Translate this page into:
Acceleration of histopathological and biochemical defense patterns in mung bean with biopriming of Amycolatopsis sp. SND-1 against Cercospora leaf spot disease
⁎Corresponding author. sreenivasankud@gmail.com (Sreenivasa Nayaka)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Failure of chemical control methods in conferring protection against Cercospora leaf spot (CLS) disease in mung bean crop led to the investigation of biocontrol agents for effective management of the CLS. The current study was focused on increased defense response (histochemical and biochemical) in mung bean plants by alginate pellet formulation of endophytic Amycolatopsis sp. SND-1 (SND-1) against CLS disease.
Methods
The sodium alginate bioformulation of SND-1 was prepared by standard method using CaCl2 and evaluated for viability and contamination. Greenhouse study of prepared SND-1 formulation was performed on mung bean plants against CLS infection. Further, the acceleration of histochemical depositions (lignin, H2O2 and phenol) by differential staining methods and biochemical defense enzymes like PAL, GLU, and POX, phenolic, flavonoid and chlorophyll contents and cell death analysis were evaluated through microscopic and spectroscopic studies.
Results
The viability assay of the prepared formulation exhibited a negligible reduction in SND-1 spores (99.5% to 86.55%) throughout the six months of storage period. In-vivo (Greenhouse) study exhibited increased growth parameters in alginate pellet formulation of SND-1challenged with Cercospora pathogen (SND-1+Pathogen) treated mung bean plants in comparison with Control (SDW) and only pathogen infested plants. Consequently, the plants with SND-1+Pathogen treatment showed significant reduction in disease severity up to 56%. Furthermore, the microscopic evaluation of histochemical defense markers depicted increased deposition of lignin (82.43±0.31%), H2O2 (47.97±0.66%), and phenol (76.74±0.79%) and cell death analysis through microscopic observation exhibited lesser dead cells of 31% in SND-1+Pathogen treatment in comparison with other treatments. Defense patterns at biochemical level hinted the elevation in activities of defense enzymes like PAL, GLU and POX at 12 and 24 h post pathogen inoculation (hpi) in SND-1+Pathogen treatment. The formulation treatment with pathogen (SND-1+Pathogen) also substantiated that enhanced total phenolic, flavonoid, and chlorophyll contents at different time intervals.
Conclusions
Overall the present investigation showed that Amycolatopsis sp. SND-1 formulation confers the protection in mung bean upon Cercospora infection and it might be an effective eco-friendly approach towards sustainable agriculture.
Keywords
Amycolatopsis SND-1
Mung bean
Cercospora leaf spot
Induced systemic resistance
Histochemical defense
Biochemical defense enzymes
1 Introduction
Mung bean is a foremost legume crop growing predominantly in Asia and worldwide. Over 7 million hectares were recorded for the mung bean crop alone (Nair et al., 2019). The seeds of mung bean are rich source of proteins, antioxidants, and fiber. The sprout contains abundant niacin, thiamine, and ascorbic acid profile. The seeds and sprouts of the mung bean are excellent source of minerals such as magnesium, phosphorus, calcium and iron (Itoh et al., 2006). A recent estimation of yield consistency on mung bean exhibited a reduction in the total production from 2.5 to 3.0 tons/hectare to 0.5 tons/hectare, and abiotic and biotic constrain were considered as significant cause of the yield reduction (Pratap et al., 2019). Among the biotic stresses the CLS disease caused by the deuteromycete fungus Cercospora canescens (C. canescens) is the most devastating entity which shows maximum yield loss. The CLS is a foliar disease and showed high yield loss in 50–70% and up to 96% in mung bean cultivation (Rafiq et al., 2022). The symptoms shows water soaked spots with grey borders and can affect leaf, petiole, pods and cause severe damage. Apart from the aggressiveness of the pathogen, the susceptible nature of the mung bean varieties and long term survival capability (Dormant mycelium in plant debris as primary inoculums) of the pathogen play significant affecting nature. The application of chemical fungicides poses chemical contamination and pollution, and repeated adaptation of these fungicides develops resistance in pathogens. An alternative eco-friendly method for controlling these diseases and improving the resistance in mung bean plants is a much-needed strategy to achieve sustainable cultivation. Biological control is the eco-friendly strategy of controlling the plant diseases that shows employment of beneficial living organism to counteract the specific or targeted plant pathogen with. This strategy includes the pathogen and disease control through the antibiosis, parasitism and resource competition (El-Tarabily et al., 2009).
