Translate this page into:
Adsorptive removal of Pb(II) ions from groundwater samples in Oman using carbonized Phoenix dactylifera seed (Date stone)
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
In the present work, the potential use of raw date stone as an inexpensive carbonized adsorbent material for removal of Pb(II) has been demonstrated.
Methods
The adsorption of Pb(II) ions onto carbonized date stone has been studied by batch adsorption method. The variables (pH, adsorbent dose and initial concentration of Pb(II) ions) of the adsorption process were optimized by response surface methodology via Box-Behnken design (BBD).
Results
The optimum values of pH, adsorbent dose and initial concentration were 5.0, 0.3 g and 5.0 mg L−1, respectively to achieve 88.50% removal efficiency. The adsorption data fitted well to both Langmuir and Freundlich isotherm models with coefficient of correlation (r) ˃0.9999. The maximum adsorption capacity of the described adsorbent material for Pb2+ ions with Langmuir model was found to be 9.03 mg g−1. Kinetic data obtained at different concentrations have been analysed using pseudo-first-order and pseudo-second-order kinetic models. The experimental data obeyed pseudo-second-order kinetic model. Gibb’s free energy change (ΔG°) was evaluated and resulted with −17.91 kJ mol−1 at 298 K, hence indicated favourable adsorption process.
Conclusion
Neghal carbonized date stone was found to be a promising natural adsorbent for removal of Pb(II).
Keywords
Pb(II) ions
Carbonized date stone
Adsorption isotherms
Ground water samples
1 Introduction
The water contaminated with heavy metals is unsafe for drinking purposes because of their toxicity (World Health Organization, 2011; Azmi et al., 2013). The region around Muscat, Oman, is rich in metallic deposits which are associated with the ophiolite rocks such as basalt that contains Fe, Ca, Na, Mg, Cu, Au, Ag and Pb. In addition, Oman copper is generally associated with high amounts of lead. The reason of high concentration of Pb(II) in selected water sources may be due to leaching from the rocks (Yaghi, 2007; Al-Raisi et al., 2014). Lead enters the human body through air, water, soil, food and dust (Schroeder and Tipton, 1968). Lead even at extremely low concentrations causes brain damage in kids (World Health Organization, 2011), anemia (Moore, 1988) and reproductive effects (Wildt et al., 1983). World Health Organization (World Health Organization, 2011) and Directorate General for specifications and measurements, Oman (OS, 2012) have set a permissible limit of 0.01 mg L−1 Pb(II) for un-bottled drinking water. Lead is sometimes released during metallurgical processes and contaminate water bodies (Mishra and Patel, 2009). Due to strict environmental protection legislation concerning public health, several treatment methods such as electrochemical treatment (Thien-Khanh et al., 2017), ion exchange (Azarudeen et al., 2015), chemical precipitation (Wang et al., 2017) and adsorption (Gupta et al., 2004; Rahman and Haseen, 2014; Rahman et al., 2020) have been used for the remediation of heavy metals from water.
Bio-sorption is a very promising field utilizing natural biological materials such as rice straw (Amer et al., 2017), eggshell (Soares et al., 2016), jatoba (Isis et al., 2017), black walnut (Lawal et al., 2017), chitosan and sodium citrate (Pu et al., 2017) for remediation of Pb(II) from water. Peanut shell-based biochar was investigated for adsorption of Pb(II) ions (Tasar and Ozer, 2020). Activated carbon prepared from Albizia lebbeck and Melia azedarach seeds were applied for adsorptive removal of Pb(II) from wastewater (Ullah et al., 2020). The potential of activated carbon derived from Reptonia buxifolia was explored for removal of Pb(II) from wastewater (Bilal et al., 2020). Obayomi et al., 2019 have prepared activated carbon from groundnut shell through chemical activation for Pb(II) sorption from aqueous solution. The equilibrium modeling for the sorption of Pb(II) onto activated carbon from olive branches was studied (Alkherraz et al., 2020). Additionally, a review on the use of naturally occurring adsorbents for removal of metal ions has also been reported (Singh et al., 2020). Date palm (Phoenix dactylifera L.) is an important plant in Oman whose date stones are waste material and easily available as a source of activated carbon. Date stones have been extensively used as carbonaceous adsorbents by physical (Al-Ghoutia et al., 2010) and chemical activations (Al-Dawsari et al., 2017). The preparation of activated carbonized date stone is easy and its lead(II) removal efficiency is higher (Abudaia et al., 2013). Fresh water resources such as well, falaj and wadi are uneven in Oman (Zidi et al., 2017). Therefore, carbonized date stone can be used to remove Pb(II) from groundwater samples to cater the need of the people.
