Translate this page into:
Acute and subacute toxicity studies of a new herbal formula induced apoptosis in the highly metastatic MDA-MB-231 cells
⁎Corresponding author. nabutaha@ksu.edu.sa (Nael Abutaha),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Breast cancer is one of the major causes of cancer deaths worldwide, and survival rate remains low even with the advancement of screening and treatment methods. Polyherbal medicines have been widely used for cancer treatment. CBBh and SNH are two new polyherbal formulations that showed anticancer effects.
MDA-MB-231 and MCF-7 cells were used for in vitro investigation. After treatments with CBBh and SNH, the proliferation and apoptosis of cells were detected by MTT assay, fluorescent microscopy, and Muse cell analyzer. The acute and subacute toxicities were studied following Organization for Economic Cooperation and Development (OECD) guidelines. Gas chromatography-mass spectrometry (GC–MS) was applied for analysis of chemical constituents of the extract.
CBBh extract inhibited proliferation of MCF-7 (IC50: 109.7 μg/mL) and MDA-MB-231 (IC50: 71.1 μg/mL) cancer cells, whereas the IC50 value for SNH was higher than 400 μg/mL against both cancer cell lines. The percentages of apoptotic cells following treatment with 200 and 300 μg/mL for 24 h were 38.9 ± 2.8% and 76.2 ± 2.1% MDA-MB231 cells respectively, versus 0.07% and 5.21% respectively in vehicle-treated cells. No acute or subacute oral toxicity was observed. CBBh extract was tolerated in concentrations up to 2000 mg/kg. Acute and subacute histopathological examination of the liver and kidneys revealed no abnormalities in their architecture.
The new polyherbal formula CBBh could suppress breast cancer through apoptosis. Therefore, our findings could provide a novel herbal formula for the treatment of breast cancer.
Keywords
Polyherbal formulations
Anticancer
Apoptosis
Toxicity
1 Introduction
Breast cancer is the second most common cancer affecting women. There were around 2 million breast cancer patients worldwide in 2018; the highest incidence was reported in Belgium and estimated to be around 113.2 out of 100,000 (World, 2020). In Saudi Arabia, around 930 patients are diagnosed with breast cancer each year (Alotaibi et al., 2018) and the incidence rate is increasing (Ibrahim et al., 2008).
Many challenges exist in treating cancer: developing target therapies, optimizing the immune system to target cancer without damaging normal tissues, alleviating undesired side effects of various cancer therapies, overcoming drug resistance, and finding additional chemotherapeutic agents (NIH, 2020). Traditional medicine is a promising option for the treatment of many illnesses, including cancer, as its practice has a long documented history of clinical success. Several anticancer compounds have been isolated from plants to serve as single chemotherapeutic drugs, such as: taxol, camptothecin, vinblastine, bleomycin, staurosporine, doxorubicin, and epothilone B (Cragg and Pezzuto, 2016). Recently, multicomponent herbal therapy has become highly recommended in cancer treatment for its ability to enhance the efficacy of chemotherapy, relieve pain, prolong patients’ life span, and reduce side effects (Fu et al., 2018).
Despite the availability of pharmacological data for the medicinal plants, few reports describe the safety profile of polyherpal extracts. Therefore, it is imperative to generate toxicological data of the test extract for assuring safety upon use. Mice share the most physiological and genetic features with humans making the mice the model of choice for biomedical research. Similarities in internal organ, endocrine, nervous, immune, cardiovascular, musculoskeletal systems have been extensively documented (Rosenthal and Brown, 2007). Mouse models have been used extensively to assess the effectiveness of new drugs, predict responses (Ward et al., 2017) and offer invaluable safety profiles for promising drugs (Takahashi et al., 2012). The information obtained from animal model investigation has an insightful effect on human health (Justice and Dhillon, 2016). The Food and Drug Administration (FDA) depends on data obtained from animal models to assess efficacy and safety of new promising drug (James, 2019). There are thousands of papers published using mice as an animal model to test the toxicity of natural products and synthetic compounds in preclinical stage (Ben Hsouna et al., 2020; Wang et al., 2020). Therefore, the goal of this study was to assess the apoptotic potential and safety of new herbal formula after acute and sub-acute administration.
2 Methods
2.1 Extraction of herbal materials
All plants were collected from Reef al Yamen Co., Al Morooj, Riyadh, Saudi Arabia. Formula SNH was prepared by mixing Saussurea costus (Asteraceae) root, Nigella sativa (Ranunculaceae) seed, and honey from local beekeeper. Formula CBBh was prepared by mixing Commiphora myrrha (Burseraceae) resin, Boswellia serrata (Burseraceae) resin, and Chinese propolis (Holista, China) according to the weight ratio of 1:1:1. Four hundred-milliliter aliquots of solvents with increasing lipophilicity (n-hexane, chloroform, ethyl acetate, and methanol) were used sequentially, and each extraction was carried out for 24 h using Soxhlet apparatus. Extracts were centrifuged to remove undissolved particles, then the solvent was collected and removed by rotary evaporation at 45 °C. The extract was dissolved in cell culture medium. A separate medium with methanol (0.1% (v/v) in place of the extract was used as a control.
2.2 Analysis of viable cell percentage and LDH assay
Cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Germany). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) overnight in flat-bottomed 24-well plates at 5 × 103 cells per well, and then incubated with SNH and CBBh at different concentrations (50–500 µg/mL) for 24 h. For each well, 100 µl of 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) (5 mg/mL) (Life Technologies, CA, USA) was pipetted and incubated for 2 h at 37 °C. Medium with methanol 0.1% (v/v) was used as a control. The formazan formed after incubation was read at 570 nm. Viable cell percentage was calculated using Origin 8 software (Northampton, MA). Results are expressed as a percentage of the control, assuming that the viability of control is 100% (Al-Zharani et al., 2019).
After treatment with CBBh extract, lactate dehydrogenase (LDH) released from cells was assessed with a commercial kit following the manufacturer’s specifications (Sigma, USA), and percent LDH released was read at 420 nm (Al-Zharani et al., 2019).
