Translate this page into:
Airborne Menace: Unveiling antibiotic resistance in bacteria around household waste containers
⁎Address: Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia. Ralbiheyri@kau.edu.sa (Raed Albiheyri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The increase in antibiotic-resistant bacteria and air contamination is a critical issue that requires significant scientific attention. This study was undertaken to isolate, identify, and detect antibiotic-resistant bacteria around garbage containers located in Jeddah city. A total of 40 plates were exposed to the air around garbage containers in major areas of the city such as Al-Salama, Al-Zahra, Al-Safa, and Al-Faisaliyah. The sample plates were taken to the laboratory within an hour for incubation. In total, 38 airborne bacteria were isolated and identified. Overall, 26.325 % of isolates showed multiple drug resistance (MDR). The highest percentage of resistance was seen against ampicillin (90 %), cefotaxime (80 %), cefuroxime (80 %), ceftrazime (80 %), augmentin (50 %), and cefoperazone (20 %). The multiple antibiotic index is seen between 0.16 and 0.5. Al-Safa and Al-Salama were regions that showed the highest contamination of airborne antibiotic-resistant bacteria. These results suggest that the selected area has a significant prevalence of multiple drug resistance to routinely used antibiotics. Due to selection pressure, contaminants like bacteria or other pollutants around garbage containers cause antibiotic resistance, which leads to a global public health crisis. Therefore, to combat antimicrobial resistance, a collaborative, multidisciplinary, and regulatory approach is required.
Keywords
Airborne bacteria
Antibiotic resistance
Waste containers
Jeddah
Saudi Arabia
1 Introduction
Globally, a considerable number of individuals are employed in the waste management sector. It is imperative to recognize that exposure to microbes poses a significant risk to those handling solid waste and discarding garbage into waste containers (Vyas et al., 2022). Organic toxic dust syndrome (ODTS), diarrhea, and respiratory system symptoms have all been associated with waste management (Poulsen et al., 1995). Solid waste generation rates have increased due to population growth, urbanization, the creation of major conurbations, and changes in society's lifestyle, which have resulted in issues with the global economy, the environment, society, and health (Wikuats et al., 2020). As Jeddah's household garbage is made up of a variety of components, such as yard waste, vegetable waste, diapers, animal waste, vacuum cleaner bags, and others, the source of exposure to domestic waste may be highly diverse (Hakami and Abu Seif, 2015; Kamli et al., 2021; Soares et al., 2023). Therefore, household garbage may unleash a variety of microbial species. Of note, antibiotics-resistant bacteria are of serious concern globally. Because of these infections, more than 700,000 deaths occur worldwide every year. By 2050, the death toll may rise to 10 million per year, making it a higher mortality rate than deaths caused by cancer (Rather et al., 2017).
(He et al., 2023). Studies on airborne bacteria have obtained a great deal of attention since some of the microorganisms in bioaerosols are thought to represent a significant risk factor for health issues (Yamamoto et al., 2012). Numerous studies have shown that several forms of airborne pathogenic microbes have adversely affected the health of humans, plants, and mammals (Chen et al., 2020). There are a plethora of pathogens detected in different bioaerosols, such as Streptococcus gallolyticus, Streptococcus mitis, Staphylococcus, Bacillus circulans, Enterococcus faecium, Staphylococcus epidermidis, Helicobacter, Arcobacter, Helicobacter, Enterobacter cloacae, Pseudomonas aeruginosa, Enterococcus haemoperoxidus, Aeromonas hydrophila, Enterococcus caselliflavus, Acinetobacter, Acinetobacter baumannii, Propionibacterium acnes, Saccharopolyspora rectivirgula, Thermoactinomyces vulgaris, Klebsiella pneumoniae, Escherichia coli, Clostridium botulinum Types C, Bacillus sp, and Ralstonia (Chen et al., 2020).
Pathogens of bacteria attract urgent attention to study the types of bacteria that are transported by air. In addition, antimicrobial resistance is a global issue with significant health. The issue may involve certain bacteria that are naturally resistant to specific antibiotics. Recently, it has been discovered that these bacteria significantly enhance the level of resistance to antibiotics commonly used in both human and veterinary medicine. (Saad B. Al-Masaudi and Saleh Mohammed Saleh Al-Maaqar, 2020).