Conventional method such as one-variable-at-a-time (OVAT) approach is frequently used to optimize the analytical methods and adsorption processes (Azmi et al., 2016; Rahman and Nasir, 2017; Rahman et al., 2016) which is considered to be time consuming and expensive. To overcome these limitations, response surface methodology (RSM) was used for optimization of process variables to achieve the best response. Recently, RSM with Box-Behnken design was used to optimize the variables of adsorption process (Rahman and Nasir 2018) and spectrophotometric method (Rahman et al., 2019). Central composite design under RSM was also utilized to optimize the variables of adsorptive removal of Ni(II) and acetaminophen using N-(((2-((2-Aminoethyl)amino)ethyl)amino)methyl)-4-sulfamoylbenzamide impregnated hydrous zirconium oxide and Ca(II)- doped chitosan/β-cyclodextrin as adsorbents, respectively (Rahman and Nasir, 2019; Rahman and Nasir, 2020).
This study described the adsorption of Pb(II) ions onto carbonized date stone using batch adsorption experiments in aqueous solutions. Adsorption data were analyzed by isotherm and kinetic models. thermodynamics studies were also performed.
2 Materials and methods
2.1 Apparatus
Atomic absorption spectrometer (Thermo-Scientific, iCE 3500 series, UK) was used to determine Pb(II) ions in test and real samples. Hanna pH meter (USA) was used to measure pH of solutions. Electrical linear plate shaker (SM-30, Edmund Bühler GmbH, Germany) was used to stir solution at 120 revolutions per minute (rpm). Electrical vacuum pump (Rocker 300, Taiwan) was used for filtration.
2.2 Standard and samples solutions
A standard aqueous solution of 100 mg L−1 lead(II) was prepared in 250 mL demineralized water and maintained at pH 5. The ground water samples were collected in new pre-cleaned polypropylene bottles (450 mL capacity) from different locations of Oman such as Al-Jarda well (Ash Sharqiyah region), Wadi Andem and Falaj Samail (Ad Dakhilliyah region) in the morning time between 7:00 to 9:00 AM. Sampling procedure was adopted as per the grab method (Canadian Council of Ministers of the Environment, 2011). Water samples from Al-Jarda well and Wadi Andem were collected from 2 directions (North and East). The properties of water samples are given in Table 1.
Parameter
Al-Garda well (East)
Al-Garda well (North)
Samail Falaj
Wadi Endam (East)
Wadi Endam (North)
pH
7.40
7.56
7.48
7.52
7.25
Total hardness (mg L−1)
340.70
355.60
327.45
345.85
378.22
Calcium (mg L−1)
80.50
74.60
85.24
78.19
87.23
Magnesium (mg L−1)
32.28
29.46
31.64
34.52
30.46
Chloride (mg L−1)
65.10
72.08
80.42
78.20
86.38
Sulfate (mg L−1)
48.28
40.19
58.41
64.72
56.35
Fe3+ (mg L−1)
2.34 × 10−1
1.81 × 10−1
2.13 × 10−1
2.42 × 10−1
2.84 × 10−1
Pb2+ (mg L−1)
7.33 × 10−2
9.73 × 10−2
2.20 × 10−1
5.65 × 10−1
5.48 × 10−1
All ground water samples were stored at 4 °C and the pH was maintained at 5 with HNO3 prior to the determination of lead(II) ions by atomic absorption spectrophotometry. Neghal (a productive date palm tree in Oman) date stones were collected from Nizwa, Oman.
2.3 Method for analysis of lead in ground water samples
The absorbance of solutions containing varying concentration of Pb(II) ions was measured at 217 nm using atomic absorption spectrometer (AAS) against reagent blank. The linear regression equation was generated using OriginPro 6.1 Software (USA). The absorbance of ground water samples was recorded (pH maintained to 5). The amount of lead ions in ground water samples was computed using linear regression equation.
2.4 Method for preparation of activated carbonized date stones
Date stones of Neghal were taken and washed properly with distilled water. The date stones were dried at room temperature. The dried date stones were soaked in hot water for at least 1 h to remove soft materials and impurities from date stones. The date stones were cleaned with hot water, then with doubly distilled water and kept under sun light for 3 h. Dried date stones were transferred into crucible and heated under fume hood with Bunsen burner flame until all of the white fumes came out. Roasted date stones were kept inside the furnace at 600 °C for 15 min and thereafter, powdered using mortar and pestle. The powdered carbonized date stones were sieved using 120 mesh sieve (particle size < 125 μm). The filtered powder was transferred into a sample tube and kept in desiccator. Carbonized date stone (5 g) was taken and washed with 5 × 50 mL of doubly distilled water. The material was filtered using vacuum pump with glass crucible (Duran No. 4) and washed with distilled water until the pH of the filtrate became 7.