Bioformulations developed with beneficial microbes (Bacteria, actinomycetes, and non-pathogenic fungi) can trigger the defense mechanisms and reduce the pathogen's multiplication and severity of diseases. Several studies reported that actinomycetes (Soil and endophytic) play an essential role as antifungal agents and elicit the induced systemic resistance in different crop plants (Bhat et al., 2022; Sreenivasa et al., 2020). Endophytic actinomycetes colonize the different tissues (obligate or facultative) in plants and create the symbiotic relationship. Furthermore, the colonized actinomycetes play significant role in plant defense enhancement and survival strategies during biotic and abiotic stresses (Gopalakrishnan et al., 2019). The development bioformulations through the encapsulation of plant growth promoting microbes exhibits the protection, prolonged and controlled release of the formulated microbes’ spores. The encapsulation of microbes through sodium alginate is the most convenient for its rapid diffusion of the spores and compounds preferable for large scale application. Moreover, the alginate bioformulations shows high adsorption, excellent carrier and harmless to the environment (Miyada et al., 2017). El-Tarabily et al. (2009) reported increased plant growth and decreased severity of Pythium disease in cucumbers by glucanase-producing endophytic actinomycetes through the acceleration of induced protection. In another study, significant plant growth enhancement was observed when plants were exposed to stress conditions with the endophytic Amycolatopsis pittospori sp. nov., Amycolatopsis sp. BCA-696, and Streptomyces rochei strain PTL2 in rice, sorghum and tomato plants (Onuma and Christopher, 2021; Gopalakrishnan et al., 2019; Ting et al., 2017; Miyada et al., 2017). In another report Sabarathanam et al. (2015) studied the significant biocontrol activity of Streptomyces sp. strain Di-944 formulation (Alginate-Kaolin-based) against damping-off severity in tomato plants. Several actinomycetes based formulations such as ‘Mycostop’ (Streptomyces griseovirids K61 strain) for soil and seed borne fungal diseases, ‘Actinovate’ registered for control of Pythium ultimum and Rhizoctonia solani and PGP trials, ‘Mykocide KIBC’ registered in South Korea for controlling of brown pacth, powdery mildew and grey mold diseases and ‘Actofit’, ‘Incide SP’ and ‘Actin’ are used for the control of diverse diseases of the crop plants in Ukraine, USA and in India were commercially available in the market (Churasia et al. 2018).
Plant defense response is initiated at the cellular level by depositing frontline defense barriers (lignin, hydrogen peroxide and phenol) in cell walls (Sudisha et al., 2020). Deposition of increased lignin in plant's cell walls was reported to block the pathogen invasion (Milan et al., 2021). Another barrier, H2O2, and phenol effectively induced the mechanism of programmed cell death of plant pathogens (Singh et al., 2021). Several researchers have found that acceleration in the activity of these enzymes can significantly counteract the pathogen. In another report, formulation of endophytic Streptomyces spp. elicited the PAL, GLU and POX resulted in the suppression of S. rolfsii in chickpea, M. phasiolina in mung bean, Phytopthora megakarya in cocoa plants (Singh et al., 2021; Sangeetha et al., 2018; Aristide et al., 2022). The S. rimosus and S. monomycini increased the phenolic and flavonoid contents that showed effective inhibition of damping-off disease causing pathogen P. drechsleri in cucumber plants (Akram et al., 2017). Considering the noteworthy literature of actinomycetes formulations, the current study performs the effect of bioformulation of endophytic Amycolatopsis sp strain SND-1 on the acceleration of plant growth against Cercospora disease in mung bean and elicitation of resistance at histochemical and biochemical levels.
2 Materials and methods
2.1 Collection of actinomycetes strain, pathogen, and mung bean variety
For the current study, the previously identified Amycolatopsis sp. strain SND-1 (SND-1) with Accession No-OM807224 and characterized for production of indole acetiac acid (IAA), gibberellic acid (GA3), Cytokinin, Siderophore, hydrogen cyanide (HCN), Ammonia and phosphate solubilization and plant growth promotion in mung bean was selected (Basavarajappa et al., 2023). The highly susceptible variety of mung bean seeds (DGGV-2) and pathogen Cercospora canescens were collected from University of Agricultural Sciences (UAS), Dharwad, Karnataka, India.
2.2 Preparation of sodium alginate pellet formulation
Final density of SND-1 spores ≈ 106 CFU mL−1 was adjusted by the cell count method. The sodium alginate pellets formulation of SND-1 was prepared according to Miyada et al., (2017). Finally, the dried SND-1 pellets were evaluated for the spores at ≈ 2×106 CFU g−1. The prepared sodium alginate SND-1 formulation was stored with the double distilled water in the sterilized container for further experimental use.
