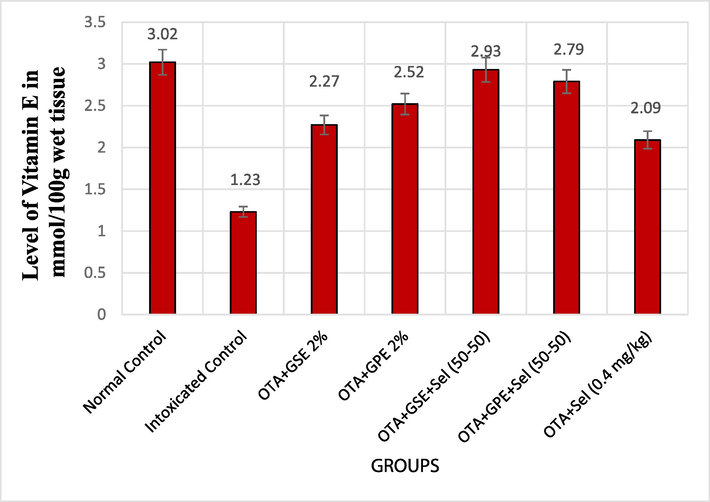
Effect of GSE, GPE and/or selenium addition on Vitamin E in OTA-induced nephrotoxic rats.
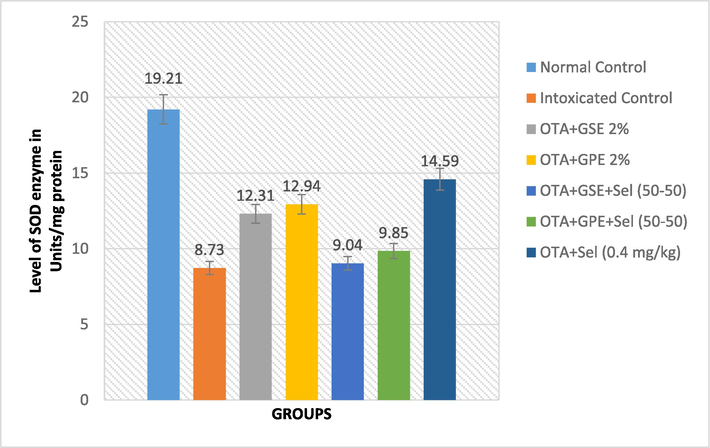
Effect of GSE, GPE and/or selenium addition on SOD enzyme in OTA-induced nephrotoxic rats.
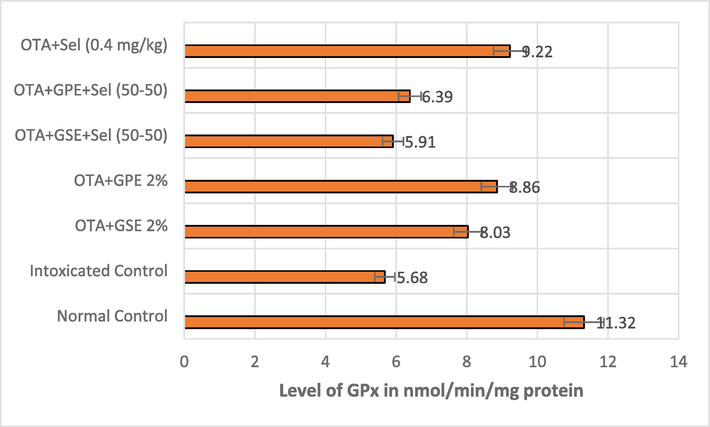
Effect of GSE, GPE and/or selenium addition on GPx enzyme in OTA-induced nephrotoxic rats.
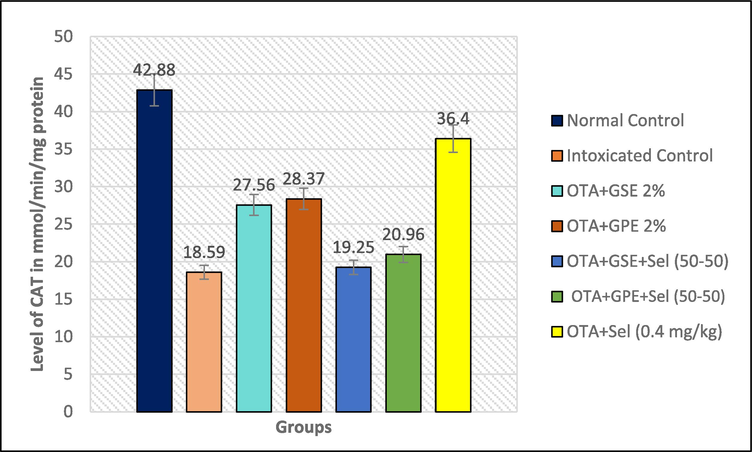
Effect of GSE, GPE and/or selenium addition on CAT enzyme in OTA-induced nephrotoxic rats.