2.3 Cell and nuclear morphology
MCF-7 and MDA-MB-231 cells were treated with CBBh extract at a concentration of 71 µg/mL for 24 h, and subsequent cell morphology was imaged using a phase contrast microscope (Leica, Germany) at 100 × magnification. Nuclear morphology was assessed by Hoechst 33258 staining as previously described (Al-Zharani et al., 2019). Cells exhibiting apoptotic features were identified under a fluorescent microscope (Evos, USA) using morphological criteria such as nuclear fragmentation and chromatin condensation.
2.4 CBBh induced apoptosis in MDA-MB-231 cells
Apoptosis was investigated by the Muse Annexin V and dead cell kit (Merck, Millipore, USA) and analyzed using a Muse cell analyzer, as reported by Abutaha (Abutaha et al., 2020). Cells were first treated with CBBh extract using two different concentrations (200 and 300 µg/mL), and the Muse cell cytometer was used subsequently to detect apoptosis.
2.5 Dual acridine orange and ethidium bromide (AO/EB) staining
MDA-MB-231 cells were incubated for 24 h with CBBh extract and stained with AO/EB (2 μg/mL). Images were immediately taken under a fluorescence microscope.
2.6 Caspase-3/7 activity assay
After incubation with or without CBBh, MDA-MB-231 cells were labeled with caspase-3/7 green ReadyProbes™ reagent (ThermoFisher, USA) under darkness in DMEM medium for 30 min at 37 °C. Then, images were taken under a fluorescence microscope.
2.7 Animal study
Healthy male albino mice weighing 25–35 g were obtained from the Zoology Department, King Saud University. Mice were housed at five animals per group/cage under conditions of 12 h light/12 h dark and ambient temperature of 25 °C. All mice were acclimatized to the laboratory environment for seven days prior to treatment. Mice were allowed ad libitum access to water and food. All procedures were carried out according to the King Saud University Animal Ethics Committee and also Organization for Economic Cooperation and Development (OECD).
2.8 Acute toxicity study
Acute oral toxicity of the hexane extract of CBBh was investigated using the ‘up-and-down’ technique of mice testing at single doses of 250, 500, and 2000 mg/kg following OECD guideline no. 407. The control group received 200 µl of corn oil only. After fourteen days of daily dosing, mice were observed for toxicity symptoms including regurgitation, diarrhea, respiratory, tremors, convulsion, and salivation. After 21 days, mice were then, euthanized by CO2 gas. Kidneys and liver were fixed in 10% formalin and further processed as reported previously (Abutaha et al., 2020), then imaged using a BP73 optical microscope (Olympus, Japan).
2.9 Subacute toxicity study
Mice were arbitrarily assigned to three groups (n = 5 per group). Groups I and II received 250 and 500 mg/kg of body weight per day, respectively, of the CBBh extract, while group III received 100 μL per day of corn oil only. The mice were dosed by oral gavage for 21 days. On day 21, the mice were killed. The kidneys and liver were excised and fixed as described in the previous section.
2.10 Gas chromatography-mass spectrometry (GC–MS) analysis
Extract was analyzed in a gas chromatograph coupled with a mass spectrometer (Perkin Elmer, Autosystem), and a volume of the extract (2 μl) was loaded into the capillary column (60 m × 0.25 mm, 0.25 µm film thickness). Oven temperature was set to 60 °C for 10 min, then increased to 220 °C with an acceleration rate of 4 °C/min, and thereafter increased to 240 °C at a rate of 1 °C/min. The flow of the helium mobile phase was set at 0.8 mL/min. The source and inlet line temperatures were fixed at 180 °C and 250 °C, respectively. MS detection was accomplished at 70 eV with a scan mass range of 35–450 m/z. Spectra were compared with the NIST, 2005 v2.1 library to identify compounds.
2.11 Statistical analysis
Results were analyzed using t-tests, and data was presented as mean ± SD. Data with p-values <0.05 were regarded as statistically significant.
3 Results
3.1 Effects of CBBh and SNH on breast cancer cell line proliferation
Viability of cancer cells after 24 h treatment with CBBh and SNH extracts was assessed by MTT and LDH assays, and significant variations were observed (Fig. 1). These results revealed that CBBh was more effective than SNH in cell line inhibition, with IC50 values of 109.7 μg/mL (MCF-7) and 71.1 μg/mL (MDA-MB-231) (Fig. 1A). In contrast, IC50 values for SNH were 444.59 μg/mL (MCF-7) and 416 μg/mL (MDA-MB-231). In this experiment, we observed significant changes in LDH release, indicating that CBBh induced cell inhibition and caused a cytotoxic effect (Fig. 1B). After confirming the inhibition of cell lines by CBBh, morphology observation was carried out to visualize the effect on the proliferation of tested cells. Cell number was dramatically depleted after CBBh incubation in both cell lines at concentrations of 110 μg/mL (MCF-7) and 71.1 (MDA-MB-231) μg/mL (Fig. 2).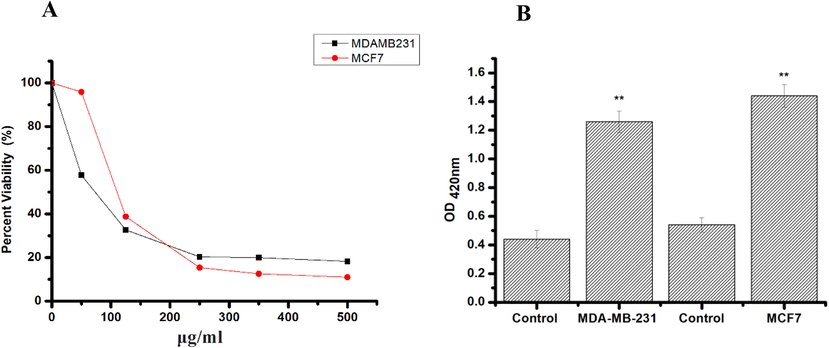
A: Cell viability percentage of MDA-MB-231, and MCF7 cells treated with CBBH extract. Values are expressed as mean ± std. dev (n = 3). B: Lactate dehydrogenase (LDH) test showing the cytotoxicity of the CBBH extract against MDA-MB-231, and MCF7 cells. The data expressed as mean ± std. dev (n = 3). *p < 0.05 compared with the control group.