2.3 Purity and viability of pellet formulation
The purity and viability assay of SND-1 formulation was done according to Miyada et al., (2017), by cell counting plate method (CFU g -1). All the viability trials were performed in triplicates for statistical significance (Miyada et al., 2017).
2.4 Pathogen inoculums preparation
Inoculums of C. canescens pathogen were prepared according to (Gopalakrishnan et al., 2019) and final spore load (105 CFU mL−1) was adjusted.
2.5 Greenhouse study
The growth promotion and biocontrol assay was conducted under controlled greenhouse (in-vivo) conditions during the pre monsoon season (May to July) with the pots (15 × 10 cm) filled with sterilized soil, farm yard manure and sand (1:1:2). The dried SND-1 alginate pellets (0.14 g) were added to the soil around the seed inoculation area by soil drench method. Then the mung bean seeds were surface sterilized with 2% sodium hypochlorite (NaOCl) for 2–5 min, and then multiple times with SDW and sowed (4 seeds per pot). The inoculation of the pathogen was performed for two weeks old mung bean plants by spraying the final spore load for 5 consecutive days at early morning conditions. The triplicate trials of each treatment were considered for statistical analysis. After 35 days, the plants belonging to individual treatments were uprooted and subjected to evaluate growth parameters. Disease severity assessed by employing the 5-class scale which includes measurements such as 0=no cerspora symptoms, 1=0–25% of Cercospora symptoms, 2=26–50% of Cercospora symptoms, 3=51–75% Cercospora symptoms and 4=76–100% Cercospora symptoms and the values were converted into percentage. Finally, each treatment's disease severity was measured using the following formula.
(R = Disease rating and N = Number of mung bean plants. H = Highest disease rating, and T = Total number of plants).
2.6 Assessment of induced systemic resistance
The induced systemic resistance in treated mung bean plants was analyzed histochemically through the localization of lignin, hydrogen peroxide (H2O2), and phenol. Biochemical defense patterns were evaluated by measuring the activity of antioxidant enzymes like PAL, POX and GLU in the leaf epidermis. Each experiment was performed with fresh leaves (1 g) from each treatment at specified time intervals of hours after post pathogen inoculation (hpi).
2.7 Microscopic observation of cell wall depositions
Deposition of lignin was assessed according to Milan et al. (2021). The leaf peelings were observed for lignin deposition (reddish brown depositions) under a microscope (OLYMPUS, CX23) at 40 X magnification and deposition percentage (cells) was calculated. The H2O2 deposition was examined by using 3, 3- Diaminobenzidine (DAB) and phenol deposition in each treatment was performed according to Milan et al. (2021) using 0.05% toluidine blue staining technique. The % cell death was evaluated using trypan blue staining method (blue color depositions).
2.8 Estimation antioxidant defense enzymes
The enzyme extract obtained from the homogenized leaf samples was used as an enzyme source, and at respective time intervals. The Phenylalanine ammonia-lyase (PAL) activity was estimated using a spectrophotometer (Shanghai Metash Instruments Co., Ltd., Shanghai, China) at 290 nm and β-1,3-glucanase (GLU) activity was evaluated spectroscopically at 540 nm using laminarin (1%). The Peroxidase (POX) quantification was done using the substrate guaiacol (25%) and the activity was at 470 nm. Peroxidase and GLU activities were expressed in units/mg protein/min (Milan et al., 2021).
2.9 Estimation of phenolic, flavonoid, and chlorophyll content
The phenolic content was quantified spectrophotometrically according to Vernon et al. (1993) by Folin-Ciocalteu (FC) method by measuring the absorbance at 765 nm. Flavonoid quantification was performed using aluminum chloride method and recorded the absorbance at 425 nm. The chlorophyll content was estimated using N, N′-dimethylformamide (DMF) and absorption spectra was recorded between 350 and 750 nm (Natale et al., 2023).
2.10 Statistical analysis
The viability assay, histochemical depositions and biochemical tests were performed in triplicates trials and results were tabulated as Mean ± Standard deviation (SD).