2.5 Experimental design
In Box-Behnken design (BBD) (Rahman and Nasir, 2018), three factors were selected: pH (A; 2.5–7.5), adsorbent dose (B; 0.10–0.40 g) and initial concentration (C; 1.5–8.5 mg L−1).Design Expert software (free trial 11.1.0.1 version) was used to generate the BBD matrix and experiments were conducted accordingly to evaluate the effect of three main independent variables on the Pb (II) removal efficiency. For response surface methodology, the experimental data were fitted to the second order quadratic model using the following equation:where y represents the predicted response, β0 is the constant coefficient. βi, βii and βij are the, linear, quadratic and interaction coefficients of input variables, respectively. Analysis of variance (ANOVA) was performed to determine the significance of model terms.
2.6 Adsorption procedure
0.3 g of carbonized date stone was transferred into different dried 100 mL stoppered conical flasks. To each flask, 50 mL of each Pb(II) ions solution (concentration range: 1.5 to 5.0 mg L−1; pH maintained to 5) was added and placed on electrical linear shaker for 2 min at 120 rpm at 25 °C. The solution was filtered using vacuum pump with glass crucible (Duran No.4) and concentration of Pb(II) in the filtrate was determined by AAS. The uptake of Pb(II) ions by carbonized date stone was calculated using the following equation:where qe is the amount of Pb(II) ions adsorbed (mg g−1), V and m are the volume of solution (litre) and mass of adsorbent (g), respectively.
The % removal of lead(II) ions was calculated using the equation:where Ci and Ce are initial and equilibrium concentrations in mg L−1 at t = 0 and t = equilibrium time, respectively.
3 Results and discussion
The SEM images of carbonized date stone (before and after adsorption of Pb(II) from aqueous standard solutions are shown in Fig. 1 a & b which revealed the adsorption of lead(II) ions on irregular and fibrous surfaces of adsorbent. The energy dispersive spectroscopic mapping was also performed (Fig. 2). Fig. 2 showed the presence of Pb(II) ions in the material after adsorption.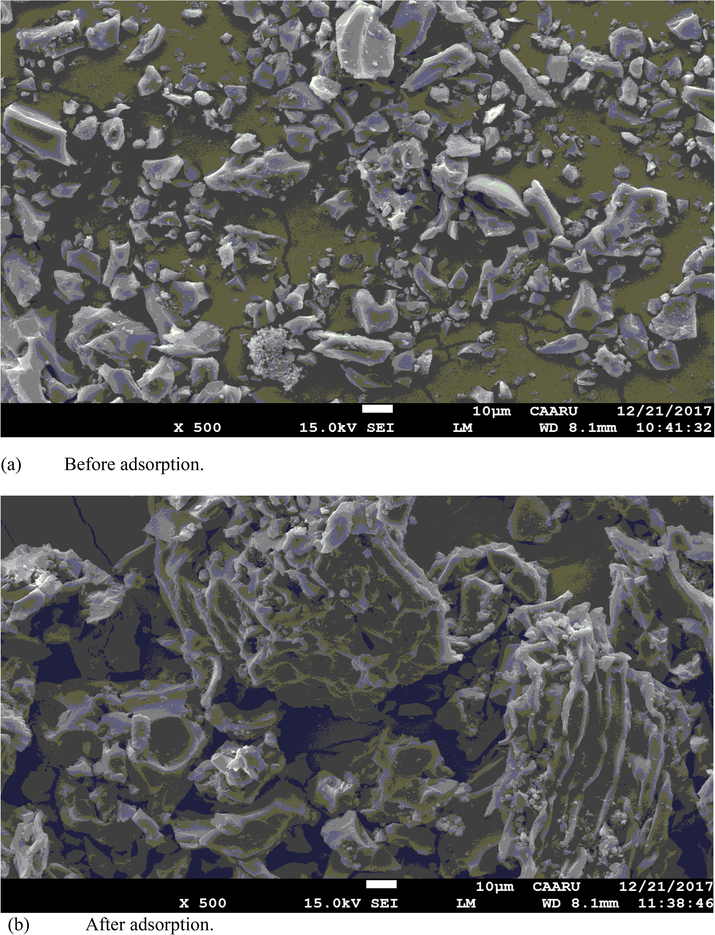
SEM images of carbonized date stone (a) before adsorption and (b) after adsorption from aqueous standard solutions.