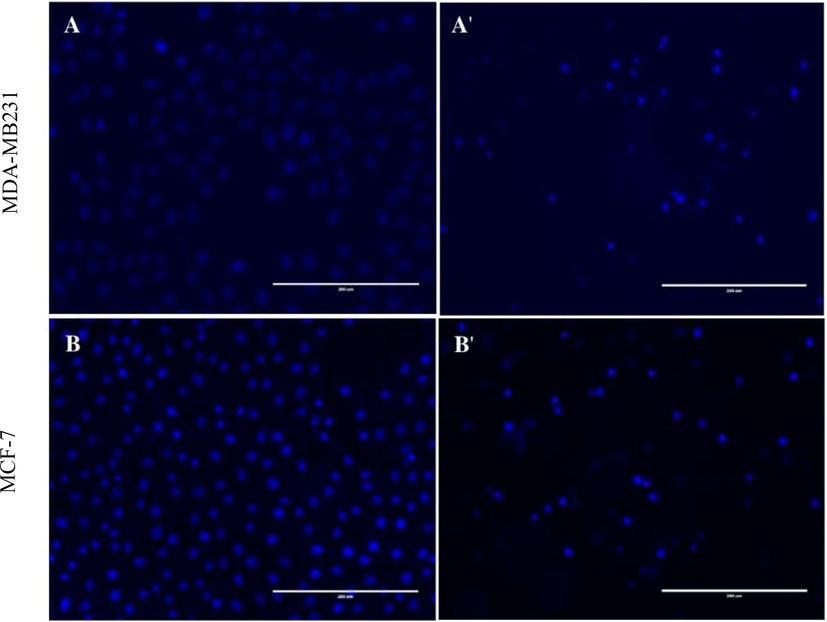
Morphological and nuclear changes in MDA-MB-231 and MCF-7 cells following 24 h treatment with 71
g/ml and 110
g/ml respectively of CBBh extract. MDA-MB-231cells (A: control, A': treated), MCF-7 cells (B: control, B': treated) (200 × magnification).
3.2 Induction of apoptosis by CBBh extract
Cell proliferation was also assessed by Hoechst 33258 staining. Cell morphology post-treatment with CBBh extract showed condensed nuclei and apoptotic bodies (Fig. 2). Furthermore, results from the Muse cell analyzer of the Muse™ Annexin-V staining assay revealed a significant increase in apoptosis.
Induction of apoptosis had a dose-related effect in CBBh extract treated MDA-MB-231 cells. The percentage of apoptotic cells post-incubation with 200 μg/mL and 300 μg/mL of CBBh extract was 37.9 ± 2.5% and 11.3 ± 1.69%, respectively, against MDA-MB231 cells. In contrast, vehicle-treated cells had post-incubation apoptotic cell percentages of 0.07% and 5.21%, respectively (Fig. 3).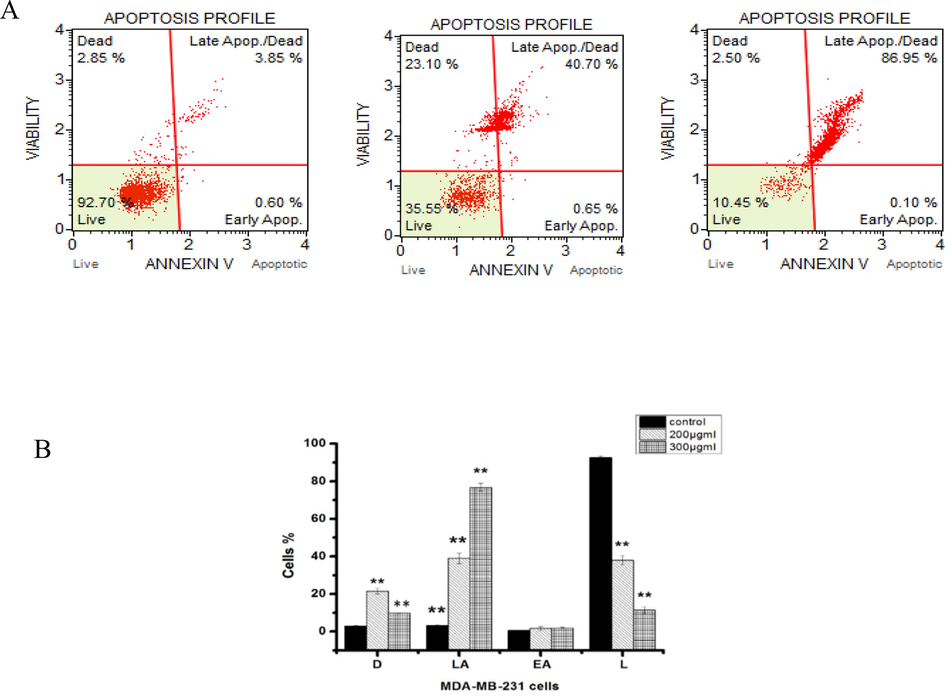
Induction of apoptosis in human MDA-MB-231 cells post-treatment with 200 and 300 µg/ml CBBh extract. Representative figures A and B show the population of viable (Annexin V-PE –, dead cell marker –), early apoptotic (Annexin V-PE +, dead cell marker –), late apoptotic (Annexin V-PE +, dead cell marker + ) and necrotic (Annexin V-PE –, dead cell marker + ) cells. (**) significantly different from control (p < 0.05).