3 Results
3.1 Purity and viability of pellet formulation
The prepared sodium-alginate pellet formulation of SND-1 exhibited alginate beads with the 0.3 mm of approximate size and stored in sterilized container with distilled water. The SND-1 pellet formulation showed there is no contamination and a slight decrease up to 6 months storage period (Fig. 1 A-F). It was recorded 2 × 106 CFU g−1 (99.5%) up to 30 days from the day of formulation preparation and reached 1.73 × 106 CFU g−1 (86.5%) after 6 months of storage period (Fig. 1 G).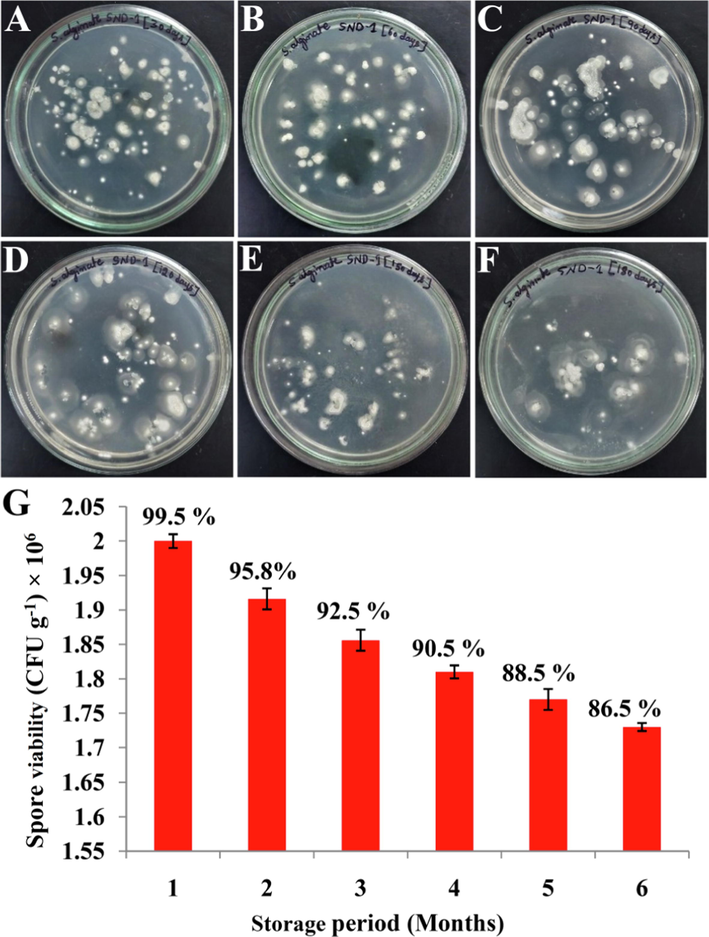
Purity and viability assay: (A-F) Spore viability of sodium alginate formulation of SND-1 strain, evaluated up to 6 months (1 month interval) of storage period. (G) Histogram representing % of viability of sodium alginate formulation of SND-1.
3.2 Greenhouse study
In-vivo growth promoting and biocontrol assay (after 35 days of the greenhouse study) showed significant increase in growth parameters and less disease severity (DSI) in alginate pellet formulation treated mung bean plants (Fig. 2A) in comparison with control and pathogen treatments (Table 1). The plants treated with SND-1+Pathogen exhibited root length 13.9 ± 0.04 cm, shoot length 30.05 ± 0.31 cm, root fresh weight 1.79 ± 0.05 g, shoot fresh weight 3.34 ± 0.55 g and 0.76 ± 0.03 g of dry weight (Fig. 2B). Whereas minimum root length of 8.3 ± 0.30 cm, shoot length of 19.93 ± 0.55 cm, root fresh weight of 0.81 ± 0.02 g, shoot fresh weight of 1.97 ± 0.03 g and 0.42 ± 0.03 g of total dry weight. In the treatment with only SND-1 formulation and Control (SDW) the moderate growth parameters were observed. The biocontrol efficacy of SND-1 alginate formulation was assayed by calculating the disease severity (Table 1) in comparison with all the treatments and very low severity 43.63% in SND-1+Pathogen was recorded. In only SND-1 formulation treatment it was observed 8.85%, and disease severity of 55.2% was noticed in Control (SDW) plants and highest 88.19% of disease severity was found in only pathogen treated plants (Fig. 2C).
Effect of SND-1 sodium alginate formulation on mung bean plants under greenhouse conditions: (A) Growth patterns of mung bean plants upon different treatments, (B) Increased root length among the different treatments and (C) Disease severity among the different treatments.