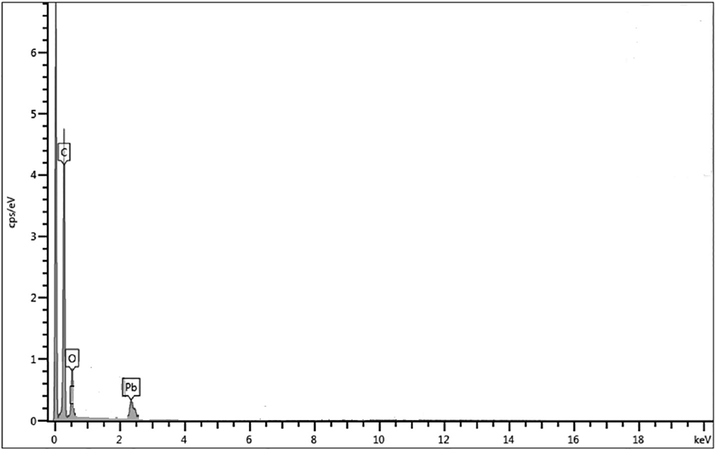
EDX spectrum of carbonized date stone after adsorption from aqueous standard solutions.
3.1 Effects of contact time and revolutions per minute
The effect of contact time on % removal of Pb(II) by carbonized date stone was studied and maximum % removal of Pb(II) was achieved in 1 min. Therefore, 2 min of contact time was recommended as the optimum contact time for further studies. The effect of rpm on percent Pb(II) removal was studied using 5 mg L−1 Pb(II) solution and 0.3 g of adsorbent. The maximum removal was obtained at 100 rpm, beyond this up to 150 rpm, the % removal was constant Hence 120 rpm was selected for further adsorption studies.
3.2 Box-Behnken statistical analysis
The proposed BBD requires 17 experimental runs to model the response surface. The experiments were conducted with different sets of input parameters and the percent removal of Pb(II) was measured for each set of independent variables. The percent removal of Pb(II) is considered as response in the model. The removal of Pb(II) with different sets of input parameters varies in the range of 54.87–88.50%.
The experimental data were fitted to second order quadratic models to obtain the empirical relationship between the predicted response and the independent variables. The second order quadratic equation in terms of coded factors is given below:
Removal = +88.5 + 5.46 A + 3.10B −1.28C + 9.00 AB − 1.20 AC − 8.26 BC − 15.43 A2 − 6.85 B2 − 6.08 C2
The results of ANOVA for the second order quadratic model showed that F-value for the model was very high (8.11 × 106) which indicated that most of the variations in the response were explained well by the model. Moreover, all the model terms have very high F-values and lower p-values (<0.0001), suggested that all model terms have significant effect on the response. Additionally, the effects of interaction terms (AB, AC and BC) and quadratic terms (A2, B2 and C2) on the precent removal of Pb(II) are also significant at 95% confidence level.
Fig. 3 (a-c) showed the three dimensional surface plots of the quadratic model. Fig. 3 (a) showed the interaction effects of adsorbent dose and pH, keeping initial concentration constant on the removal efficiency of Pb(II). It was observed that the removal efficiency increased with increasing pH and adsorbent dose. The uptake of lead ions decreased as the acidity of the contact solution increases. The pH of the aqueous solution governs the adsorption process as