The effect of GSE and GPE with/without selenium on the serum non-enzymatic biomarker GSH is presented in Fig. 1b. The OTA-induced nephrotoxicity rats fed with GSE+Sel (50–50) and GPE+Sel (50–50) recorded the lowest level of GSH (10.51 and 10.89 nmol/mg protein), which wason par with OTA intoxicated control rats. The nephrotoxicity rats fed with selenium alone increased the GSH level to 14.82n mol/mg protein.
The influence of the treatments GSE and GPE with/without selenium on the non-enzymatic antioxidant vitamin E is depicted in Fig. 1c. The OTA intoxicated control rats showed a minimum level of 1.23 mmol/100 g wet tissue of vitamin E compared to normal control rats. Moreover, treating OTA-induced nephrotoxicity rats with GSE+Sel (50–50) and GPE+Sel (50–50) has significantly increased the vitamin E level to 2.93 and 2.79 mmol/100 g, respectively, compared to OTA intoxicated control rats. Also, the other treatments like GSE, GPE, and selenium alone showed an increased level of vitamin E compared to OTA intoxicated control rats.
The single and combined effect of GPE, GSE, and Selenium on the SOD biomarker is illustrated in Fig. 2a. In general, the combined effect of GSE+Sel (50–50) and GPE+Sel (50–50) lowers the SOD level to 9.04 and 9.85 Units/mg protein, which is more similar to OTA intoxicated control rats. However, the treatments of GSE, GPE and Selenium alone showed an increased level of SOD of 12.31, 12.94 and 14.59 units/mg protein, whereas the control rats showed 19.21 units/mg protein.
A similar trend was noted in the level of GPx enzyme in the OTA intoxicated rats treated with the combination of GSE+Sel (50–50) and GPE+Sel (50–50) thereby recording 5.91 and 6.91 nmol/mg protein. This reduction was on par with the effect of OTA intoxicated rats that showed 5.68 nmol/mg protein. However, the treatments of GSE, GPE and Selenium alone on nephrotoxicity rats had some significant effect on GPx level by increasing it to 8.03, 8.86, and 9.22 nmol/mg protein, and normal control rats showed 11.32n mol/mg protein (Fig. 2b).
A drastically high level of CAT (42.88 mMol/mg protein) enzyme was produced in the normal control rats, and a lower CAT enzyme level was noted in all other treatments. The combined effect of GSE+Sel (50–50) and GPE+Sel (50–50) and GSE, GPE and selenium alone showed only less effect on the CAT level in OTA induced nephrotoxicity rats, which was described in Fig. 2c. It was observed that the different combinations GSE+Sel (50–50), GPE+Sel (50–50) and the individual treatments of GSE, GPE, and Selenium showed a CAT level of 19.25, 20.96 and 27.56, 28.57, 36.40 nmol/mg protein, respectively.
3.5 Histopathological effect of GPE and GSEand/or selenium on the kidney tissues of OTA-induced nephrotoxic rats
Microscopically histopathological examination (H and EX 200) of kidney tissues is depicted in Fig. 3 (Photos 1–4). It was observed from Photo 1 that the kidneys of the normal rat group showed glomerular and tubular histological structure normally, whereas the kidneys of OTA intoxicated control rats (Photo 2) showed more distortions like inflammatory reaction and cloudy swelling of the epithelial lining of the collecting tubules leading to different grades of the lumen obliteration. The rats fed with a combination of GPE2%+Sel (50–50) displayed only mild histological alteration with patent lumens (Photo 3),whereas the combination of GSE 2 %+Sel (50–50) showed a normal histological pattern similar to the normal control (Photo 4). It was inferred from all the above results that the treatment of GSE 2 %+Sel (50–50) in OTA induced nephrotoxicity rats positively reduces renal damage.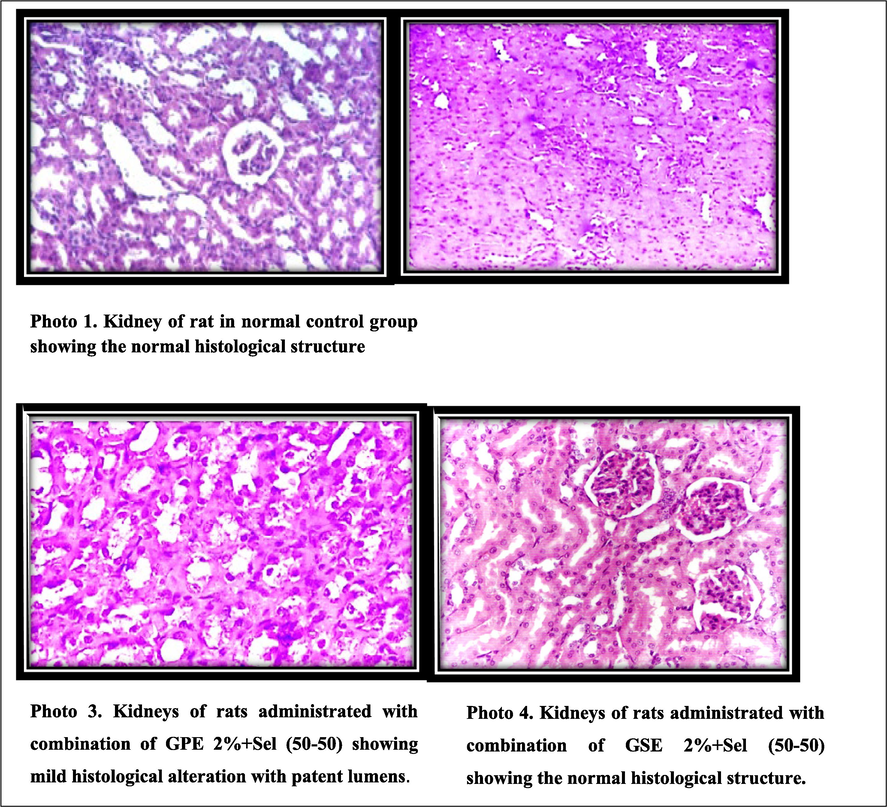
