Translate this page into:
Acetylcholinesterase inhibitory activity and iron-induced lipid peroxidation reducing potential of Pimpinella stewartii leaves in male wistar rats
⁎Corresponding authors. armankhan0301@gmail.com (Ameer Khusro), talhabmb@bgctub.ac.bd (Talha Bin Emran) waseem_bnu57@yahoo.com (Talha Bin Emran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study was aimed to determine the potentialities of Pimpinella stewartii leaves extracts fractions towards the inhibition of acetylcholinesterase (AChE) activity as well as reduction of iron-induced lipid peroxidation in male Wistar rats (age – 50 days; weight – 280–300 g). Initially, animals were divided into 17 groups with 7 rats in each group. Gallic acid and quercetin were used as standards. The AChE activities of aqueous and ethanolic extract fractions of P. stewartii leaves were determined using standard protocol. Results showed that ethyl acetate extract fractions significantly (P = 0.01) inhibited the enzyme in a concentration dependent manner when compared to the aqueous extract fractions and the control group. Gallic acid and quercetin depicted higher inhibition of AChE activities than ethyl acetate extract fractions. Additionally, the IC50 values for aqueous extract fractions, ethyl acetate extract fractions, gallic acid, and quercetin were estimated as 72.6 ± 2.72, 30.41 ± 0.56, 8.4 ± 0.22, and 6.82 ± 0.2 µg/mL, respectively. Furthermore, the lipid peroxidation activities of extracts fractions were assessed by estimating thiobarbituric acid reactive substances (TBARS) production. Both the extracts fractions showed significant protection against lipid peroxidation but the ethyl acetate extract fractions exhibited comparatively higher reductions in TBARS levels (P = 0.001). However, gallic acid and quercetin revealed higher reductions of pro-oxidants than the extracts fractions. In conclusion, findings of this study demonstrated pronounced AChE inhibitory activity and reduced lipid peroxidation trait of ethyl acetate extract fractions of P. stewartii leaves, and thus, suggested its paramount role as therapeutics against Alzheimer disease.
Keywords
Acetylcholinesterase activity
Alzheimer disease
Lipid peroxidation
Pimpinella stewartii leaves
Therapeutics
1 Introduction
In order to re-establish the levels of acetylcholine in the brain, several cholinesterase inhibitors are used. This is the most frequently used treatment of Alzheimer disease (AD) which is based on cholinergic hypothesis (Perry, 1986; Asefy et al., 2021). These cholinergic inhibitors are associated with a fewer benefits and several side effects (Van Marum, 2008). In order to cope with these side effects, researchers are working on designing and synthesis of such drugs having no or lesser adverse effects (Francis et al., 1999; Van Marum, 2008; Dastmalchi et al., 2009). With the passage of time, the demand for alternative treatment of AD has significantly increased.
Various natural dietary phyto-constituents have got attention of the researchers due to their anti-inflammatory, antioxidant, and anti-amyloidogenic effects for the treatment of AD as an alternative source of existing drugs (Singh et al., 2008; Sun et al., 2010; Kamran et al., 2020). Medicinal plants have a potential role in resolving the issues related with the cerebral impairment (Kennedy et al., 2002; Akhondzadeh et al., 2003; Muzaffar et al., 2021; Eftekhari et al., 2021; Ahmad et al., 2022). Previous studies revealed that the medicinal plant’s ethnopharmacological assessment might be a useful tool in discovering newer candidate therapy for AD treatment (Dastmalchi et al., 2007; Tewari et al., 2018). Numerous medicinal plants including Rosmarinus officinalis (Perry et al., 1999), Salvia officinalis (Akhondzadeh et al., 2003), Ginko biloba (Dos Santos-Neto et al., 2006), and Melissa officinalis (Ahmad et al., 2018) have been investigated for their neuroprotective effects and their therapeutic effectiveness in AD. Various plants’ extracts are used to make tea that has beneficial effects in calming nerves as well as rectifying sleep disorders (Wheatley, 2005). It has also been investigated that certain plant extracts exhibit a promising antioxidant effect against various pro-oxidant agents in brain homogenates (Pereira et al., 2009).
