Translate this page into:
Alkaloids as chemotaxonomic markers from the Philippine endemic Uncaria perrottetii and Uncaria lanosa f. philippinensis
⁎Corresponding author at: College of Science, University of Santo Tomas, Espana, Manila 1015, Philippines. matan@ust.edu.ph (Mario A. Tan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Phytochemical investigation on the aerial parts of Uncaria perrottetii led to the isolation of three pentacyclic oxindole alkaloids uncarine A or isoformosanine (1), uncarine E or isopteropodine (2), and rauniticine-allo-oxindole A (3). Five oxindole alkaloids, isomitraphylline (4), mitraphylline (5), uncarine B or formosanine (6), uncarine F (7), corynoxine (8), and uncarine D or speciophylline (9), were isolated from the leaves of Uncaria lanosa f. philippinensis. Their structures were determined by spectroscopic techniques and in comparison with the literature data. These compounds proved to be important chemotaxonomic markers in the genus Uncaria.
Keywords
Rubiaceae
Uncaria
Oxindole alkaloids
Chemotaxonomy
Uncarine
1 Introduction
The genus Uncaria Schreb. (Rubiaceae) has been described commonly as lianas with characteristic paired fang hooks at the nodes (Ridsdale, 1978). The species are widely distributed among tropical areas such as Southeast Asia, Africa, and South America (Ridsdale, 1978). Uncaria species possessed interesting chemical structures including alkaloids, terpenes, quinovic acid glycosides, flavonoids and coumarins (Heitzman et al., 2005). Biological activities associated with various Uncaria species include cytotoxicity, anti-inflammatory, antiviral, immune-stimulant, antioxidant, CNS-related response, vascular, hypotensive, mutagenicity and antibacterial properties (Heitzman et al., 2005). A total of 34 Uncaria species are recorded worldwide (Ndagijimana et al., 2013). In the Philippines, the genus Uncaria was represented by ten species in which two, U. nervosa Elmer and U. perrottetii (A. Rich.) Merr. are endemic (Ridsdale, 1978). U. perrottetii is an interesting Philippine endemic as it is the only species in the genus that bears a deeply bifid laciniate stipule and a distinct violet colored shoot system.
In our continuing studies on the endemic Philippine Rubiaceae species (Tan et al., 2012; Tan et al., 2014a,b), we herein report the isolation and identification of oxindole alkaloids from the leaves of U. perrottetii and U. lanosa f. philippinensis. The structures were elucidated based on 1D and 2D NMR, MS and by comparison with literature data. This is the first phytochemical study on U. perrottetii and U. lanosa f. philippinensis. The isolated alkaloids also served as chemotaxonomic markers for the different Uncaria species.
2 Materials and methods
2.1 General considerations
1H and 13C NMR spectra were recorded on 400 MHz or 500 MHz Agilent NMR spectrometer. HRESI-MS were recorded on a Thermo Fischer Scientific Exactive spectrometer. Silica gel (Merck 7734 for gravity CC or Merck 9385 for flash CC) was used for column chromatography. Solvents for chromatography were analytical grade.
2.2 Plant material
Fresh leaves of U. perrottetii were collected from Lanuza, Surigao del Sur, Philippines on May 2016 while fresh leaves of U. lanosa f. philippinensis from Mt. Mingan, Aurora, Philippines on April 2016. Identity of the specimens was confirmed by the first author (JECO) using characters distinctive of the two species, U. perrottetii by having deeply bifid laciniate stipules and distinct violet colored shoot system and U. lanosa f. philippinensis by possessing deltoid calyx lobes. Voucher specimens of U. perrottetii (USTH 012900) and U. lanosa f. philippinensis (USTH 013915) were deposited at the UST Herbarium, Research Center for the Natural and Applied Sciences, University of Santo Tomas.
2.3 Extraction and isolation
The air-dried, ground leaves of U. perrottetii (1.2 kg) were percolated with distilled MeOH (14 L) for three days at room temperature and filtered. The combined filtrate was concentrated under reduced pressure to obtain the crude extract (86 g). The crude extract was dissolved in 1 M HCl and extracted thrice with EtOAc. The aqueous acid layer was basified to pH 8–9 and extracted exhaustively with CHCl3. The CHCl3 layer was dried with anhydrous Na2SO4 and concentrated under reduced pressure affording the crude base. The crude base was subjected to silica gel gravity CC using a gradient of hexane-EtOAc (neat hexane, 9:1, 4:1, 7:3, 3:2, 1:1, neat ethyl acetate) resulting to five pooled fractions. Fraction 2, with distinct positive orange spots in Dragendorff, was re-purified by silica gel flash CC using isocratic hexane-EtOAc (3:2) to afford alkaloids 1 (uncarine A or isoformosanine, 3 mg) and 2 (uncarine E or isopteropodine, 4 mg). Alkaloid 3 (rauniticine-allo-oxindole A, 3 mg) was obtained from fraction 3 by silica gel flash CC using isocratic hexane-EtOAc (1:1).