Histopathological assessment of the kidneys of OTA-induced nephrotoxic rats by the treatment of GSE, GPE and/or selenium (Microcroscopical examination at H and EX 200).
4 Discussion
Nephrotoxicity is one of the most prevalent kidney issues that arise when a drug or toxin is ingested by the body. OTA affects the human kidneys by causing a deleterious effect on human health when accidentally consuming OTA-contaminated food (Fuchs and Peraica 2005). OTA controls histone modifications, DNA methylation, and transcriptional variables to generate kidney damage eventually leading to inflammation, glomerular and tubular damage, and even renal fibrosis (Khoi et al., 2021). According to Khanal et al. (2009), the extract from grape seed (GSE) is a complex polyphenolic mixture including flavonoids, non-flavonoids, and pro-anthocyanidins that exhibits multi-organ protection in several experimental conditions. Therefore, in the current investigation, the ameliorative effect of GPE and GSE along with or without combining selenium was evaluated for its nephroprotective effects in OTA induced nephrotoxicity in Wistar rats.
Body weight is the major indicator for determining the harmful effects of various medications or toxins on animals (Wang et al., 2016).The biological effect of GPE and GSE with/without selenium on food ingestion increase in body and liver weight in OTA induced nephrotoxicity rats was determined. The OTA induced nephrotoxicity rats treated with GSE+Sel (50–50) showed a maximum increase in feed intake and body weight gain and decreased liver weight to a level similar to normal rats. These effects may be to the reduction of toxicity by OTA by this treatment. The results obtained are consistent with the effects reported by Damiano et al. (2020) and El-Shafie et al. (2015). Damiano et al. (2020) determined that OTA administration significantly reduced body weight in rats; however, the combined treatment with curcumin significantly increased the body weight of rats. Herein, we examined the impact of grape seed and peel extract with/without selenium on TG, HDL, LDL-c, VLDL-c andTC since these are theone of modifiable risk factors responsible for chronic renal disorders mainly dyslipidemia. In the condition of dyslipidemia, there will be elevation in triglycerides, low-density lipoprotein and low high-density lipoprotein. The results showed that the OTA induced nephrotoxicity rats specified a high rise in TG, LDL-c VLDL-c and TC and a significant drop in HDL level in relation to normal control. However, the treatment GSE+Sel (50–50) was significantly on par with the normal control, thereby returning to the vicinity of normal in control rats. The cause of this impact in our results might be due to presence of polyphenolics, flavonoids, and unsaturated fatty acids in GPE and GSE (El-Shafie et al., 2015).Moreover, the OTA-induced rats fed with GPE and GSE showed increased body weight, HDL-C and decreased levels of TG, LDL-C, VLDL-c and TC.