it affects the surface charge of adsorbents and influences the degree of ionization of metal ions in solution. The maximum removal efficiency was achieved with 0.30 g of adsorbent at pH 5.0. The combined effect of pH and initial concentration on the present removal of Pb(II) is shown in Fig. 3 (b). As can be seen in Fig. 3 b that the removal efficiency increased with increasing concentration up to 5 mg L−1. The interactive effect of adsorbent dose and initial concentration on the removal efficiency is displaced in Fig. 3 (c). The removal efficiency increased with increasing both adsorbent dose and initial concentration and maximum percent removal was obtained with 0.3 g adsorbent using initial concentration of 5 mg L−1.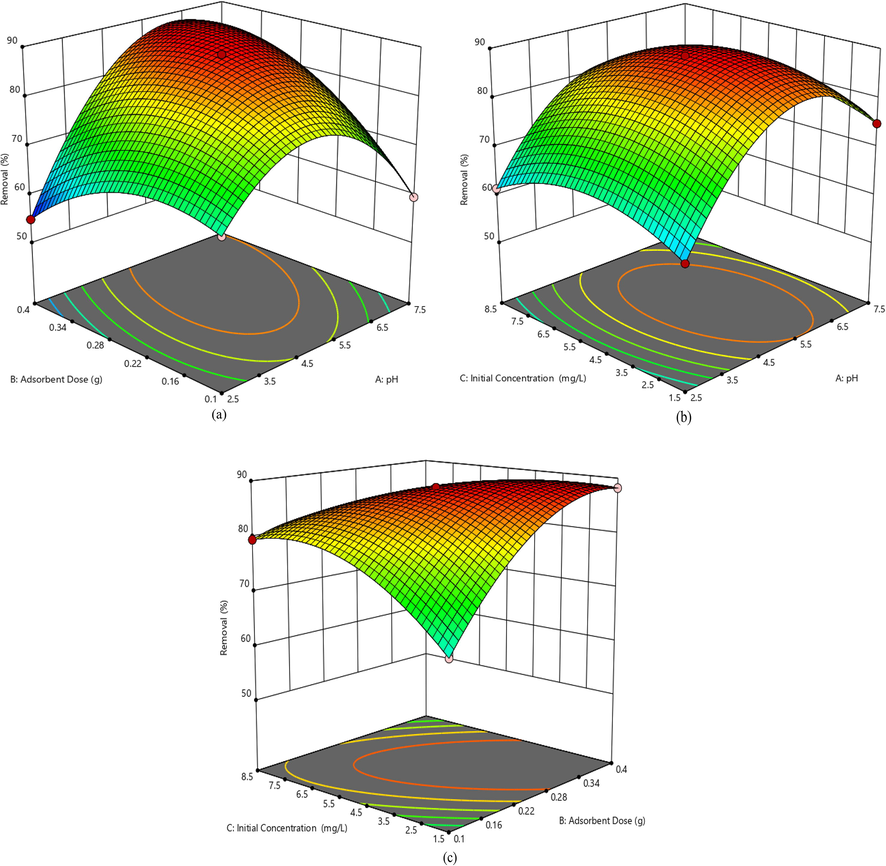
Response surface plots showing the effects of (a) pH and adsorbent dose, (b) pH and initial concentration and (c) adsorbent dose and initial concentration on the percent removal of Pb(II).
For the numerical optimization, the input variables were given specific ranged values while response was set at maximum. Under these conditions, the optimum values of each independent variable were obtained using Design Expert software. The maximum removal was 88.50% at an initial pH of 5.0, Pb(II) ions concentration of 5 mg L−1 and adsorbent dose of 0.3 g. Experiments were conducted under these conditions. The results are very close to the predicted values.
3.3 Adsorption isotherms
Equilibrium data were analysed by Langmuir and Freundlich isotherm models.
The Langmuir model is described by the following equation (Langmuir, 1918):where qm is the amount adsorbed in mg g−1 (maximum adsorption capacity); KL is the Langmuir constant in L mg−1. The graph between 1/qe and 1/Ce was plotted (Fig. 4) which yielded qm and KL values of 9.03 mg g−1 and 0.157 L mg−1, respectively with R2 = 0.9999. Carbonized date stone has the advantage of removing lead(II) ions in 1.0–2.0 min whereas reported methods (Mishra and Patel, 2009; Abudaia et al., 2013) required longer time to remove lead(II) ions.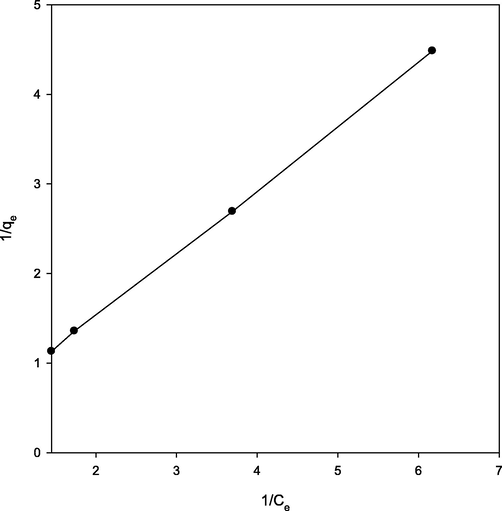
Langmuir adsorption isotherm for adsorption of Pb(II) ions at 298 K.
RL, the separation factor was calculated with KL value using the following expression:where Ci is the initial concentration in mg L−1. The value of RL predicted the nature of adsorption. Here, RL values were in the range of 0.515–0.809 at 298 K demonstrated 0 < RL < 1, hence the adsorption was favourable at room temperature.