The air-dried, ground leaves of U. lanosa f. philippinensis (1.9 kg) were percolated with distilled MeOH (16 L) for three days at room temperature and filtered. The combined filtrate was concentrated under reduced pressure obtaining the crude extract (100 g). The crude extract was dissolved in 1 M HCl and extracted thrice with EtOAc. The resulting aqueous acid layer was basified to pH 8–9 and extracted exhaustively with CHCl3. The CHCl3 layer was dried with anhydrous Na2SO4 and concentrated under reduced pressure to obtain the crude base. The crude base was subjected to silica gel flash CC using gradient elution of hexane-EtOAc (neat hexane, 3:1, 1:1, 1:3, neat EtOAc) resulting to thirteen pooled fractions. Several fractions were isolated as pure compounds including fraction 1 (isomitraphylline, 4, 45 mg), fraction 2 (mitraphylline, 5, 38 mg), fraction 6 (formosanine, 6, 21 mg), fraction 10 (uncarine F, 7, 23 mg), fraction 12 (corynoxine, 8, 4 mg), and fraction 13 (uncarine D, 9, 7 mg).
3 Results and discussion
Chromatographic purification of the crude base of U. perrottetii yielded uncarine A or isoformosanine (1), uncarine E or isopteropodine (2), and rauniticine-allo-oxindole A (3) (Fig. 1). This is the first report of alkaloids 1 and 3 from U. perrottetii while uncarine E (2) was previously identified from this plant (Lleander et al., 1974). The presence of 2 was also identified from U. bernaysii, U. donisii, U. guaianensis, U. homolalla, U. laevigata, U. lanosa, U. longiflora, U. orientalis, U. roxburghiana, U. scandens, U. sinensis, U. sterrophylla, U. tomentosa, and U. veluntina (Arbain et al., 1993; Aquino et al., 1997; Kam et al., 1991; Lee et al., 1999a, 1999b; Phillipson et al., 1978; Tanahashi et al., 1997; Wagner et al., 1985). From the 34 species, alkaloid 3 was previously identified from U. elliptica (Phillipson and Supavita, 1983). Uncarine A (1) was previously identified from U. attenuata, U. cordata, U. gambir, U. hirsuta, U. laevigata, U. orientalis, and U. sessilifructus (Phillipson et al., 1978; Wu and Chan, 1994).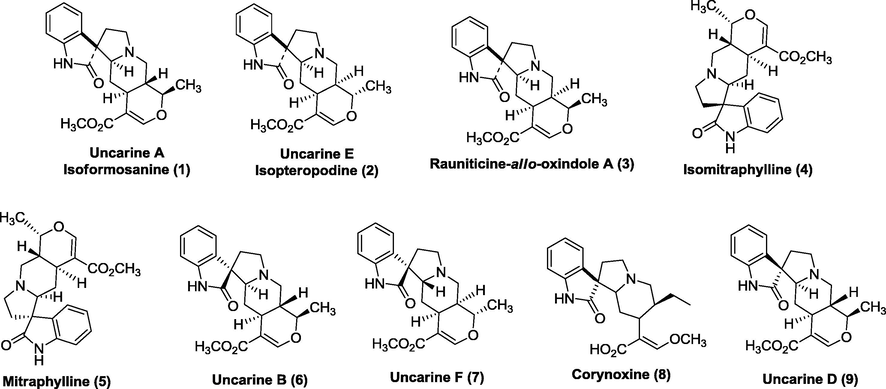
Structures of isolated alkaloids.
U. lanosa f philippinensis has elaborated isomitraphylline (4), mitraphylline (5), formosanine or uncarine B (6), uncarine F (7), corynoxine (8), and uncarine D or speciophylline (9) (Fig. 1). Isomitraphylline (4) was isolated from 18 species namely U. africana, U. bernaysii, U. callophylla, U. elliptica, U. guianensis, U. hirsuta, U. homomalla, U. laevigata, U. lancifolia, U. lanosa, U. longiflora, U. orientalis, U. perrottetii, U. scandens, U. sessilifructus, U. sterrophylla, U. tomentosa, and U. veluntina (Diyabalanage et al., 1997a, 1997b; Phillipson et al., 1978; Tantivatana et al., 1979; Wagner et al., 1985). The presence of mitraphylline (5) was perceived in U. africana, U. attenuata, U. bernaysii, U. callophylla, U. elliptica, U. gambir, U. guianensis, U. hirsuta, U. homomalla, U. laevigata, U. lancifolia, U. lanosa, U. longiflora, U. orientalis, U. perrottetii, U. scandens, U. sessilifructus, U. sterrophylla, U. tomentosa, and U. veluntina (Phillipson et al., 1978; Wagner et al., 1985; Tantivatana et al., 1979; Tantivatana et al., 1980; Herath et al., 1978). The species U. attenuata, U. elliptica, U. gambir, U. hirsuta, U. laevigata, U. orientalis, and U. sessilifructus were known to contain uncarine B (6) (Phillipson et al., 1978; Wu and Chan, 1994; Tantivatana et al., 1980; Herath et al., 1978). Uncarine F (7) was previously identified from U. bernaysii, U. donisii, U. homomalla, U. lanosa, U. longiflora, U. orientalis, U. perrottetii, U. roxburghiana, U. scandens, U. sessilifructus, U. sinensis, U. sterrophylla, and U. veluntina (Phillipson et al., 1978; Tanahashi et al., 1997). Six species contained corynoxine B (8) including U. attenuata, U. cordata, U. kunstleri, U. macrophylla, U. sessilifructus, and U. sterrophylla (Phillipson et al., 1978; Lee et al., 2000). Uncarine D (9) was isolated from U. attenuata, U. bernaysii, U. donisii, U. guianensis, U. homomalla, U. laevigata, U. lanosa, U. longiflora, U. orientalis, U. perrottetii, U. roxburghiana, U. scandens, U. sinensis, U. sterrophylla, U. tomentosa, and U. veluntina (Arbain et al., 1993; Phillipson et al., 1978; Tanahashi et al., 1997).