It is interpreted from our study that the GPE and GSE with/without selenium have a substantial effect on kidney functions, as evidenced by alteration in blood serum urea nitrogen, uric acid, albumin and creatinineof OTA induced nephrotoxicity.The results revealed that the administration of GSE+Sel (50–50) lowers uric acid, urea nitrogen, and creatinine levels and increases albumin levels compared to intoxicated control. Yet, the maximum increase in albumin level was more evidenced in the alone selenium treated nephrotoxicity. There are also reports indicating that the intake of high doses of selenium alone will result in albuminuria (Zhang et al., 2023). In fact, our study suggested that selenium might act as a cofactor of antioxidant enzymes, and therefore it is safe to be administered along with GSE rather than alone (Zachara, 2015).
Interestingly, there are no significant alterations noted in serum antioxidant biomarkers such as GSH, SOD, GPx, and CAT in the OTA-induced nephrotoxicity rats fed with GSE+Sel (50–50) and GPE+Sel (50–50). Nevertheless, most treatments showed a positive effect in reducing MDA levels and increasing vitamin E levels. Incidentally, the effect of GSE+Sel (50–50) and GPE+Sel (50–50) on GSH, SOD, GPx, and CAT levels was significantly similar to the OTA-induced nephrotoxicity rats rather than control rats. This insignificant result might be due to the addition of co-factor selenium to GPE and GSE, as the nephrotoxicity rats fed with selenium alone increased the GSH, SOD, GPx, and CAT levels, which was closely comparable to normal control rats. Excessive selenite on its own causes toxicity in liver asseleniumbind toa prosthetic group in important oxidative stress-related enzymes such as glutathione peroxidase and TR that necessary for reducing oxidative stress (Ranmali Ranasinghe et al., 2023). The enzymatic activity of CAT, GPx, and SOD might be affected by excess selenium, indicating the presence of cell membrane damage. selenium also enhances lipid peroxidation in a dose-dependent way (Talbi et al., 2019).
It is also well evinced from the current study that the combined effect of GSE 2 %+Sel (50–50) on kidney tissues of OTA-induced nephrotoxicity rats displayed normal histological pattern (normal glomerular and tubular histological structure) as similar to the normal control, whereas the kidneys of OTA intoxicated control rats exposed more distortions and cell inflammations.Indeed, similar effects on the histological data were reported by Charradi et al. (2013), who reported that the grape skin and seed extract (GSSE) is protective against high-fat diet imposed renal damage. Moreover, proved that the ethanol extract obtained from unripe pods and leaves of Orchid tree named Bauhinia purpurea had curative effects on renal injury by protecting rat kidneys from gentamicin-induced histopathological changes. In addition, numerous biological effects and health-promoting qualities of GSSE have been reported, including antioxidants, cholesterol reduction, and anti-tumor capacities (Charradi et al., 2013; Talbi et al., 2019; Šikuten et al. 2020).
5 Conclusion
In conclusion, our results suggested that the supplementation of the GSE 2 % in combination with selenium (50–50) treatment had the most beneficial effect on lowering renal dysfunction in OTA-induced nephrotoxicity rats, thereby revealing the possible nephroprotective and curative efficacy. Therefore, from the perspective of its potential use in issues related to renal toxicity, GSE, along with selenium, could be widely used as a dietary supplement.
CRediT authorship contribution statement
Mohamed Farouk Elsadek: Writing – original draft, Resources, Methodology, Investigation, Conceptualization. Tse-Wei Chen: Software, Project administration, Formal analysis. Khalid S. Al-Numair: Writing – review & editing, Validation, Supervision, Project administration. Tasneem Sobhy Fahmy: Writing – review & editing, Investigation, Formal analysis, Data curation.
Acknowledgment
The authors would like to extend their sincere appreciation for funding this work through the Researchers Supporting Project number (RSP2024R349), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chitosan nanoparticles plus quercetin suppress the oxidative stress, modulate DNA fragmentation and gene expression in the kidney of rats fed ochratoxin A-contaminated diet. Food Chem. Toxicol.. 2017;99:209-221.
- [Google Scholar]
- Enzymatic determination of high density lipoprotein cholesterol: Selected Methods. Clin. Chem.. 1983;10:91-99.
- [Google Scholar]
- Renoprotective effect of red grape (Vitis vinifera L.) juice and dark raisins against hypercholesterolaemia-induced tubular renal affection in albino rats. Folia Morphol.. 2019;78(1):91-100.
- [Google Scholar]
- Effects of antioxidant combinations on the renal toxicity induced rats by gold nanoparticles. Molecules. 2023;28(4):1879.