Treatments
Root length (cm)
Shoot length (cm)
Root fresh weight (g)
Shoot fresh weight (g)
Total dry weight (g)
Disease
Severity
(%)
Control (SDW)
10.6 ± 0.3
27.06 ± 0.25
1.21 ± 0.02
2.23 ± 0.15
0.59 ± 0.03
55.2
Control+Pathogen
8.3 ± 0.30
19.93 ± 0.55
0.81 ± 0.02
1.97 ± 0.03
0.42 ± 0.03
88.19
SND- 1
11.27 ± 0.29
27.62 ± 0.52
1.53 ± 0.28
2.62 ± 0.25
0.62 ± 0.04
8.85
SND-1+Pathogen
13.9 ± 0.04
30.05 ± 0.31
1.79 ± 0.05
3.34 ± 0.55
0.76 ± 0.03
43.63
3.3 Evaluation of cell wall depositions
Microscopic observation of stress-responsive defense markers such as lignin, H2O2 and phenol deposition resulted maximum deposition of lignin 82.43 ± 0.31% at 24 hpi in SND-1+Pathogen treatment and in only SND-1 formulation treated plants it was found that 68.07 ± 0.27% at 24 hpi. Lignin localization in Control+Pathogen treatment showed 33.16 ± 0.27% lignin at 24 hpi and Control (SDW) plants exhibited a very low accumulation of 27.17 ± 0.30% at 24 hpi (Fig. 3A). The early deposition of H2O2 was observed at 4 hpi and reached 47.97 ± 0.66% at 24 hpi was observed in SND-1+Pathogen treatment and in SND-1 formulation only it was found that 43.08 ± 0.19% at 24 hpi (Fig. 3B). The plants received Control+Pathogen showed maximum 23.28 ± 0.43% of deposition of H2O2 at 24 hpi and in control (SDW) treatment it was exhibited 21.12 ± 0.18% of H2O2 accumulation at 24 hpi.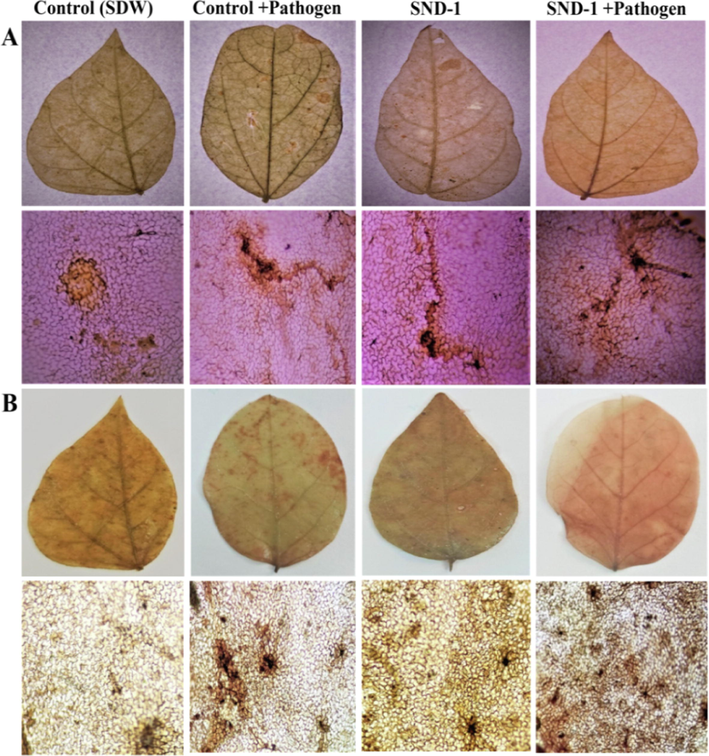
Histochemical depositions in treated mung bean plants: (A) Lignin deposition in leaves and leaf epidermis (reddish brown deposition along the cell wall) stained with phloroglucinol at 24 hpi. (B) Visualization of hydrogen peroxide (H2O2) deposition (brown color deposition along the cell wall) in leaves and leaf epidermis upon staining with DAB at 24 hpi.