The results of AO/EB staining of MDA-MB-231 cells incubated with CBBh are presented in Fig. 4. Nuclear DNA of live and dead cells was stained with AO, whereas the nuclear DNA of cells that have lost their membrane integrity was stained with EB [29]. In this study, live cells were uniformly stained green, cells in early apoptosis appeared green with fragmentation in the chromatin, cells in late apoptosis appeared orange with fragmentation in the chromatin, and nuclear necrotic cells appeared red with no fragmentation in the chromatin (Fig. 4).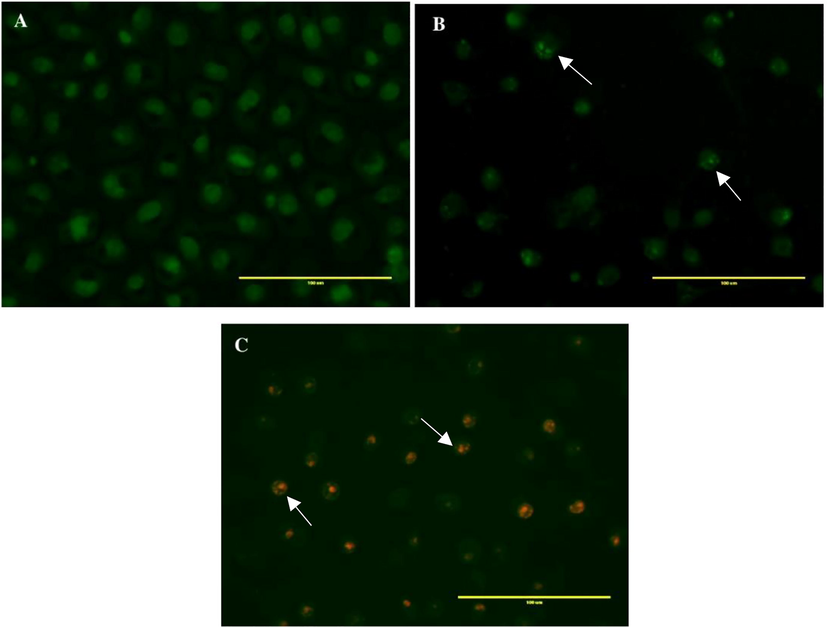
Acridine orange/ethidium bromide staining of MDA-MB-231 cells to evaluate apoptosis induced by CBBh extract. (A) negative control; (B) 71 µg/ml; (C) 142 µg/ml. Colors indicate live cells (uniformly green), early apoptotic cells (unevenly green with DNA fragmentation), and late apoptotic cells (yellow-orange with DNA fragmentation).
To elucidate whether CBBh extract induced cell death via apoptosis, caspase-3/7 activity was assessed. Treatment of MDA-MB-231 cells with CBBh at 71 µg/mL exhibited an increase in caspase-3/7 expression compared with the control, as shown by the bright green fluorescence (Fig. 5).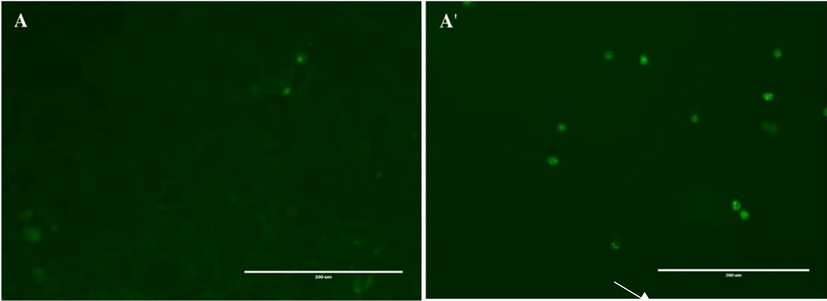
Activation of caspases 3 and 7 in MDA-MB-231 cells post-treatment with 71
g/ml of CBBh extract for 24 h. Activated caspase-3/7 was identified, then images were captured using fluorescence microscopy at 200 × magnification (A: control, A': treated).
3.3 Acute toxicity study
CBBh extracts showed no signs of acute oral toxicity or mortality in the treated groups during the study period. Compared with the kidney control group (Fig. 6A), the histopathology of kidneys treated with CBBh at 2000 mg/kg (Fig. 6B) showed normal renal architecture associated with mild congestion of intertubular blood vessels and mild infiltration of inflammatory cells (Fig. 6C and D).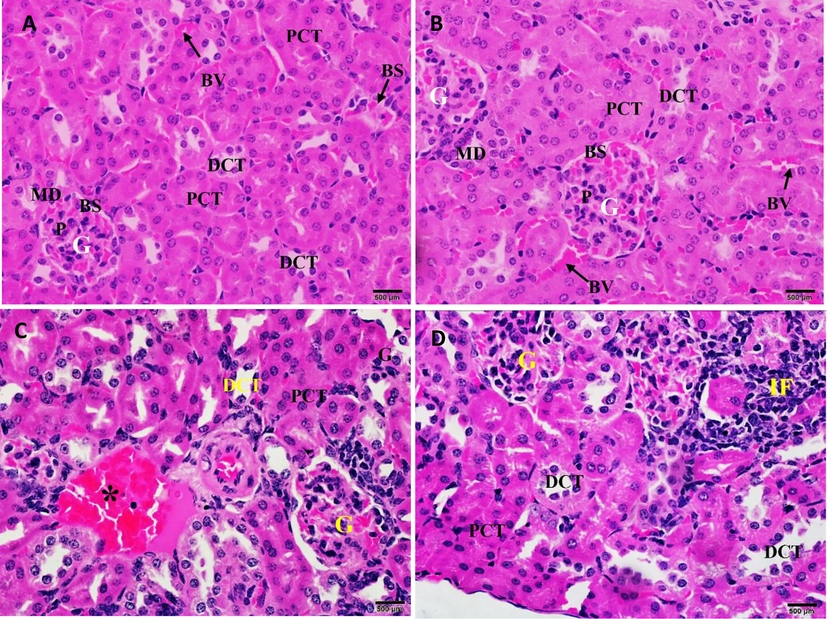
(A) Photomicrograph of the kidney section of control mice showing normal renal architecture. (B) Photomicrograph of the kidney section of mice treated with 2000 mg/kg of CBBh extract showing normal renal architecture associated with (C) mild congestion of intertubular blood vessels (*) and (D) mild infiltration of inflammatory cells (IF). PCT = proximal convoluted tubule, DCT = distal convoluted tubule, G = glomerulus, BS = Bowman’s space, BV = blood vessels, MD = macula densa, and P = podocyte. (H & E 400 × ).