Freundlich isotherm model was also investigated using the following equation (Rahman and Nasir, 2019):where Kf and n are related to adsorption capacity and intensity of adsorption, respectively. The graph between ln qe and ln Ce was plotted (Fig. 5), yielded Kf and n values of 1.25 and 1.06, respectively with R2 = 0.9998 which demonstrated the favourable adsorption.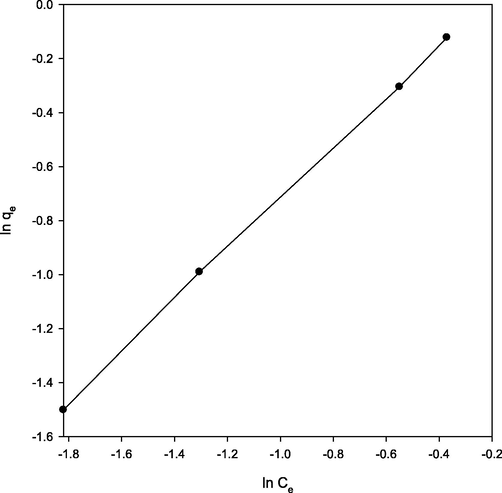
Freundlich adsorption isotherm for Pb(II) adsorption at 298 K.
The values of R2 ˃ 0.9999 were found for both Langmuir and Freundlich isotherm models. Therefore, both the models were able to describe the adsorption process very well.
3.4 Adsorption thermodynamics
The orientation and feasibility of the adsorption of lead(II) ions onto carbonized date stone was evaluated at room temperature by Gibb’s free energy, ΔG° using following expressions:
Kc = qe × 1000/Cewhere Kc is the equilibrium constant, R is universal gas constant (8.314 JK−1mol−1), T is the absolute temperature in Kelvin. If ΔG ° reaches to −20 kJ mol−1, would be the basis for electrostatic interaction between adsorbent sites and metal ions (Yao et al., 2010). Here, ΔG° was found to be −17.91 kJ mol−1. Hence, the adsorption of lead(II) ions on to carbonized date stone was spontaneous, feasible and resulted in physical adsorption process. As the temperature for adsorption of lead(II) ions increased (greater than 298 K), the sorption decreased. Hence, the sorption of lead(II) ions is endothermal in nature.
3.5 Adsorption kinetics
The Lagergren’s first-order kinetic model (Rahman and Nasir, 2019) was applied using following equation:where qt and k1 refer to metal adsorbed per gram of adsorbent at time, t in min and rate constant in min−1, respectively. ln (qe -qt) was plotted against t (Fig. 6), resulted with slope and intercept of −2.06 and −1.51, respectively. qe was calculated and found to be 0.22 which is much smaller than the experimental qe (0.737 mg g−1). Hence, the adsorption of Pb(II) ions on to carbonized date stone did not obey Lagergren first-order kinetic model.
Pseudo-first-order reaction plot for Pb(II) adsorption onto carbonized date stone.
The adsorption data were then analysed by a pseudo-second order kinetic model (Rahman and Nasir, 2019) using the equation:where k2 is the pseudo-second-order rate constant in g mg−1 min−1.
The adsorption rate, ho in mg g−1 min−1 at t → 0 was calculated by:
A curve between t/qt and t was plotted (Fig. 7) which provided slope and intercept of 1.33 and 0.077, respectively. qe, k2, and ho were calculated and found to be 0.752 mg g−1, 22.85 g mg−1 min−1 and 12.98 mg g−1 min−1, respectively. The calculated qe (0.752 mg g−1) was close to qe, exp (0.737 mg g−1) and hence the adsorption of Pb(II) ions onto carbonized date stone was best described by pseudo second-order kinetic model. This suggested that the multilayer adsorption occurred onto the surface of adsorbent.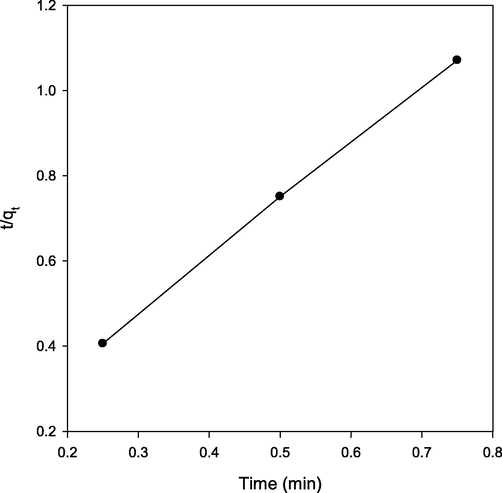
Pseudo-second-order reaction plot for Pb(II) adsorption onto carbonized date stone.