Pimpinella stewartii (Dunn) E. Nasir, synonyms Eriocycla stewartii, or Pituranthos stewartii is a member of family Apiaceae. This family is backed by 434 genera and 3700 species. It is called as carrot family. It forms a remarkable group of plants producing flowers. Most of the members of this family are aromatic in nature and bears hallow stems, flowers, and tapped roots. In Pakistan, it is mostly dispersed in Haripur, Abbottabad, and Murree hills on 700 to 2500 m height. The plant grows wildly alongside the edges of fields cultivated with other crops and on dry or rocky areas. Its fruits are used as carminative and for the treatment of stomach diseases (Afzal et al., 2009). The plant’s proximate mineral, nutritional composition, phenolic, and in vitro antioxidant activity is reported in recent decade. The antimicrobial activity of essential oil of P. stewartii was reported by Syed et al. (1986). Another report showed that the methanolic extract of P. stewartii is a broad-spectrum antibacterial agent (Gul and Hassan, 2016). Considering the pivotal medicinal applications of P. stewartii, this study was investigated to determine the potentialities of P. stewartii extracts not only to inhibit acetylcholinesterase (AChE) but also reduce iron-induced lipid peroxidation in male Wistar rats.
2 Materials and methods
2.1 Ethical approval
The study approval was granted by the ethical committee of Centre of Biotechnology and Microbiology, University of Peshawar, Pakistan (No: 119/UOP/Biotech).
2.2 Extraction and fractionation
P. stewartii was collected from District Abbottabad, Pakistan and was identified in Department of Botany, University of Peshawar, Pakistan. Leaves were cleaned, washed, and shade dried at ambient temperature. After drying, leaves were powered using a grinder. Two hundred grams of powder were mixed with 1 L of ethanol (70% v/v) and kept in rotator shaker for a week. After required incubation period, the filtrate was collected and the concentrated product was obtained using a rotary evaporator (Heidolph, HeiVAP Core, Germany). Further, a suspension of ethanolic extract in distilled water was prepared and fractionated with n-butanol, ethyl acetate, and dichloromethane. The aqueous extract was obtained by hot water treatment and was prepared immediately before use. Sterile capped vials were used to store the extracts at 4 °C until further use.
2.3 Animals and experimental design
Male Wistar rats (age – 50 days; weight – 280–300 g) were obtained from National Institute of Health, Islamabad, Pakistan. The animals were housed in steel cages. All the cages were cleaned regularly in order to ensure healthy animals. The animals were allowed free access to the standard pellet diet and also the water was provided ad libitum. The cages were kept in a well aired room having a 12 h light dark cycle. The animals selected for the experiment were divided into 4 different groups that were further divided into various sub-groups (7 rats in each group). Group 1 was assigned as control group, Group 2 was administered with eserine, Group 3 was administered with ethanol only, and Group 4 was administrated with various concentrations of extracts fractions (aqueous fraction with 4 sub-groups: ethyl acetate fraction with 4 sub-groups). There were 7 rats in each group and sub group. Altogether, 17 groups were designed for this study. The treatment was given intra-peritoneal. The mixing of all the compounds was done separately in accordance with the standard protocol. The animals were treated according to the guidelines of the Brazilian College of Animal Experimentation (COBEA), affiliated with the International Council for Laboratory Animal Science (ICLAS).
2.4 AChE inhibitory activity
The AChE activity was demonstrated as per the modified protocol of Rocha et al. (Rocha et al., 1993). In order to determine AChE activity, animals from each group were sacrificed by blowing their heads (without anaesthesia), and the cerebral tissues were quickly incised and kept on ice. The tissues were homogenised using Tris HCl buffer (50 mM; pH 7.5) and the homogenised product was centrifuged at 4000 × g for 10 min. After centrifugation, the supernatant was collected to pursue AChE activity.
The AChE activity was evaluated in a reaction mixture that contained 200 µL of acetylcholinesterase solution (0.415 U/mL prepared in 0.1 M phosphate buffer; pH 8.0), 100 µL of DTNB [5,5′-dithio-bis(2-nitrobenzoic)] acid solution, 500 µL of phosphate buffer (pH 7.0), and 50 µL of supernatant. The reaction mixture was incubated at room temperature for 20 min, following the addition of 100 µL of 0.05 M acetylcholine iodide solution as substrate. The absorbance was read at 412 nm using UV–Visible spectrophotometer (Shimadzu UV-1780) and the inhibitory activity (%) was estimated as:
2.5 Lipid peroxidation assessment
Since free radicals damage the brain tissues, cerebral tissues were used for thiobarbituric acid reactive substances (TBARS) assay. The lipid peroxidation assay was assessed by estimating TBARS production using the methodology of Puntel et al. (2007). Plant extracts fraction (100 µL), purified gallic acid, and quercetin were mixed separately into the freshly prepared 5 mM of sodium nitroprusside (SNP), Fe2SO4 (10 mM), and 2 mM of 3-nitropropionic acid (3-NPA). Pro-oxidants were used in order to induce oxidative stress which causes lipid peroxidation in the body. Following incubation time, acetate buffer, 8.1% SDS, and TBA was pipetted and a further incubation was done for 60 min at 100 °C. The reaction of TBA with malondialdehyde can be assessed with the production of light pink color. After cooling the reaction mixture, the absorbance was read at 532 nm.