Except for rauniticine-allo-oxindole A (3), all the alkaloids from this study were identified from at least six different Uncaria species. Thus, the isolated alkaloids may provide as chemotoxanomic markers for the genus Uncaria.
Löfstrand et. al. (2014), supported the monophyly (PP = 1.00; BS = 100%) of Uncaria indicating the distinctness of members of the genus from other members of the family Rubiaceae. It must be noted that only members of Uncaria exhibit the following character combinations: (1) lianescent growth habit and (2) presence of paired fang hooks at the nodes. This distinctness can be directly correlated to the presence of unique oxindole alkaloids in Uncaria, which are of chemotaxonomic significance. Phylogenetically, Löfstrand et al. suggested that members of the genus can be subdivided into an Asian and Afro-Neotropical clades. It is no surprise that U. perrottetii and U. lanosa f. philippinensis share oxindole alkaloids with other members, as they are representatives from the strictly Asian clade. These alkaloids, therefore, maybe be used to delimit members of the clade.
Acknowledgement
The Philippine Tropical Forest Conservation Foundation (PTFCF) of the Philippines is acknowledged for the funding of the field collection.
References
- Triterpenes and quinovic acid glycosides from Uncaria tomentosa. Phytochemistry. 1997;45:1035-1040.
- [Google Scholar]
- A quinovic acid glycoside from Uncaria elliptica. ACGC Chem. Res. Commun.. 1997;6:29-32.
- [Google Scholar]
- Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry. 2005;66:5-29.
- [Google Scholar]
- Chemical investigation of Ceylonese plants. Part 33. Three new ursane carboxylic acids from Uncaria thwaitesii. Phytochemistry. 1978;17:1979-1981.
- [Google Scholar]
- Studies on Malaysian Uncaria. Part 4. Dimeric indole alkaloids from Uncaria callophylla. Phytochemistry. 1991;30:3441-3444.
- [Google Scholar]
- Uncarinic acids: phospholipase C. 1 inhibitors from hooks of Uncaria rhynchophylla. Bioorg. Med. Chem. Lett.. 1999;9:1429-1432.
- [Google Scholar]
- Bioactive indole alkaloids from the bark of Uncaria guianensis. Planta Med.. 1999;65:759-760.
- [Google Scholar]
- Inhibition of phospholipase Cg1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J. Nat. Prod.. 2000;63:753-756.
- [Google Scholar]
- Three isomeric alkaloids from Uncaria perrottetii (A. Rich) Merr. Uncaria ferrea F. Vill. non D.C. Philos. J. Sci.. 1974;103:75-80.
- [Google Scholar]
- A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia. 2013;86:35-47.
- [Google Scholar]
- Phylogeny and generic delimitations in the sister tribes Hymenodictyeae and Naucleeae (Rubiaceae) Syst. Bot.. 2014;39:304-315.
- [Google Scholar]
- Alkaloids of Uncaria. Part V. Their occurrence and chemotaxonomy. Lloydia. 1978;41:503-570.
- [Google Scholar]
- Alkaloids from Uncaria species. Part 8 Alkaloids of Uncaria elliptica. Phytochemistry. 1983;22:1809-1813.
- [Google Scholar]
- Chemotaxonomic implications of the absence of alkaloids in Psychotria gitingensis. Biochem. Syst. Ecol.. 2012;45:20-22.
- [Google Scholar]
- Iridoids and a norsesquiterpenoid from the leaves of Villaria odorata. Nat. Prod. Commun.. 2014;9:1229-1230.
- [Google Scholar]
- Chemotaxonomic relevance of the constituents from the leaves of Rothmannia merrillii. J. Chem. Pharm. Res.. 2014;6:779-781.
- [Google Scholar]
- Alkaloids of Uncaria. Part 7. Alkaloids of U. attenuata (U. salaccensis) from N.E Thailand. Planta Med.. 1980;40:299-301.
- [Google Scholar]
- Alkaloids from Uncaria tomentosa and their phagocytosis enhancement effect. Planta Med.. 1985;51:419-423.
- [Google Scholar]
- Constituents of leaves of Uncaria hirsute Haviland. J. Chin. Chem. Soc.. 1994;41:209-212.
- [Google Scholar]