3.6 Application to environmental water samples
The carbonized date stone was applied for remediation of lead(II) ions in groundwater samples. The removal percentage of lead(II) ions in well, falaj and wadi water samples were about 78% (Table 2).
Ground water samples
Pb(II) in mg L−1
% removal of Pb(II)
RSDa (%)
Before adsorption
After adsorption
Al-Garda well (East)
7.33 × 10−2
1.61 × 10−2
78.04
2.55
Al-Garda well (North)
9.73 × 10−2
2.11 × 10−2
78.31
2.75
Samail falaj
2.20 × 10−1
4.80 × 10−2
78.18
1.56
Wadi Endam (East)
5.65 × 10−1
1.20 × 10−1
78.76
2.28
Wadi Endam (North)
5.48 × 10−1
1.20 × 10−1
78.10
2.45
The presence of other ions in real water samples slightly interfered with the adsorption of lead(II) ions under the optimized conditions. Hence, the % removal of lead(II) ions in ground water samples was found to be less than that obtained using Box-Behnken statistical analysis.
4 Conclusions
The investigation concluded that the Neghal carbonized date stone seems to be a promising natural adsorbent. The material was stable and selective for the remediation of lead(II) ions in real samples at trace level.
Adsorption process was described well by both Langmuir and Freundlich isotherm models at room temperature. ΔG ° was −17.91 kJ mol−1, hence favoured the feasibility of adsorption process.
Acknowledgement
The authors are thankful to Dean, Heads of Applied Sciences and Chemistry Section, Higher College of Technology, Muscat, Oman for the facilities. The authors are grateful to the higher-up of the Ministry of ManPower (Higher College of Technology) Muscat, Sultanate of Oman for support to carry out this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adsorption of Pb (II) and Cu (II) from aqueous solution onto activated carbon prepared from dates stones. Int. J. Environ. Sci. Develop.. 2013;4:191-195.
- [Google Scholar]
- Assessment of heavy metals in leachate of an unlined landfill in the Sultanate of Oman. Int. J. Environ. Sci. Dev.. 2014;5:60-63.
- [Google Scholar]
- Development of activated carbon from Phoenix dactylifera fruit pits: Process optimization, characterization, and methylene blue adsorption. Desal. Water Treat.. 2017;62:273-281.
- [Google Scholar]
- Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater.. 2010;176:510-520.
- [Google Scholar]
- Removal of Pb(II), Zn(II), Cu(II) and Cd(II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int.. 2020;6:11-20.
- [Google Scholar]
- Removal of lead(II) from aqueous solutions using rice straw. Water Sci. Technol.. 2017;76:1011-1021.
- [Google Scholar]
- Heavy and toxic metal ion removal by a novel polymeric ion-exchanger: synthesis, characterization, kinetics and equilibrium studies. J. Chem. Technol. Biotechnol.. 2015;90:2170-2179.
- [Google Scholar]
- Utility of cefixime as a complexing reagent for the determination of Ni(II) in synthetic mixture and water samples. Environ. Monit. Assess.. 2013;185:4647-4657.
- [Google Scholar]
- Optimized and validated spectrophotometric method for the determination of palladium(II) in synthetic mixture and automobile workshop area samples. J. Assoc. Arab Univ. Basic Appl. Sci.. 2016;19:29-36.
- [Google Scholar]
- Removal of Pb(II) from wastewater using activated carbon prepared from the seeds of Reptonia buxifolia. J. Serb. Chem. Soc.. 2020;85:S120-S125.
- [Google Scholar]
- Canadian Council of Ministers of the Environment, 2011. Protocols Manual for Water Quality Sampling in Canada, p.43.
- Removal of lead and chromium from wastewater using bagasse fly ash-A sugar industry waste. J. Colloid Interface Sci.. 2004;275:398-402.
- [Google Scholar]
- Preparation of biosorbents from the Jatoba (Hymenaea courbaril) fruit shell for removal of Pb(II) and Cd(II) from aqueous solution. Environ. Monit. Assess.. 2017;189:1-16.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [Google Scholar]
- Application of black walnut (Juglans nigra) husk for the removal of Pb(II) ion from aqueous solution. Water Sci. Technol.. 2017;75:2454-2464.