This study aimed to investigate the presence of airborne bacteria that might affect household waste collectors and throwers during waste collection or throwing trash and learn more about how airborne bacteria are transported from outdoors around the waste.
2 Material and methods
2.1 Sample collection
The present study was undertaken in four areas (Al-Salama, Al-Zahra, Al-Safa, and Al-Faisaliyah) of Jeddah city. The laboratory work was carried out in the Department of Biological Sciences, King Abdulaziz University, Jeddah. The agar plates were exposed directly to outdoor air around the garbage containers in these areas at different time points such as one min, two mins and ten mins. The temperature around was approximately 22.5 °C–28.5 °C and humidity of 51 %. After exposure, the plates were closed using parafilm and transferred to the laboratory within an hour in a box. Then, the plates were carefully incubated at 37 °C for 24 to 48 h to see any visible colonies.
2.2 Isolation, and identification of airborne bacteria
The plates were checked after 24–48 h incubation at 37 °C and visible colonies were picked for isolation and identification of pathogenic and nonpathogenic bacteria. Of note, the cultivated bacterial colonies were counted as CFU per mL, and a single colony was picked carefully and streaked on nutrient agar. The procedure was repeated until colonies of uniform size and shape were obtained. Finally, a single purified colony was taken and cultured in NB broth at 37 °C for 24 h. The culture was preserved by adding 16 % glycerol and stored at −80 °C.
2.3 Antimicrobial susceptibility tests
Following CLSI guidelines, the conventional Kirby-Bauer disk diffusion method was used to determine the isolates' antibiotic susceptibility profile after the bacteria were isolated and identified from each sample taken (CLSI, 2017). Briefly, Mueller Hinton Agar Media (MHA) was inoculated with bacteria (106 colony-forming units/mL) (Clinical and Laboratory Standards Institute, CLSI, 2014). The following 12 commercial antibiotics were used; Ampicillin (AMP) 10 mcg, amikacin (AK) 20 mcg, augmentin (AMC) 30 mcg, cephotaxime (CTX) 30 mcg, ciprofloxacin (CIP) 5 mcg, cefuroxime (CXM) 30 mcg, cefoperazone (CPZ) 75 mcg, ceftazime (CAZ), gentamicin (GEN), netilmincin (NET) 30 mcg, ofloxacin (OF) 5 mcg, norfloxacin (NX) 10 mcg. The discs were purchased from Micromaster Laboratories Pvt. Ltd. Maharashtra, India. Following molecular identification, each pure colony was spread on MH agar, and antibiotic discs were placed using sterile forceps. The plates were then incubated at 37 °C for 24–48 h. Finally, the zone of inhibition was measured around each disc.
2.4 MAR index
The MAR index was checked by using the formula (a/b), where “a” is the number of antibiotics a particular bacterium shows resistance and “b” depicts the total number of antibiotics tested against each bacterium.
2.5 Molecular characterization of bacterial isolates
For molecular characterization, a QIAGEN kit was used to extract the genomic DNA from each bacterial isolate by following the guidelines of the manufacturer. In brief, 5 mL of each isolate was cultured overnight in NB broth. Approximately, 1.75 mL culture was taken into an ependof tube and subjected to centrifugation for 5 mins at 15000 X g. The supernatant was discarded and the pellet lysis enzymatic buffer was added (180 μL), and vortexed for 20 s. The solution was incubated at 37 °C for 30 mins. After incubation, proteinase K and alkaline lysis buffer of 25 μL and 200 μL were added, respectively. Following this, the mixture was again incubated for 20 mins at 56 °C. Further, 200 μL of 100 % ethanol was added, and the solution was transferred to a mini spin column then it was centrifuged at 15000 X g for 1 min. Following this, the filtrate was collected and discarded. Then, 500 μL of AW2 was added and it was again centrifuged at 15000 X g for 3 min. After discarding the filtrate, 100 μL of water (nuclease-free) was added the and column was again centrifuged at 15000 X g for 1 min. The collected DNA sample was carefully stored at −20 °C.