- [Google Scholar]
- Arbillaga, L., Vettorazzi, A., Ana, G.G., Joost, H.M., van Delft, José Antonio, García-Jalón, Adela Lopez de Cerain., 2008. Gene expression changes induced by ochratoxin A in renal and hepatic tissues of male F344 rat after oral repeated administration. Toxicol. Appl. Pharm. 230, 197–207.
- Plasma α-tocopherol in man at various time intervals after ingesting free or acetylated tocopherol. Nutr. Rep. Int.. 1980;21:531-536.
- [Google Scholar]
- Histological Techniques (4th Ed.). London: Oxford University Press, New York, USA; 1979.
- High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J. Physiol. Sci.. 2013;63:445-455.
- [Google Scholar]
- Red orange and lemon extract prevents the renal toxicity induced by ochratoxin A in rats. J. Cell. Physiol.. 2020;235:5386-5393.
- [Google Scholar]
- The kidney, a cardio vascular risk marker, and a new target for therapy. Kidney Int.. 2005;68:S25-S29.
- [Google Scholar]
- Albumin standards and the measurement of serum albumin with bromcresol green. Clinicachimica Acta.. 1971;31(1):87-96.
- [Google Scholar]
- Curative effect of orally consumed Aloe vera juice on Ochratoxin A-induced nephrotoxicity in rats. ProgrNutr.. 2015;17(2):128-136.
- [Google Scholar]
- Uric acid measurements with enzymatic colorimetric method. Medicinal Clin Chem.. 1980;26:227-273.
- [Google Scholar]
- Estimation of the concentration of low density lipoprotein. Clin. Chem.. 1972;18:499-502.
- [Google Scholar]
- Ochratoxin A in human kidney diseases. Food Addit. Contam.. 2005;22(Suppl. 1):53-57.
- [Google Scholar]
- Procyanidin content of grape seed and pomace and total anthocyanin content of grape pomace as affected by extrusion processing. J. Food Sci.. 2009;74:174-182.
- [Google Scholar]
- Ochratoxin A Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci.. 2021;22:11237.
- [Google Scholar]
- Drug-Induced Nephrotoxicity and Its Biomarkers. Biomolther.. 2012;20(3):268.
- Studies on glutathione and glutathione characterization of erythrocytes glutathione peroxidase. J. Lab. Clin. Med.. 1979;70:158-169.
- [Google Scholar]
- Critical period and minimum single oral dose of ochratoxin A for inducing developmental toxicity in pregnant Wistar rats. ReprodToxicol.. 2006;22:679-687.
- [Google Scholar]
- Cytoprotective remedies for ameliorating nephrotoxicity induced by renal oxidative stress. Life Sci.. 2023;318:121466
- [Google Scholar]
- AIN-93. Purified diets for laboratory rodents. Final report of the American Institute of Nutrition AdHoc writing committee on the reformulation of the AIN-76- A Rodent diet. J. Nutr.. 1993;123:1939-1951.
- [Google Scholar]
- Research Progress on the toxic antagonism of selenium against Mycotoxins. Biol. Trace Elem. Res.. 2019;190:273-280.
- [Google Scholar]
- Antioxidant and antiglycation properties of Passifloraalataand Passiflora edulis extracts. Food Chem.. 2007;100:719-724.
- [Google Scholar]
- The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. BiochemBiophys Acta.. 2002;1588:149-158.
- [Google Scholar]
- An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem.. 1989;179:8-18.
- [Google Scholar]
- Effects of selenium on oxidative damage and antioxidant enzymes of eukaryotic cells: wine Saccharomyces cerevisiae. Journal of Applied Microbiol.. 2019;26(2):555-566.
- [Google Scholar]
- Extraction of victoria and red globe grape seed oils using supercritical carbon dioxide with and without ethanol. J. Food Lipids. 2008;15:356-369.
- [Google Scholar]
- Grape seed powder improves renal failure of chronic kidney disease patients. EXCLI J.. 2016;15:424-433.
- [Google Scholar]
- Wahlefeld, A.W., 1974. Enzymatic Determination of Triglycerides. Methods of Enzymatic Analysis. Vol. 5, HU. Bergmeyer, Ed. Academic Press, New York,1831-1835.
- Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and bio-distribution of minerals (Zn, Fe, cu, Mn) in mice. PLoS One.. 2016;11:e0164434.
- [Google Scholar]
- Precision toxicology shows that troxerutin alleviates ochratoxin A-induced renal lipotoxicity. FASEB J.. 2019;33:2212-2227.
- [Google Scholar]
- Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem.. 2015;68:131-151.
- [Google Scholar]
- Excess selenium intake is associated with microalbuminuria in female but not in male among adults with obesity: Results from NHANES 2009–2018. Front. Nutr.. 2023;10:51.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103453.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