2.6 Statistical analyses
The data was analysed statistically using one-way ANOVA, followed by DMRT (Duncan's Multiple Range Test) (for TBARS) and Bonferroni’s multiple comparison tests (for AChE activity). P value < 0.05 was considered significant. All the data included in this study was presented as mean ± standard error of mean (mean ± SEM).
3 Results and discussion
3.1 AChE inhibitory activity
Results demonstrated that the aqueous extract fraction was unable to inhibit AChE activity at varied concentrations (Fig. 1A). On the other hand, ethyl acetate fraction significantly (P = 0.01) inhibited the enzyme in a concentration dependent manner in comparison to the control group (Fig. 1B). Gallic acid and quercetin showed higher inhibition of AChE activities than the ethyl acetate extract fractions (Fig. 1C and 1D). The IC50 values for aqueous extract fractions, ethyl acetate extract fractions, gallic acid, and quercetin were estimated as 72.6 ± 2.72, 30.41 ± 0.56, 8.4 ± 0.22, and 6.82 ± 0.2 µg/mL, respectively (Fig. 2).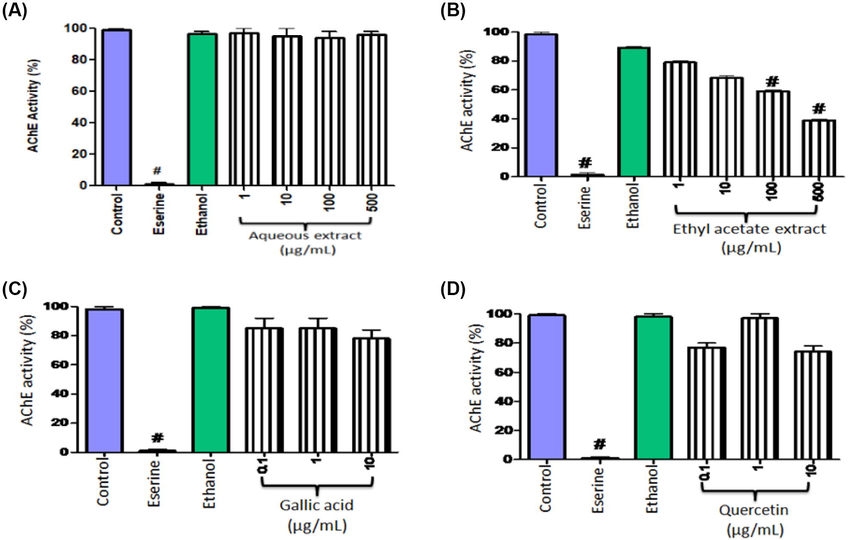
AChE inhibitory activity (%) of aqueous extract fractions (A), ethyl acetate extract fractions (B) of P. stewartii, gallic acid (C), and quercetin (D). Eserine was used as positive control. Data were a representative of mean ± SEM.
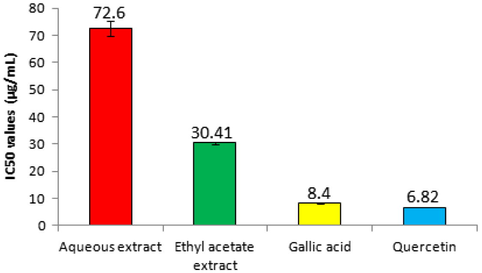
IC50 values for aqueous extract fractions, ethyl acetate extract fractions, gallic acid, and quercetin.
Acetylcholine is an important neurotransmitter distributed widely throughout the nervous system (Halder and Lal, 2021). Cholinesterase is classified into AChE and butyrylcholinesterase. AChE is a member of α/β hydrolases family. AChE causes the hydrolysis of acetylcholine, both in synapses and neuromuscular intersection, which results in nerve impulse termination (Asefy et al., 2021). It is reported that AChE is a good candidate for drug designing in AD treatment; as in AD there is a deficiency of acetylcholine (Zhou et al., 2021). After its transport to synapses, acetylcholine is hydrolysed into choline and acetate (Voet and Voet, 1995; Colović et al., 2013). Current strategy is to focus on the blockage of the AChE function in order to increase the quantity of acetylcholine. Most acetylcholinesterase drugs being used to treat AD are AChE inhibitors.