The deposition of phenol was noticed early (at 4 hpi) and maximum phenol accumulation 76.74 ± 0.79% at 24 hpi was observed in SND-1+Pathogen received plants (Fig. 4A). A fair amount of phenol deposition with 45.33 ± 0.34% and 37.00 ± 0.26% was detected in only SND-1 formulation as well as in Control+pathogen treated plants. The Control (SDW) treated plants showed delayed accumulation of phenol 33.48 ± 0.45% 24 hpi. Trypan blue staining method revealed that highest percentage of cell death (65%) in pathogen treated and 53% in Control (SDW) mung bean plants (Fig. 4B). Where as in only SND-1 formulation treatment 41% of cell death and significant reduction in cell death 31% was observed SND-1+Pathogen treatment. The time course analysis of histochemical depositions was graphically represented in Fig. 5 A-C.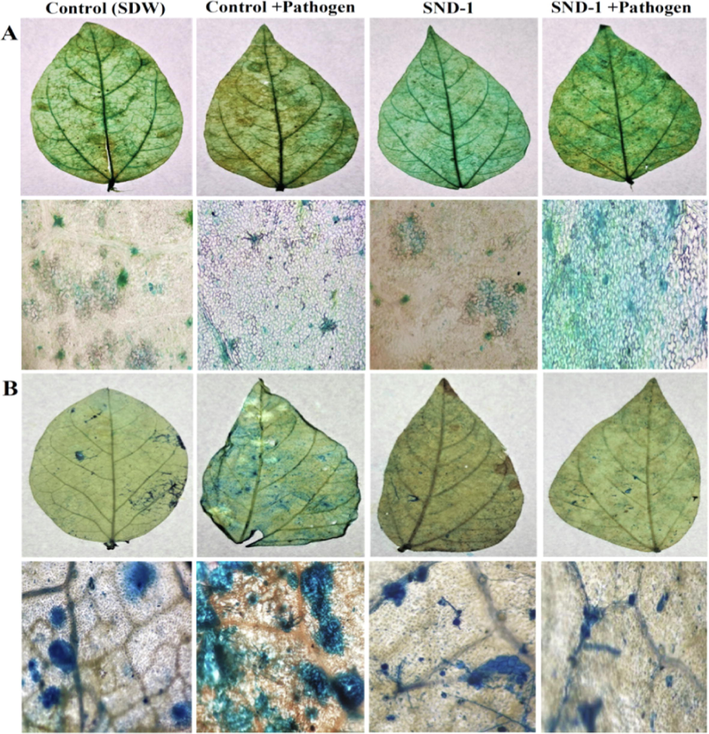
Histochemical deposition and cell death analysis in mung bean plants: (A) Deposition of phenol in mung bean leaves and leaf epidermis (greenish blue deposition along the cell wall) upon staining with toluidine blue at 24 hpi. (B) Detection of cell death in mung bean leaves and leaf epidermis staining with trypan blue (dark blue stain represents dead cells). The results were displayed by considering the necrosis spot and compared with complete leaf blade surface.
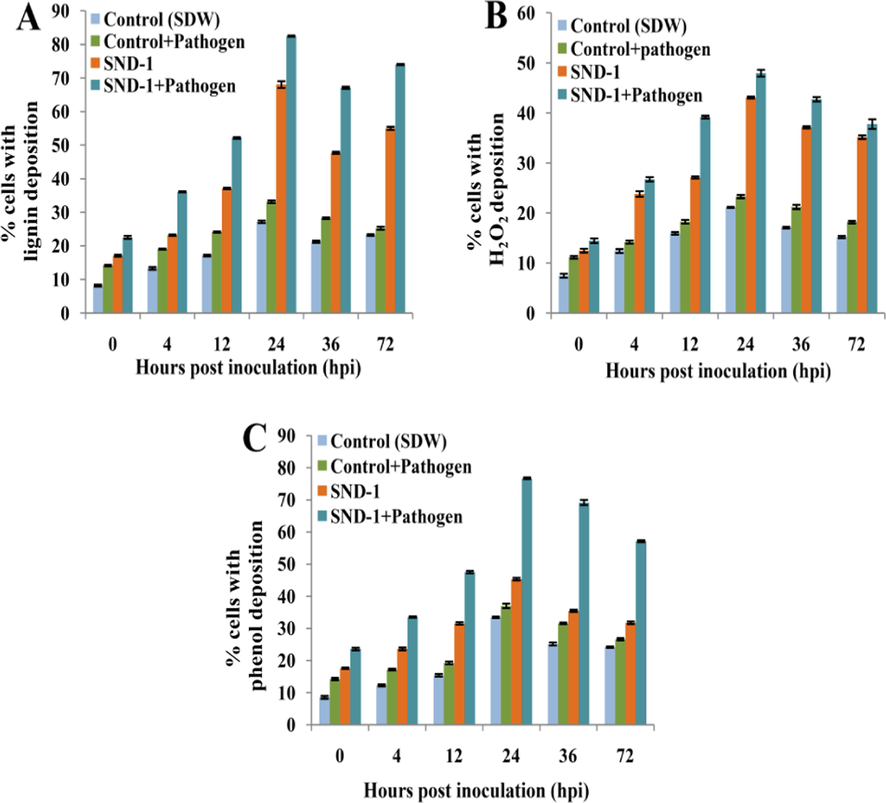
Histogram representing histochemical depositions in treated mung bean plants: (A) Time course analysis of lignin deposition. (B) Time course analysis of H2O2 deposition. (C) Time course analysis of phenol deposition.