Compared with the liver control group (Fig. 7E), the histopathological investigation of the livers of treated mice showed normal hepatic architecture (Fig. 7F) associated with mild hepatic degenerative changes (Fig. 7G), infiltration of inflammatory cells, and congestion of dilated blood vessels (Fig. 7H).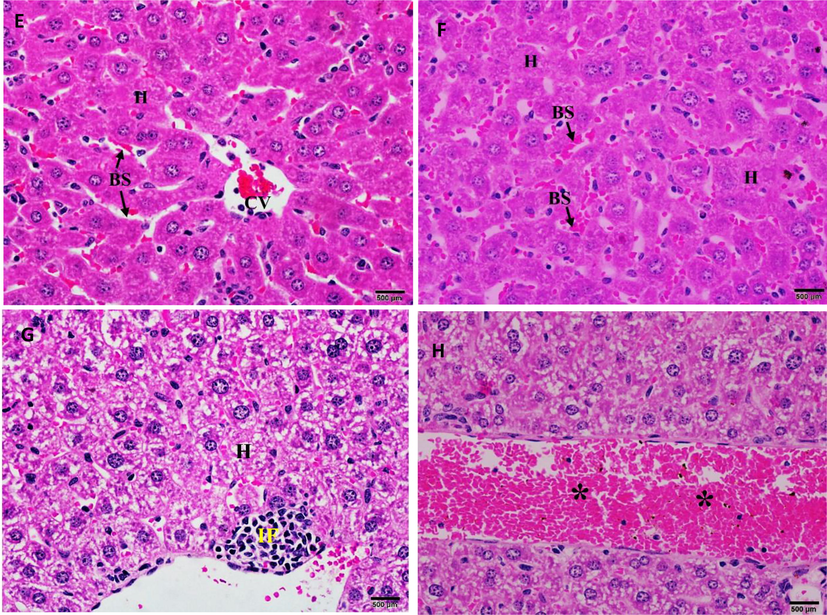
(E) Photomicrograph of the control mice liver showing normal morphology of the hepatic lobule with normal central vein (CV) bounded by an intact endothelium (arrow head). Parallel cords of hepatocytes (H) are separated by sinusoidal spaces (BS). (F) Photomicrograph of the liver sections of mice treated with 2000 mg/kg of CBBh extract showing normal morphology associated with (G) mild hepatic degenerative changes, (H) infiltration of inflammatory cells (IF), and congestion of dilated blood vessel (*). (H & E 400×).
3.4 Subacute toxicity study
Histopathological investigation of kidney sections in the control group (Fig. 8A) showed normal renal architecture. Kidney sections of mice treated with CBBh extract at 500 mg/kg (Fig. 8B) showed normal Bowman’s capsule, glomerulus, and distal and proximal convoluted tubules, but were associated with infiltration of inflammatory cells and dilation of intertubular blood vessels (Fig. 8C), mild dilatation of glomerular blood capillaries (Fig. 8D), edema (Fig. 8E), and congestion of intertubular blood vessels (Fig. 8F).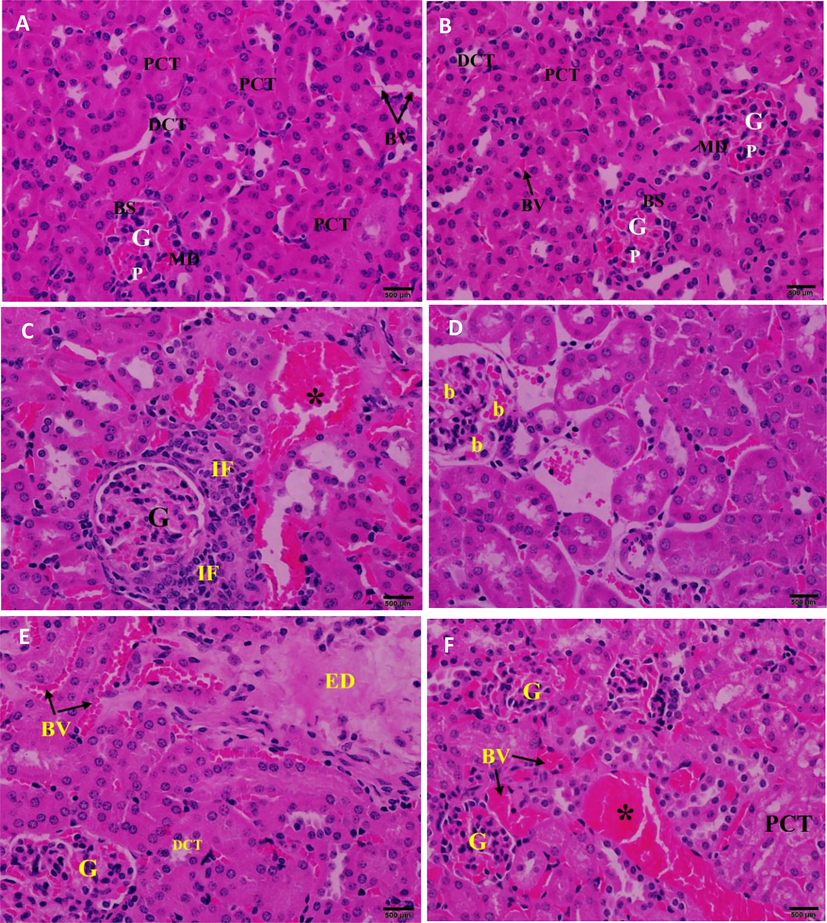
(A) Photomicrograph of the kidney section of control mice showing normal renal architecture. (B) Photomicrograph of the kidney section of mice treated with 500 mg/kg of CBBh extract showing normal renal architecture (C) with infiltration of inflammatory cells (IF), dilation of intertubular blood vessels (*), (D) mild dilatation of glomerular blood capillaries (b), (E) edema (ED), (F) congestion of intertubular blood vessels (*). PCT = proximal convoluted tubule, DCT = distal convoluted tubule, G = glomerulus, BS = Bowman’s space, BV = blood vessels MD = macula densa, and P = podocyte. (H & E 400×).