Several in vitro and in vivo studies have revealed the role of medicinal plants in treating neurological disorders (Ratheesh et al., 2017; Faheem et al., 2022). Suganthy et al. (2009) reported AChE inhibitory potential of Avicennia officinalis methanolic extract producing an IC50 value of 1.24 ± 0.011 mg/mL and butyryl cholinesterase inhibition with an IC50 value of 0.911 ± 0.007 mg/mL. Similarly, the ethanolic extract of Acacia catechu seeds exhibited promising AChE inhibition potential with an IC50 value of 204.38 ± 2.54 μg/mL and recommended for the brain disorders management (Thangavelu and Ramasamy, 2015). In another study, the hydro-alcoholic extract of Hemidesmus indicus exerted significant AChE inhibitory activity with an IC50 value of 28.4 ± 0.92 μg/mL (Kadiyala et al., 2014). Additionally, the aqueous and the methanolic extract of H. indicus roots also exhibited in vitro AChE inhibitory activity (Kundu and Mitra, 2013). In the present work, the ethyl acetate extract fractions of P. stewartii inhibited AChE activity significantly. Therefore, P. stewartii could be considered to assess its possibility as potent alternative candidate for designing effectual drug towards the treatment of AD.
3.2 Lipid peroxidation assessment
Results of TBARS levels induced by various pro-oxidant agents are illustrated in Table 1. In fact, the TBARS content measured the degree of peroxidation. Both the fractions were found to be protective against lipid peroxidation but ethyl acetate extract fraction reduced the TBARS levels significantly (P = 0.001) in comparison to the aqueous extract fraction. However, gallic acid and quercetin exhibited higher reductions of pro-oxidants than ethyl acetate and aqueous extract fractions. Findings of this context were found in consistence with the report of Tripathi et al. (1997). Rubiadin, (a dihydroxy anthraquinone) showed its remarkable anti-lipid peroxidation activity induced by FeSO4 and t-butyl hydroperoxide in a concentration dependent manner. The inhibitory activity was estimated maximum in Fe+2 induced lipid peroxidation. In this investigation, quercetin was found to be more potent as compared to the extracts and gallic acid. This might be due to the lipophilic characteristic of quercetin which enhanced its potency to block lipid peroxidation. Data represent mean ± SEM; SNP - Sodium nitroprusside; FeSO4: Iron (II) sulphate; 3-NPA − 3-nitropropionic acid.
P. stewartii
Pro-oxidants
SNP
FeSO4
3-NPA
Aqueous extract fraction
540.4 ± 2
480.8 ± 24.6
620.2 ± 16.8
Ethyl acetate extract fraction
380.4 ± 12.4
410 ± 16.6
392.8 ± 2
Gallic acid
106.82 ± 0.32
114.54 ± 0.22
118.6 ± 0.44
Quercetin
98.6 ± 0.22
90.2 ± 0.24
101.4 ± 0.1
4 Conclusion
In a nutshell, ethyl acetate extract fractions of P. stewartii leaves possessed promising AChE inhibitory activities with an IC50 value of 30.41 ± 0.56 µg/mL. Additionally, both the extracts fractions were found to be protective against lipid peroxidation but ethyl acetate extract fraction reduced the TBARS levels significantly (P = 0.001) as compared to aqueous fraction. P. stewartii leaves could be considered an ideal candidate for designing new drugs that can be beneficial in the treatment of AD and anti-oxidative conditions.
Acknowledgements
We are grateful to Dr. Philip (Ireland) and Dr. John Stephen (Austria) who critically analysed the data for improving this manuscript. This work is funded by Taif University Researchers Supporting Project number (TURSP-2020/75), Taif, Saudi Arabia.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication. We confirm that the manuscript has been read and approved by all named authors.
References
- Ethno-botanical studies from Northern Pakistan. J. Ayub Med. Coll. Abbottabad. 2009;21:52-57.
- [Google Scholar]
- Neuroprotective effects of Melissa officinalis on oxygen and glucose deficiency induced damage in rat's brain cortex slices. Int. J. Pharmacol.. 2018;14:781-786.
- [Google Scholar]
- Lipid peroxidation reduction and hippocampal and cortical neurons protection against ischemic damage in animal model using Stellaria media. Saudi J. Biol. Sci.. 2022;29:1887-1892.
- [Google Scholar]
- Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry. 2003;74:863-866.
- [Google Scholar]
- Melatonin hormone as a therapeutic weapon against neurodegenerative diseases. Cell. Mol. Biol.. 2021;67:99-106.