The 16 s rRNA gene was amplified using universal primers, 27F (5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ) 1492R (5ʹ-AAGGAGGTGATCCAGCCGCA-3ʹ) for all isolates following the methoddescribedrbed elsewhere (Adedokun et al., 2014; Ahmad Rather et al., 2013; I.A. Rather et al., 2015; Irfan A. Rather et al., 2015). The sequences were run using the BLASTN search engine. The reference sequences were used to construct a phylogenetic tree. The sequences were also submitted in GenBank for accession number.
3 Results
3.1 Isolation and identification
A total of thirty-eight bacteria were isolated from the 40 air samples examined. These samples were obtained from different districts, including Al-Salama, Al-Safa, Al-Zahra, and Al-Faisaliyah districts in Jeddah, Saudi Arabia (Table 1). All the strains were isolated and purified using non-selective media and purified using a Nutrient Agar (NA) medium. The meteorological data, including temperature and relative humidity (RH), are reported as the average values over a 1-hour sampling period for each sample. The molecular characterization was carried out using 16S rRNA gene sequencing. The isolates belong to different genera according to the results of this study as shown in Fig. 1.
Airborn Isolates
Isolates per location
(%)
Al-Salama
Al-Zahra
Al-Safa
Al-Faisaliyah
(10 plates)
(10 plates)
(10 plates)
(10 plates)
Kocuria spp.
3
0
2
0
5 (13.15 %)
Glutamicibacter protophormiae
0
1
1
0
2 (5.26 %)
Nocardioides jiiishulii
0
0
0
1
1 (2.6 %)
Pontibacter spp.
1
2
1
0
4 (13.15 %)
Streptococcus spp.
0
1
0
0
1 (2.6 %)
Aerococcus urinaeequi
0
0
2
1
3 (7.9 %)
Mesobacillus maritimus
1
0
0
0
1 (2.6 %)
Bacillus spp.
3
0
3
1
7 (18.15)
Lysobacter defluvii
0
0
0
1
1 (2.6 %)
Massilia agri
0
0
1
0
1 (2.6 %)
Pseudomonas spp.
0
1
1
0
2 (5.2 %)
Acinetobacteria schindleri
0
1
0
0
1 (2.6 %)
Franconibacter helveticus
0
1
0
0
1 (2.6 %)
Mixta spp.
1
0
1
0
2 (5.2 %)
Pseudorhizobium halotolerans
0
0
0
1
1 (2.6 %)
Brevundimonas spp.
1
1
0
0
2 (5.26 %)
Sphingomonas spp.
1
0
1
1
3 (7.9 %)
Total Isolates
11
8
13
6
38 (100 %)
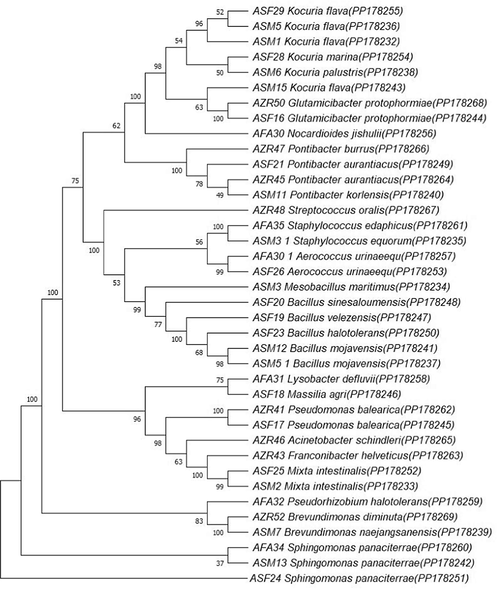
Phylogenetic tree of airborne bacteria isolated near waste containers in Jeddah, Saudi Arabia.