Histopathological investigation of the liver sections in the control group (Fig. 9G) displayed normal central veins, blood sinusoids, and portal triad, including branches of the hepatic artery and hepatic portal vein. Mice treated with CBBh extract at 500 mg/kg (Fig. 9H) showed normal hepatic structure with normal central veins, hepatic cord, hepatocytes, and blood sinusoids, but were associated with granulomas of inflammatory cells (Fig. 9I), mild infiltration of inflammatory cells, and congestion of the central veins (Fig. 9J).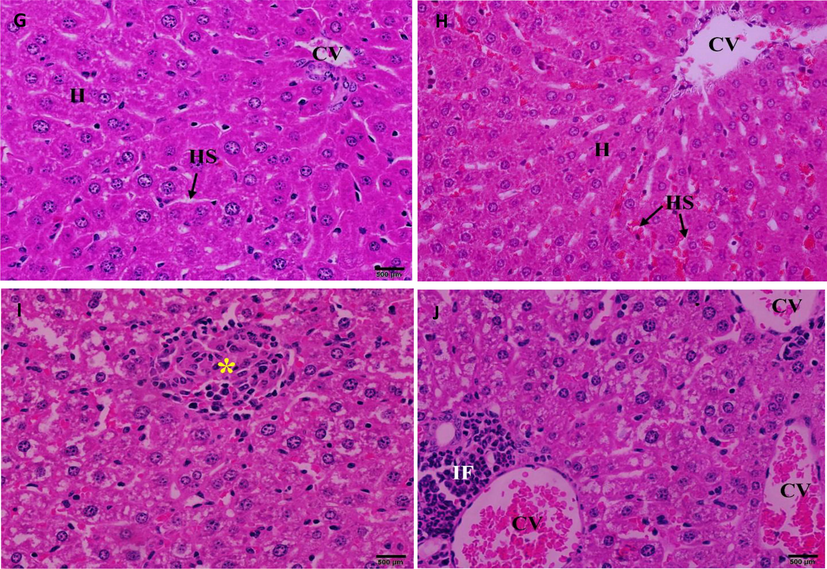
(G) Photomicrograph of the liver sections of control mice showing normal hepatic architecture. (H) Photomicrograph of the liver section of mice treated with 500 mg/kg of CBBh extract showing normal morphology of the hepatic lobule associated with (I) granulomatous of inflammatory cells (*), (J) mild infiltration of inflammatory cells (IF), and congestion of central vein (CV). (H & E 400×).
3.5 Gas chromatography-mass spectrometry (GC–MS) analysis
GC–MS chromatograms of CBBh extract showed the existence of 39 different phyto-chemotypes (Table 1) by comparison of their mass spectrum fragment with the NIST database library. Four major phyto-chemotypes were recorded, namely, 1,3,5-trimethyl-benzene (3.06%), 3-[(e)-2-phenyl-1-propenyl]cyclohexanone (3.55%), alpha- pinene (8.07%), and 6,10,14-trimethylpentadecan-2-one (37.36%) (Table 1).
Compound Name
Chemical formula
Molecular weight (g/mol)
RT (min)
Area%
1
2-methyl- heptane
C8H18
114.23
4.06
2.090
2
3-methyl-heptane
C8H18
114.23
4.18
2.730
3
2,4-dimethyl-Heptane
C9H20
128.25
4.62
0.530
4
1,3-dimethyl benzene
C8H10
106.16
5.94
1.150
5
Alpha- thujene
C10H16
136.234
6.89
0.680
6
Alpha- pinene
C10H16
136.238
7.05
8.070
7
3-Methyl-4-methylenebicyclo (3.2.1)oct-2-ene
C10H14
134.22
7.41
1.160
8
1-ethyl-2-methyl benzene
C9H12
120.19
7.55
1.400
9
1-ethyl-4-methyl-benzene
C9H12
120.19
7.60
0.640
10
Beta terpinene
C10H16
136.23
7.73
0.680
11
1,3,5-trimethyl-benzene
C9H12
120.19
8.14
3.06
12
D-limonene
C10H16
136.23
8.70
0.170
13
Gamma-terpinene
C10H16
136.23
9.17
0.150
14
1-octanol
C₈H18O
130.23
9.43
0.330
15
Alpha.-pinene oxide
C10H16O
152.23
10.12
0.760
16
Bicyclo[3.1.1]heptan-3-ol
C7H12O
112.17
10.63
0.340
17
Bicyclo[3.1.1]hept-3-en-2-ol
C7H10O
110.15
10.69
0.750
18
Bicyclo[3.1.1]hept-3-en-2-one
C7H8O
108.14
11.61
0.430
19
2-phenylethyl ester of acetic acid
C10H12O2
164.204
12.21
0.210
20
Beta-elemene
C15H24
204.35
14.20
0.250
21
(-)-Ar-curcumene
C15H22
202.33
15.85
0.250
22
Curzerene
C15H20O
216.32
16.17
2.770
23
3-[(e)-2-phenyl-1-propenyl]cyclohexanone
C15H18O
214.3
18.38
3.550
24
Alpha.-eudesmol
C15H26O
222.37
18.82
0.480
25
(-)-Caryophyllene oxide
C15H24O
220.35
19.13
0.280
26
Bicyclo[3.2.0]hept-2-ene-6-one
C7H8O
108.14
19.30
0.240
27
Arsonic acid
AsH3O3
125.944
19.47
0.860
28
1 h-cycloprop[e]azulen-4-ol
C11H8O
156.18
20.37
0.150
29
Tetradecanoic acid
C14H28O2
228.3709
21.82
0.700
30
1,5-Cyclodecadiene, 1,5-dimethyl-8-(1-methylethenyl)
C15H24
204.35
22.34
0.880
31
Heptadecanoic acid
C17H34O2
270.45
22.50
0.570
32
Cembrene-c
C20H32
272.5
22.80
0.430
33
Hexadecanoic acid
C16H32O2
256.43
23.11
1.210
34
11-eicosaenoic acid
C20H38O2
310.51
23.72
0.520
35
6,10,14-Trimethylpentadecan-2-one
C18H36O
268.5
24.57
37.360
36
Dodecanoic acid
C12H24O2
200.3178
25.05
1.060
37
1,2-benzenedicarboxylic acid
C8H6O4
166.14
27.38
2.160
38
Beta.-phenylethyl n-decanoate
C18H28O2
276.4
30.00
0.750
39
3.alpha.,5.alpha.-cyclo-ergosta-7,9(11),22 t-triene-6.beta.-ol
C28H42O
394.63248
30.75
0.210
4 Discussion
Cancer treatment has gradually shifted from single-drug to multi-drug approaches due to deleterious effects of single-drug use, combined with low efficacy and increased incidence of drug resistance. Interest in polyherbal formulations has increased due to higher therapeutic efficacy and minimal side effects, presuming that polyherbal formulations work synergistically in different pathways (Karole et al., 2019). Cancer, like many diseases, is not caused by one single pathway or a single mutated gene (Bishop, 1987), and polyherbal extracts are more beneficial to patients than single-herb treatments (Kim et al., 2017). The importance of this investigation lies in the anticancer properties of these polyherbal formulas that have not been previously studied.