3.4 Estimation of antioxidant defense enzymes
The PAL activity in SND-1+Pathogen was initiated at early (0 h) after pathogen inoculation and maximum activity 45.48 U was observed at 24 hpi (Fig. 6A). The only SND-1 formulation treated plants showed 42.25 U of PAL activity at 24 hpi. PAL activity in Control+Pathogen treated plants showed no activity up to 4 hpi and maximum activity 9.54 U was recorded at 24 hpi. Where as in Control (SDW) treatment the highest PAL activity was recorded 4.79 U at 24 hpi. The varied activity of GLU was found in performed treatments and highest GLU activity 14.54 U at 24 hpi was exhibited in plants treated with SND-1+Pathogen at 24 hpi (Fig. 6B). A progressive increase in GLU activity and maximum 12.13 U was recorded in SND-1 alone treatment at 24 hpi. The plants treated with Control+Pathogen showed 9.05 U of GLU and in control (SDW) showed delayed activity of GLU 6.02 U at 24 hpi and was declined drastically at later time intervals. The increased 21.21 U of POX activity at 24 hpi was obtained in the SND-1+pathogen treatment (Fig. 6C). Whereas SND-1 only treatment exhibited maximum POX activity 19.17 U at 24 hpi respectively. Control+Pathogen treatment displayed 5.2 U of POX activities at 24 hpi, whereas Control (SDW) treatment the elevated POX activity 3.32 U was observed at 24 hpi and later there was gradual decrease in the POX activity was noted up to 72 hpi.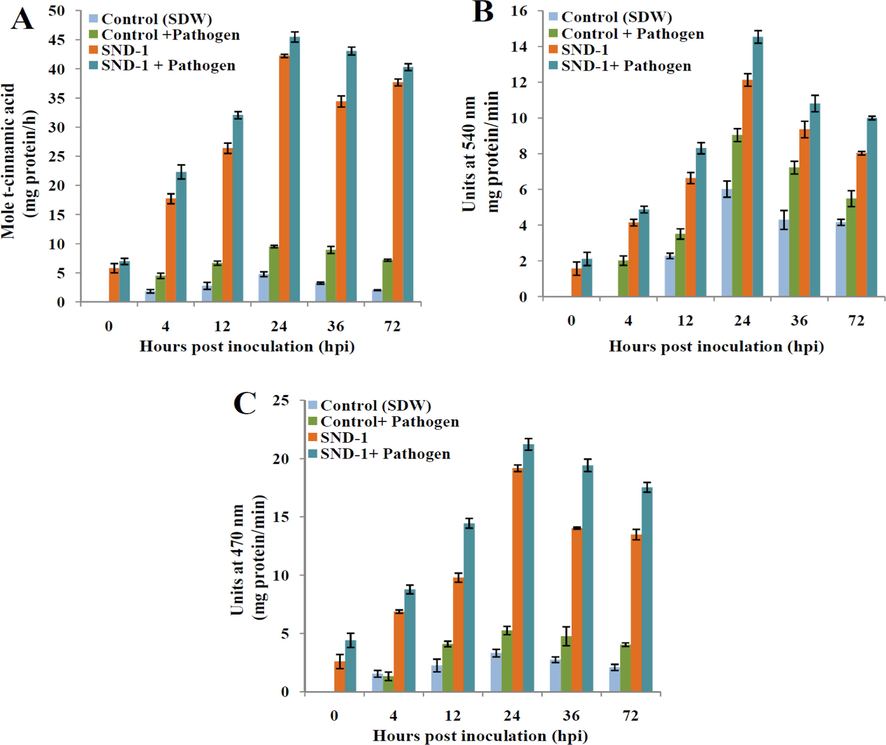
Temporal expression of antioxidant enzymes (biochemical defense) in SND-1 formulation treated mung bean plants: (A) Phenylalanine ammonia-lyase (PAL), (B) β 1, 3-glucanase (GLU) and (C) Peroxidase (POX).