3.2 Antibiotics susceptibility profile
The antibiotic sensitivity test of isolates was conducted against twelve antibiotics. These antibiotics are often used in Saudi Arabia. Fig. 2 depicts the antimicrobial susceptibility pattern against twelve commercially available antibiotics. Out of thirty-eight airborne isolates, a vast number showed sensitivity. However, many showed resistance to one or more than one antibiotics (Fig. 2 and Fig. 3). Therefore, the outcomes of this study focused on ten bacteria that showed resistance against one or more than one antibiotic tested. The isolates included Kocuria flava, Mesobacillus maritimus, Kocuria palustris, Aerococcus urinaeequi, Kocuria marina, Staphylococcus edaphicus, Franconibacter helveticus, Acinetobacter schindleri, Streptococcus oralis, and Brevundimonas diminuta.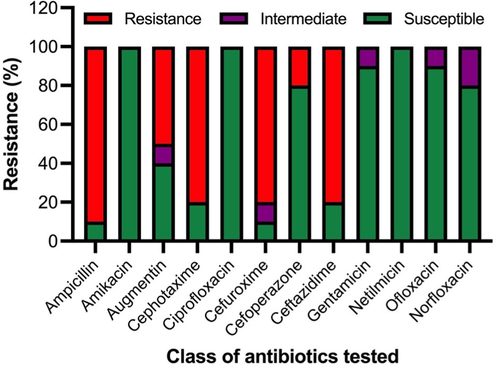
Antibiotic susceptibility profile of airborne isolates against twelve different antibiotics.
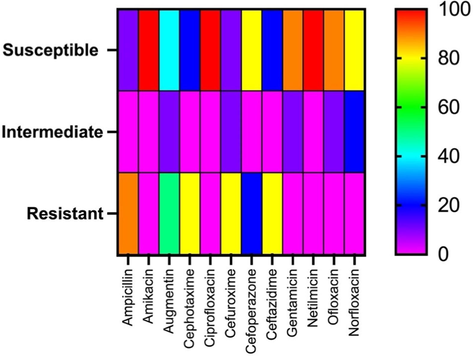
Heatmap of isolates showing susceptibility, intermediate susceptibility, and resistance against twelve antibiotics tested.
Further, it was seen that from the four different locations selected for sample collection, the Al-Salama district was the most contaminated one (30.43 %), followed by the Al-Safa (27.5 %), then the Al-Zahra district (21.7 %), while the lowest contamination was seen in Al-Faisaliyah (20.3 %) sample plates (Fig. 4). These results underpin that there is a variable number of bacteria (colonies) that varies from one neighborhood to another and from one region to another. Similar results were observed by other researchers in studies conducted in the Taif neighborhoods (Mahdy and El-Sehrawi, 1997), and other regions of the Kingdom of Saudi Arabia (Angelakis et al., 2014; Hameed and Habeeballah, 2013). According to Jedlicka et al., the air current was demonstrated to be sufficient to allow the airborne escape of microorganisms contained in the container, including pathogenic bacteria (Jedlicka et al., 2012).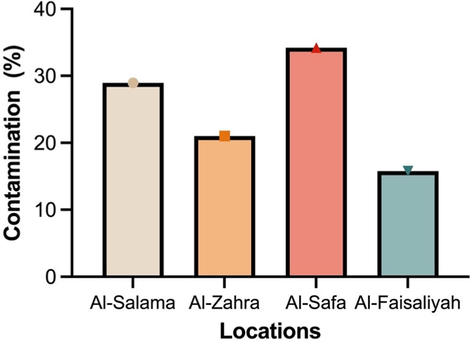
Region-based contamination with airborne isolates resistant to multiple antibiotics.
Of note, it is reported that not only in Jeddah but also in Makkah al-Mokaarama, Taif, and other parts of the world, such as Japan, the United Kingdom, Mexico, and Germany, the bacteriological contamination of waste containers is a significant issue (Büchner et al., 2021; Jedlicka et al., 2012; Madsen et al., 2016). The results of this study are in agreement with previous studies conducted reseachers elsewhere (Calderón-Ezquerro et al., 2022; Saha and Rao, 2017; Sultan et al., 2020), as well as Mesobacillus maritimus identified by(Patel and Gupta, 2020).