LDH release is an excellent indicator of cytotoxicity because it is a signal of irreversible cell death caused by membrane injury (Aslantürk, 2018). The present study revealed a significant (p ≤ 0.05) increase in LDH release when breast cancer cells were incubated with CBBh and SNH extracts, as compared to the vehicle control. MTT and LDH release are attributed to the cytotoxic nature of the extracts, which confirms their anticancer potential (Sivalokanathan et al., 2006).
Polyherbal extracts that induce apoptosis are promising candidates for cancer treatment, and different polyherbal extracts can induce apoptosis in different types of cancer cells (Maliyakkal et al., 2013). Apoptosis causes a series of characteristic morphological and nuclear features like blebbing, cell shrinkage, apoptotic body formation, nuclear fragmentation, chromatin condensation, and cytoplasmic vacuolization, compared to a vehicle control. Microscopic examination of MCF-7 and MDA-MB-231 cells showed the same apoptotic features as described in Fig. 2.
The AO/EB staining images displayed early and late apoptosis in CBBh extract treated cells. Early apoptosis was detected through the binding of AO to fragmented DNA, showing a bright green fluorescence using an extract dosage of 71 µg/mL. However, a higher dosage of 142 µg/mL of extract led to late-stage apoptosis, as revealed by the reddish-orange color attributed to the binding of EB to denatured DNA. Additionally, to confirm the above results quantitatively, Muse™ Annexin V and dead cell analysis were carried out. The results indicated that the number cells in early- and late-stage apoptosis increased with a lower number of viable cells. Quantitative data revealed that CBBh extract induced cell death and shifted most of the cancer cells into late apoptosis.
The caspase system plays a crucial role in induction, transduction, and amplification of apoptotic signals. Further investigation of the possible association of caspases in apoptosis induction by CBBh extract on tested cell lines revealed that 24 h of CBBh treatment induced DEVDase (caspase-3/7) activity. The activation of caspases 3 and 7 during apoptosis results in the induction of caspase-activated DNase, resulting in DNA fragmentation, which is a feature of apoptosis (McIlroy et al., 1999). Activation of caspases after treatment with CBBh extract permits the appearance of apoptotic DNA fragments that were also observed using florescence microscopy. Hence, the cytotoxic effects of the extract on cancer cell lines tested was mediated through apoptosis with activation of caspase-3/7.
Many nations rely on traditional medicines due to their availability, affordability, history of traditional use, and the belief that herbal medicines are safer than prescribed drugs (Zhang, 2017). Polyherbal treatments are known for their multi-target effects in the treatment of many diseases, including cancer (Karole et al., 2019). However, there is a need to evaluate the safety of herbal formulations, even if they have been shown to have pharmacological effects. Although there are many polyherbal extracts available, only a few have been evaluated for their efficacy and safety (Cheng et al., 2009). Toxicity studies are needed, especially for polyherbal formulas that are used in the treatment of chronic conditions.
In this study, a single dose (2000 mg/kg of body weight) of CBBh extract revealed no mortality or abnormal clinical signs; therefore, the LD50 was higher than 2000 mg/kg, and the treatment can be regarded as safe. This result was in agreement with Clarke and Clarke (Clarke and Clarke, 1967), who stated that any drug with oral LD50 greater than 1000 mg/kg of body weight could be considered low toxicity and safe. Likewise, the present study agrees with previously published studies in which polyherbal formulas proved to be non-toxic in animal experiments (Chen et al., 2018; Duraipandi et al., 2019; Issuriya et al., 2019).
Single-plant extracts and propolis, the constituents of CBBh, have been proven to be relatively safe on laboratory animals and in clinical trials. Boswellia serrata extract, when studied in male and female rats, exhibited no adverse effects in terms of clinical chemistry, hematology, and histopathological assessments, when administered at a dosage of 1.5 g/kg/day for 90 days (Lalithakumari et al., 2006). B. serrata has also been used in clinical trials (0.1 g/day) and was shown to be effective and safe in knee osteoarthritis treatment (Sengupta et al., 2008). Propolis extract caused no mortality or signs of toxicity in mice at doses up to 5 g/kg of body weight over eight weeks, and did not cause any histopathological changes to the liver or kidneys (Rao et al., 2001).
The presence of different bioactive molecules validates the use of polyherbal extracts as therapeutic agents. Chemotyping is essential in medicinal chemistry for identifying the components responsible for certain pharmacological properties. Chemotyping techniques facilitate the comparison and identification of chemical properties of target compounds, the discovery of new biomarkers, and the effective and efficient evaluation of bioactivity levels (Dumarey et al., 2008). By using GC–MS we were able to determine and validate the use of polyherbal formulas, such as CBBh, as therapeutic agents in cancer treatment.
Some of the compounds identified by GC–MS analysis were found to possess anticancer activity (Table 1). GC–MS analyses carried out on CBBh extract have revealed several important anticancer compounds, such as hexadecanoic acid against human colorectal carcinoma (HCT-116) (Ravi and Krishnan, 2017); 1,2-benzenedicarboxylic acid against HepG2, MCF-7, HaCaT, and NIH 3 T3 cells (Krishnan et al., 2014); β-elemene against brain tumor cells (A172, U-87MG and CCF-STTG1) (Li et al., 2013); D-limonene against lung cancer cells (A549 and H1299) (Yu et al., 2018); terpinene against HepG2, K562, and B16-F10 tumor cell lines (Sobral et al., 2014); α-pinene against hepatoma carcinoma cells (BEL-7402) (Chen et al., 2015), curzerene against human lung adenocarcinoma cells (SPC-A1) (Ward et al., 2017); and curcumin against pancreatic cancer (PANC1 and BxPC3) (Zhu and Bu, 2017).