- [Google Scholar]
- Removal of lead and zinc ions by low cost adsorbents. J. Hazard. Mater.. 2009;168:319-325.
- [Google Scholar]
- Development of low-cost bio-adsorbent from agricultural waste composite for Pb(II) and As(III) sorption from aqueous solution. Cogent Eng.. 2019;6:1687274.
- [Google Scholar]
- Omani standard for un-bottled drinking water, 2012. 8th edition, Directorate General for Specifications and Measurements, Sultanate of Oman, pp. 1-26.
- Novel highly porous magnetic hydrogel beads composed of chitosan and sodium citrate: an effective adsorbent for the removal of heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. Int.. 2017;24:16520-16530.
- [Google Scholar]
- Equilibrium modeling, kinetic, and thermodynamic studies on adsorption of Pb(II) by a hybrid inorganic–organic material: Polyacrylamide Zirconium(IV) iodate. Ind. Eng. Chem. Res.. 2014;53:8198-8207.
- [Google Scholar]
- Experimental design approach for optimization of Pb(II) removal from aqueous solution using poly-o-toluidine/stannic(IV) triethanolamine as adsorbent. Environ. Technol. Innov.. 2020;17:100634
- [Google Scholar]
- Development of Zr(IV)—Doped polypyrrole/zirconium (IV) iodate composite for efficient removal of fluoride from water environment. J. Water Process Eng.. 2017;19:172-184.
- [Google Scholar]
- Application of Box-Behnken design and desirability function in the optimization of Cd(II) removal from aqueous solution using poly(o-phenylenediamine)/hydrous zirconium oxide composite: equilibrium modeling, kinetic and thermodynamic studies. Environ. Sci. Pollut. Res.. 2018;25:26114-26134.
- [Google Scholar]
- N-(((2-((2-Aminoethyl)amino)ethyl)amino)methyl)-4-sulfamoylbenzamide impregnated hydrous Zirconium Oxide as a novel adsorbent for removal of Ni(II) from aqueous solutions: Optimization of variables using central composite design. ACS Omega. 2019;4:2823-2832.
- [Google Scholar]
- Effective removal of acetaminophen from aqueous solution using Ca (II)-doped chitosan/β-cyclodextrin composite. J. Mol. Liq.. 2020;301:112454
- [Google Scholar]
- Spectroscopic study of charge transfer complexation between doxepin and π–acceptors and its application in quantitative analysis. J. Mol. Liq.. 2016;222:944-952.
- [Google Scholar]
- Application of Box-Behnken design and desirability function in the optimization of spectrophotometric method for the quantification of WADA banned drug: Acetazolamide. J. Mol. Liq.. 2019;274:270-277.
- [Google Scholar]
- Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ.. 2020;709:135895
- [Google Scholar]
- Evaluation of eggshell-rich compost as biosorbent for removal of Pb(II) from aqueous solutions. Water Air Soil Pollut.. 2016;227:1-16.
- [Google Scholar]
- A thermodynamic and kinetic evaluation of the adsorption of Pb(II) Ions using peanut (Arachis Hypogaea) shell-based biochar from aqueous media. Polish J. Environ. Stud.. 2020;29:293-305.
- [Google Scholar]
- Electrochemical treatment of heavy metal-containing wastewater with the removal of cod and heavy metal ions. J. Chin. Chem. Soc.. 2017;64:493-502.
- [Google Scholar]
- The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells. Soil Water Res.. 2020;15:30-37.
- [Google Scholar]
- Silica aerogel-supported hydrozincite and carbonate-intercalated hydrotalcite for high-efficiency removal of Pb(II) ions by precipitation transformation reactions. Nanoscale Res. Lett.. 2017;12:1-10.
- [Google Scholar]
- Effects of occupational exposure to lead on sperm and semen. In: Clarbson T.W., Nordberg G.F., Sager P.R., eds. Reproductive and Developmental Toxicity Of Metals: Proceeding of Joint Meeting in Rochester. New York: Plenum Press; 1983. p. :279-300.
- [Google Scholar]
- World Health Organization Guidelines for drinking-water quality 4th edition 2011 Switzerland Geneva.
- Heavy metal levels in tap water in Batina Region. Oman. Int. J. Environ.Pollut.. 2007;31:219-229.
- [Google Scholar]
- Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J. Hazard. Mater.. 2010;174:137-143.
- [Google Scholar]
- Assessment of Groundwater Quality in Al-Buraimi. Sultanate of Oman: JMES; 2017. p. :1266-1276.