3.3 Multiple antibiotic resistance
To check the multiple antibiotics resistance pattern, the ten selected isolates were subjected to tests against twelve antibiotics, which are mostly used in Saudi Arabia. Of note, all the isolates (n = 10; 100 %) showed resistance to one or more one antibiotics. However, nine isolates showed resistance against ampicillin, and only one strain Aerococcus uninaeequi was found susceptible against ampicillin. Additionally, eight isolates (n = 8; 80 %) such as Kocuria flava, Mesobacillus maritimus, Aerococcus urinaeequi, Kocuria marina, Franconibacter helveticus, Acinetobacter schindleri, Streptococcus oralis, and Brevundimonas diminuta showed resistance against cephotaxime, cefuroxime, and ceftazidime. While as augmentin resistance was observed against five isolates (n = 5; 50 %), such as Mesobacillus maritimus, Staphylococcus edaphicus, Acinetobacter schindleri, Streptococcus oralis, and Brevundimonas diminuta. Of note, all ten isolates (n = 10; 100 %) showed susceptibility against amikacin, ceprofloxacine, and netilmicin. Overall, the highest MAR index was shown by Brevundimonas diminuta (0.5), followed by Streptococcus oralis (0.41), Acinetobacter schindleri (0.41), Mesobacillus maritimus (0.33), Staphylococcus edaphicus (0.33), Kocuria marina (0.33), Kocuria palustris and Aerococcus urinaeequi with 0.16, respectively. Fig. 5 shows the distinctively observed MAR index patterns among the selected isolates. (See Fig. 6).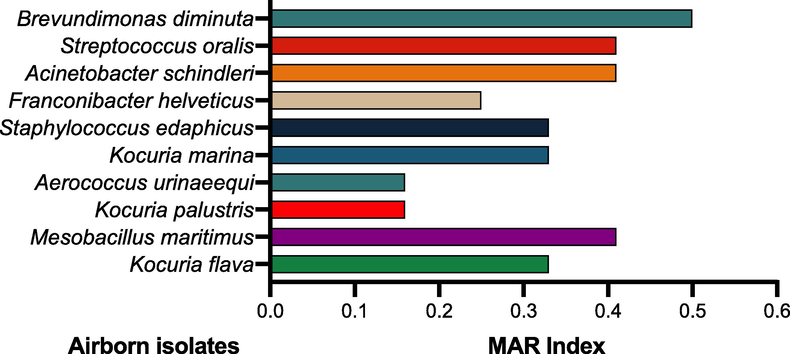
Multiple Antibiotic Resistance (MAR) index values of airborne isolates against twelve tested antibiotics.
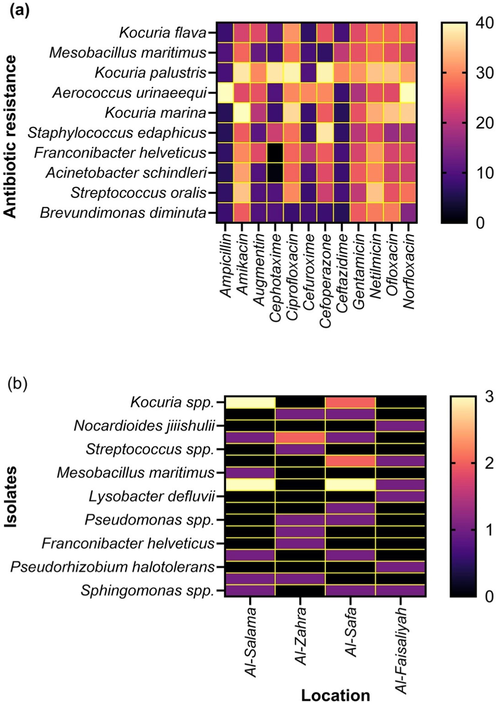
Heatmap of antibiotics-resistant strains. (a) Strain-based antibiotics susceptibility. (b). Number of isolates per location.
4 Discussion
The current research focuses on the characterization of multidrug-resistant airborne bacteria isolated from Jeddah, Saudi Arabia. Unfortunately, there is a dramatic increase in multidrug-resistant bacteria throughout the world, especially in underdeveloped countries, where antibiotics are available over the counter (Magiorakos et al., 2012; Soares et al., 2023). Besides, the use of antibiotics in livestock is yet another factor for an increase in antibiotic resistance. Of note, overdosing of pesticides and other chemicals in agricultural production further imbalances the microflora of soil. This in turn affects humans, wildlife, and many other creatures.