Our data suggest that CBBh extract may serve as a source for the development of anticancer drug for breast cancer. Further investigation and additional toxicological studies are needed to reveal the exact mechanism of action of CBBh extract.
Acknowledgements
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1442 -0037).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chem.. 2020;18(1):472-481.
- [Google Scholar]
- Apoptotic induction and anti-migratory effects of Rhazya Stricta fruit extracts on a human breast cancer cell line. Molecules. 2019;24(21):3968.
- [Google Scholar]
- Breast cancer mortality in Saudi Arabia: Modelling observed and unobserved factors. PloS one. 2018;13(10):e0206148.
- [Google Scholar]
- In vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages. InTech 2018
- [Google Scholar]
- Lobularia maritima leave extract, a nutraceutical agent with antioxidant activity, protects against CCl4-induced liver injury in mice. Drug Chem. Toxicol. 2020:1-14.
- [Google Scholar]
- Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci.. 2015;127(3):332-338.
- [Google Scholar]
- Acute and chronic toxicity of a polyherbal preparation–Jueyin granules. BMC Complement. Altern. Med.. 2018;18(1)
- [CrossRef] [Google Scholar]
- Systematic review of Chinese herbal medicine for functional constipation. World J. Gastroenterol.: WJG. 2009;15(39):4886.
- [CrossRef] [Google Scholar]
- Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Principles Practice. 2016;25(Suppl. 2):41-59.
- [Google Scholar]
- Exploration of linear multivariate calibration techniques to predict the total antioxidant capacity of green tea from chromatographic fingerprints. J. Chromatogr. A. 2008;1192(1):81-88.
- [Google Scholar]
- Acute and sub-acute toxicity studies of a patented anti-anxiety poly herbal formulation. J. Pharmacol. Toxicol.. 2019;14:9-17.
- [Google Scholar]
- Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: A review on experimental and clinical evidences. Front. Pharmacol.. 2018;9:1394.
- [Google Scholar]
- The present and the future of breast cancer burden in the Kingdom of Saudi Arabia. Med. Oncol.. 2008;25(4):387-393.
- [Google Scholar]
- Safety and antioxidant potential of traditional thai poly-herbal tea “phy-blica-d” used as a rejuvenation formula. Pharmacog. Res.. 2019;11(3):295.
- [Google Scholar]
- James, V., 2019. Animal Models In Drug Discovery.Accessed 21 November 2020, https://www.taconic.com/taconic-insights/quality/animal-models-drug-discovery.html.
- Using the mouse to model human disease: increasing validity and reproducibility. The Company of Biologists Ltd.; 2016.
- Polyherbal formulation concept for synergic action. A review. J. Drug Delivery Ther.. 2019;9(1-s):453-466.
- [Google Scholar]
- SRVF, a novel herbal formula including Scrophulariae Radix and Viticis Fructus, disrupts focal adhesion and causes detachment-induced apoptosis in malignant cancer cells. Sci. Rep.. 2017;7(1):1-14.
- [Google Scholar]
- Krishnan, K., Mani, A., Jasmine, S., 2014. Cytotoxic activity of bioactive compound 1, 2-benzene dicarboxylic acid, mono 2-ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 3(4), 246.
- Safety and toxicological evaluation of a novel, standardized 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-enriched Boswellia serrata extract (5-Loxin®) Toxicol. Mech. Methods. 2006;16(4):199-226.
- [Google Scholar]
- β-Elemene promotes cisplatin-induced cell death in human bladder cancer and other carcinomas. Anticancer Res.. 2013;33(4):1421-1428.
- [Google Scholar]
- Cytotoxic and apoptotic activities of extracts of Withania somnifera and Tinospora cordifolia in human breast cancer cells. Int. J. Appl. Res. Nat. Prod.. 2013;6(4):1-10.
- [Google Scholar]
- Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene. 1999;18(31):4401-4408.
- [Google Scholar]
- NIH, 2020. https://www.cancer.gov/research/areas/treatment. Accessed.
- Effect of oral administration of bark extracts of Pterocarpus santalinus L. on blood glucose level in experimental animals. J. Ethnopharmacol.. 2001;74(1):69-74.
- [Google Scholar]
- Cytotoxic potential of N-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J. Cell Biol. 2017;12:20-27.
- [Google Scholar]
- The mouse ascending: perspectives for human-disease models. Nat. Cell Biol.. 2007;9(9):993-999.
- [Google Scholar]
- A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther.. 2008;10(4):R85.
- [Google Scholar]
- Effects of Terminalia arjuna bark extract on apoptosis of human hepatoma cell line HepG2. World J. Gastroenterol.: WJG. 2006;12(7):1018.
- [Google Scholar]
- Sobral, M.V., Xavier, A.L., Lima, T.C., de Sousa, D.P., 2014. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014.
- Assessment of benzene-induced hematotoxicity using a human-like hematopoietic lineage in NOD/Shi-scid/IL-2Rγ null mice. PloS One. 2012;7(12):e50448
- [Google Scholar]
- Toxicological evaluation of the ultrasonic extract from Dichroae radix in mice and wistar rats. Sci. Rep.. 2020;10(1):1-12.
- [Google Scholar]
- Reproducibility of histopathological findings in experimental pathology of the mouse: a sorry tail. Lab Animal. 2017;46(4):146.
- [Google Scholar]
- World, 2020. https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics.
- D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther.. 2018;11:1833.
- [Google Scholar]
- Effect of HBM rehabilitation exercises on depression, anxiety and health belief in elderly patients with osteoporotic fracture. Psychiatria Danubina. 2017;29(4):466-472.
- [Google Scholar]
- Zhu, Y., Bu, S., 2017. Curcumin induces autophagy, apoptosis, and cell cycle arrest in human pancreatic cancer cells. Evid.-Based Complement. Altern. Med. 2017.