- [Google Scholar]
- Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol.. 2013;11:315-335.
- [Google Scholar]
- Plants as potential sources for drug development against Alzheimer’s disease. Int. J. Biomed. Pharm. Sci.. 2007;1:83-104.
- [Google Scholar]
- Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem.. 2009;17:867-871.
- [Google Scholar]
- The use of herbal medicine in Alzheimer's disease-a systematic review. Evid-Based Compl. Alt. Med.. 2006;3:441-445.
- [Google Scholar]
- Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem.. 2021;14:103106
- [CrossRef] [Google Scholar]
- A comprehensive review on antiepileptic properties of medicinal plants. Arab. J. Chem.. 2022;15:103478
- [CrossRef] [Google Scholar]
- The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66:137-147.
- [Google Scholar]
- Anti-infective potential of a medicinal plant Pimpenella stewartii used traditionally in hazara. J. Ayub Med. Coll. Abbottabad. 2016;28:555-558.
- [Google Scholar]
- Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front. Immunol.. 2021;12
- [CrossRef] [Google Scholar]
- Screening of siddha medicinal plants for its in-vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity. Pharmacogn. Mag.. 2014;10:S294.
- [Google Scholar]
- Kamran, M., Kousar, R., Ullah, S., Khan, S., Umer, M.F., Rashid, H.U., 2020. Taxonomic distribution of medicinal plants for Alzheimer’s Disease: a cue to novel drugs. Int. J. Alzheimer’s Dis. 2020. Article ID 7603015.
- Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm) Pharmacol. Biochem. Behav.. 2002;72:953-964.
- [Google Scholar]
- Flavoring extracts of Hemidesmus indicus roots and Vanilla planifolia pods exhibit in vitro acetylcholinesterase inhibitory activities. Plant Food Hum. Nutr.. 2013;68:247-253.
- [Google Scholar]
- Clinical investigation on the impact of Cannabis abuse on thyroid hormones and associated psychiatric manifestations in the male population. Front. Psychiatry. 2021;12:730388
- [CrossRef] [Google Scholar]
- Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res.. 2009;34:973-983.
- [Google Scholar]
- Medicinal plants and Alzheimer's disease: from ethnobotany to phytotherapy. J. Pharm. Pharmacol.. 1999;51:527-534.
- [Google Scholar]
- Oxalate modulates thiobarbituric acid reactive species (TBARS) production in supernatants of homogenates from rat brain, liver and kidney: effect of diphenyl diselenide and diphenyl ditelluride. Chem. Biol. Interact.. 2007;165:87-98.
- [Google Scholar]
- Role of medicinal plants in neurodegenerative diseases. Biomanufacturing Rev.. 2017;2:1-16.
- [Google Scholar]
- Effects of early undernutrition on kinetic parameters of brain acetylcholinesterase from adult rats. Acta Neurobiol. Exp.. 1993;53:431.
- [Google Scholar]
- Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem.. 2008;56:4855-4873.
- [Google Scholar]
- Cholinesterase inhibitory effects of Rhizophora lamarckii, Avicennia officinalis, Sesuvium portulacastrum and Suaeda monica: mangroves inhabiting an Indian coastal area (Vellar Estuary) J. Enzyme Inhib. Med. Chem.. 2009;24:702-707.
- [Google Scholar]
- Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol.. 2010;41:375-383.
- [Google Scholar]
- Antimicrobial activity of the essential oils of the Umbelliferae family Part III. Pimpinella anisum, Pimpinella acuminata and Pimpinella stewartii. Pak. J. Sci. Ind. Res.. 1986;29:352-356.
- [Google Scholar]
- Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front. Aging Neurosci.. 2018;10:3.
- [CrossRef] [Google Scholar]
- In vitro acetyl cholinesterase inhibitory assay of Acacia catechu willd ethanolic seed extract. Pharmacogn. J.. 2015;7:280-282.
- [Google Scholar]
- Rubiadin, a new antioxidant from Rubia cordifolia. Indian J. Biochem. Biophys.. 1997;34:302-306.
- [Google Scholar]
- Current and future therapy in Alzheimer’s disease. Fundam. Clin. Pharmacol.. 2008;22:265-274.
- [Google Scholar]
- Serine protease: In; Biochemistry. 1995;5:390.
- Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J. Psychopharmacol.. 2005;19:414-421.
- [Google Scholar]
- Kinetics-driven drug design strategy for next-generation acetylcholinesterase inhibitors to clinical candidate. J. Med. Chem.. 2021;64:1844-1855.
- [Google Scholar]