Our study shows the highest antibiotic resistance against ampicillin. The high resistance was also seen against most commonly used antibiotics such as cefuroxime, cefrazidime and augmentin. These results underpin the intervention of antibiotic stewardship. Several studies have also reported high levels of resistant bacteria throughout the world (Sabri et al., 2020; Yu et al., 2020; Yuan et al., 2024).
The geographical variation in the distribution of antibiotic-resistance genes is a complex issue. Many factors contribute to this variation, including the use of antibiotics, the environment, and the genetic makeup of bacteria. However, one of the main causes of antimicrobial pollution is from antibiotic-producing wastewater and hospitals. Globally, contaminated water bodies are mainly responsible for antibiotic-resistant crises. Therefore, it becomes compulsory to have effective wastewater management, monitoring hospital wastes, sewage, and other waste dupping sources. Failing this would result in spreading of antibiotic resistant bacteria.
The environmental spread of antibiotic-resistant bacteria has been widely documented. According to Pruden et al., wastewater treatment plants, agricultural activities, and urban waste are significant sources of antibiotic residues and resistant bacteria (Pruden et al., 2006). These sources contribute to the selection pressure that drives the evolution of resistance (Kümmerer, 2009). The role of waste disposal sites, particularly garbage containers, as reservoirs of resistant bacteria has been highlighted in several studies. For instance, Czekalski et al. found that waste sites often harbor high concentrations of antibiotic-resistant genes due to the accumulation of human and animal waste (Czekalski et al., 2014).
Airborne transmission is a critical pathway for the dissemination of antibiotic-resistant bacteria. Many researchers have reported that bacteria can become aerosolized through activities such as waste handling and traffic (Gandolfi et al., 2015; Wang et al., 2020). Once airborne, these bacteria can travel significant distances, posing a risk to human health. Several studies reported, that the presence of antibiotic-resistant bacteria in urban air was linked to nearby waste treatment facilities and heavy traffic areas (Banchón et al., 2021; Rosas et al., 2018).
The prevalence of antibiotic-resistant bacteria in urban air environments has been documented in various global contexts. Researchers have identified multiple antibiotic-resistant bacteria in the air of several Chinese cities, noting a strong correlation with industrial activities and urban waste management practices (Al-Saleh et al., 2022; Banchón et al., 2021; Mao et al., 2019; Zhuang et al., 2021). Similarly, research conducted in European cities has shown high levels of airborne antibiotic-resistant bacteria in areas with dense human activity and inadequate waste management (Soares et al., 2023).
Of note, in the past number of reports have been published highlighting the possible risks of antibiotic-resistant pathogens (Czekalski et al., 2014; Mao et al., 2019; Sabri et al., 2020; Sultan et al., 2020; Yu et al., 2020; Yuan et al., 2024). The present situation has become more alarming due to an increase in the number of antibiotic-resistant pathogens. New studies published recently reveal the presence of new microbial species resistant to antibiotics, and there are limited options to treat infections caused by these microbes (Nogrady, 2023). Since no new antibiotics have been discovered to combat antibiotic-resistant crises. Therefore, the decrease in antibiotic use in addition to its awareness about its misuse could help to tackle this issue of antibiotics.
The findings from Jeddah city underscore the urgent need for improved waste management practices and robust environmental monitoring to mitigate the spread of antibiotic-resistant bacteria. The high prevalence of multiple drug-resistant bacteria around garbage containers suggests that these sites are critical hotspots for the development and dissemination of resistance. Future research should focus on identifying specific sources of contamination, understanding the mechanisms of airborne transmission, and developing strategies to reduce environmental antibiotic resistance.
5 Conclusion
The increasing prevalence of antibiotic-resistant bacteria in the environment, especially around urban waste sites is concerning. Air contamination has become a critical issue that requires urgent and thorough investigation by the scientific community. Here, we examined the pervasiveness of antibiotic-resistant bacteria from air around garbage containers in Jeddah city of Saudi Arabia. Several strains showed multidrug resistance patterns. Given these factors, it is crucial to conduct studies in natural aquatic environments to better understand the epidemiological scenarios where resistance mechanisms may have clinical relevance. Thus, the research area has a significant prevalence of multiple drug resistance to routinely used antibiotics. Due to selection pressure, the presence of contaminants like bacteria or other pollutants around garbage containers causes resistance to antibiotics. Taking antibiotic-resistant organisms close to waste containers seriously should not be overlooked as a source of novel antibiotic-resistant genes. Therefore, proper waste containers should be established to prevent exit germs, and improved sanitary measures should be practiced to limit the spread of pathogenic microbes that are resistant to antibiotics used commonly. Following these measures would help to curb the spread of antibiotic-resistant microbes and protect both the enironment and human health.
CRediT authorship contribution statement
Raed Albiheyri: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation and characterization of lactic acid bacteria from Nigerian fermented foods and their antimicrobial activity. J. Pure Appl. Microbiol.. 2014;8
- [Google Scholar]
- Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Lett. Appl. Microbiol.. 2013;57
- [CrossRef] [Google Scholar]
- Susceptibility of multidrug-resistant enteric pathogenic diarrheal Bacteria to Saudi Honey. journal of King Abdulaziz University. Science. 2020;32:47-64.
- [CrossRef] [Google Scholar]
- Trends in methicillin-resistant Staphylococcus aureus in the Gulf Cooperation Council countries: antibiotic resistance, virulence factors and emerging strains. East. Mediterr. Health J. 2022
- [CrossRef] [Google Scholar]
- MALDI-TOF mass spectrometry and identification of new bacteria species in air samples from Makkah, Saudi Arabia. BMC Res. Notes. 2014;7
- [CrossRef] [Google Scholar]
- Airborne Bacteria from Wastewater Treatment and Their Antibiotic Resistance: A Meta-Analysis. J. Ecol. Eng.. 2021;22
- [CrossRef] [Google Scholar]
- Do closed waste containers lead to less air contamination than opened? A clinical case study at Jena University Hospital, Germany. Waste Manage.. 2021;136
- [CrossRef] [Google Scholar]
- A review on airborne microbes: The characteristics of sources, pathogenicity and geography. Atmosphere (Basel) 2020
- [CrossRef] [Google Scholar]
- CLSI, 2017. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and La. InternatioCLSI. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.nal journal of STD & AIDS 18.
- Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. ISME J.. 2014;8
- [CrossRef] [Google Scholar]
- Calderón-Ezquerro, M. del C., Gómez-Acata, E.S., Brunner-Mendoza, C., 2022. Airborne bacteria associated with particulate matter from a highly urbanised metropolis: A potential risk to the population’s health. Front. Environ. Sci. Eng. 16. DOI: 10.1007/s11783-022-1552-5.
- Spatio-temporal variability of airborne bacterial communities and their correlation with particulate matter chemical composition across two urban areas. Appl. Microbiol. Biotechnol.. 2015;99
- [CrossRef] [Google Scholar]
- Household Solid Waste Composition and Management in Jeddah City, Saudi Arabia: A planning model. International Research Journal of Environment. Sciences. 2015;4
- [Google Scholar]
- Air Microbial Contamination at the Holy Mosque, Makkah, Saudi Arabia. Curr. World Environ. J.. 2013;8
- [CrossRef] [Google Scholar]
- On the triad of air PM pollution, pathogenic bioaerosols, and lower respiratory infection. Environ. Geochem. Health 2023
- [CrossRef] [Google Scholar]
- Genome-driven discovery of enzymes with industrial implications from the genus aneurinibacillus. Microorganisms. 2021;9
- [CrossRef] [Google Scholar]
- Antibiotics in the aquatic environment - A review - Part I. Chemosphere 2009
- [CrossRef] [Google Scholar]
- Waste Workers’ Exposure to Airborne Fungal and Bacterial Species in the Truck Cab and during Waste Collection. Ann. Occup. Hyg.. 2016;60
- [CrossRef] [Google Scholar]
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.. 2012;18
- [CrossRef] [Google Scholar]
- Airborne bacteria in the atmosphere of El-Taif region, Saudi Arabia. Water Air Soil Pollut.. 1997;98
- [CrossRef] [Google Scholar]
- Comparison of culturable antibiotic-resistant bacteria in polluted and non-polluted air in Beijing, China. Environ. Int.. 2019;131
- [CrossRef] [Google Scholar]
- Patel, S., Gupta, R.S., 2020. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus bacillus: Proposal for six new genera of bacillus species, peribacillus gen. nov., cytobacillus gen. nov., mesobacillus gen. nov., neobacillus gen. nov., metabacillus gen. nov. and alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 70. DOI: 10.1099/ijsem.0.003775.
- Poulsen, O.M., Breum, N.O., Ebbehøj, N., Hansen, Å.M., Ivens, U.I., van Lelieveld, D., Malmros, P., Matthiasen, L., Nielsen, B.H., Nielsen, E.M., Schibye, B., Skov, T., Stenbaek, E.I., Wilkins, C.K., 1995. Collection of domestic waste. Review of occupational health problems and their possible causes. Sci. Total Environ. DOI: 10.1016/0048-9697(95)04524-5.
- Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Tech.. 2006;40
- [CrossRef] [Google Scholar]
- Antiviral mode of action of lactobacillus plantarum yml009 on influenza virus h1n1. Bangladesh J. Pharmacol.. 2015;10
- [CrossRef] [Google Scholar]
- Rather, Irfan A., Bajpai, V.K., Park, Y.-H., 2015. In Vitro and In Vivo Inhibition of Atopic Dermatitis (AD) by a Novel Probiotic Isolate Lactobacillus sakei Probio-65. DOI: 10.1007/978-3-319-23213-3_2.
- Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci.. 2017;24
- [CrossRef] [Google Scholar]
- Rosas, I., Calderón, C., Salinas, E., Lacey, J., 2018. Airborne Microorganisms in A Domestic Waste Transfer Station, in: Aerobiology. DOI: 10.1201/9781315136943-8.
- Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng.. 2020;8
- [CrossRef] [Google Scholar]
- Biosurfactants- a current perspective on production and applications. Nat. Environ. Pollut. Technol. 2017
- [Google Scholar]
- Soares, G.G., Campanini, E.B., Ferreira, R.L., Damas, M.S.F., Rodrigues, S.H., Campos, L.C., Galvão, J.D., Fuentes, A.S. da C., Freire, C.C. de M., Malavazi, I., Pitondo-Silva, A., Cunha, A.F. da, Pranchevicius, M.-C. da S., 2023. Brevundimonas brasiliensis sp. nov.: a New Multidrug-Resistant Species Isolated from a Patient in Brazil . Microbiol. Spectr. 11. DOI: 10.1128/spectrum.04415-22.
- Bacterial isolates harboring antibiotics and heavy-metal resistance genes co-existing with mobile genetic elements in natural aquatic water bodies. Saudi J Biol Sci. 2020;27
- [CrossRef] [Google Scholar]
- Municipal solid waste management: Dynamics, risk assessment, ecological influence, advancements, constraints and perspectives. Sci. Total Environ. 2022
- [CrossRef] [Google Scholar]
- Characteristics of microbial activity in atmospheric aerosols and its relationship to chemical composition of PM2.5 in Xi’an, China. J. Aerosol Sci. 2020;146
- [CrossRef] [Google Scholar]
- Assessment of airborne particles and bioaerosols concentrations in a waste recycling environment in Brazil. Sci. Rep.. 2020;10
- [CrossRef] [Google Scholar]
- Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J.. 2012;6
- [CrossRef] [Google Scholar]
- Is there emergence of β-lactam antibiotic-resistant streptococcus pyogenes in China? Infect Drug Resist. 2020;13
- [CrossRef] [Google Scholar]
- Antibiotics and antibiotic resistance genes in aquatic environments in China: Occurrences and risks. Kexue Tongbao/Chin. Sci. Bull. 2024
- [CrossRef] [Google Scholar]
- Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021
- [CrossRef] [Google Scholar]