3.5 Estimation of total phenolic, flavonoid, and chlorophyll contents
The highest phenolic content 3.64 ± 0.11 mg at 24 hpi was recorded in plants treated with SND-1+Pathogen (Fig. 7A). A fair phenolic content of 3.46 ± 0.05, 2.70 ± 0.05, and 1.73 ± 0.06 mg/g fresh weight at 24 hpi was recorded in only SND-1 formualtion, Control+Pathogen and control SDW plants. The maximum flavonoid 1.01 ± 0.06 mg was observed in mung bean plants with the treatment of SND-1+Pathogen at 24 hpi (Fig. 7B). Plants treated with only SND-1 formulation, Control+Pathogen and Control (SDW) plants offered 0.80 ± 0.02, 0.60 ± 0.01 and 0.43 ± 0.02 mg/g fresh weight of flavonoid content. The chlorophyll content 1.16 ± 0.03 mg was observed SND-1+Pathogen treatment and 0.82 ± 0.02 mg/g fresh weight in only SND-1 formulation treatment. A fair amount of chlorophyll 0.6 ± 0.02 mg/g fresh weight in Control (SDW) treated and lowest chlorophyll content 0.4 ± 0.01 mg/g fresh weight was exhibited by Control+Pathogen treated mung bean plants (Fig. 7C).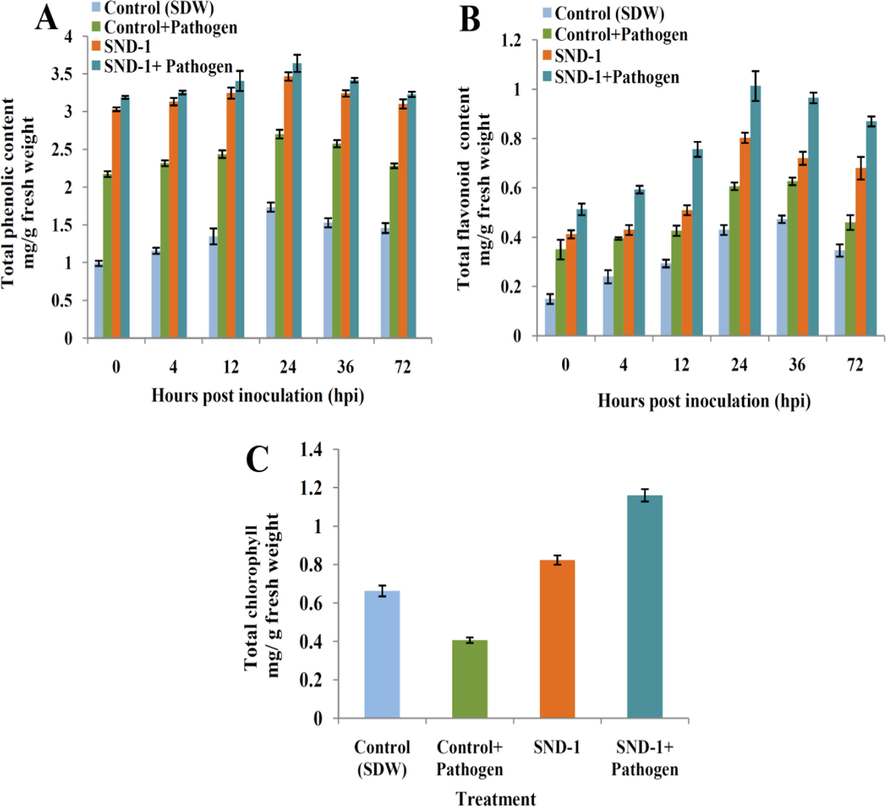
Histogram representing (A) Total phenolic content, (B) Total flavonoid content and (C) Total chlorophyll contents in treated mung bean plants.
4 Discussion
The formulated actinomycetes products were excellent alternatives of chemical products and proven for their biocontrol, growth enhancement and ISR with beneficial actinomycetes in different crop plants (Chakraborty et al., 2022). Furthermore, the chemical fungicides creates the tremendous negative impacts on soil microflora and reduction in soil nutrient profile results the decreased soil quality. The Amycolatopsis sp. SND-1 was previously isolated from Cleome chellidonii Linn. plant and characterized for its in-vitro plant growth and potential antagonistic action against Cercospora pathogen was utilized for extended biocontrol efficacy in the sodium alginate pellet formulation. The prepared SND-1 formulation showed clear distribution of spores, purity and viability test depicted no contamination and also a negligible decrease in the viability of spores up to six months of storage period. Similar study on Streptomyces rochei PTL2 alginate formulation displayed reduction from 2 × 106 CFU g−1 to 1.7 × 106 CFU g−1 of viability of spores at 2 months intervals up to one year of storage period (Miyada et al., 2017).
The greenhouse study resulted significant increase in growth parameters of mung bean and minimum disease severity in SND-1 formulation challenged with C. canescens pathogen when compared with untreated plants. The results were compared with the studies, where alginate formulation of Streptomycetes sp. Di-944 and Streptomyces palmae CMU-AB204T exhibited significant biocontrol activity up to 94% against Rhizoctonia solani and 96 to 97% against Ganoderma boninense infections, and increased the growth in tomato and palm plants up to 30 to 50 cm in comparison with the control and pathogen treatments (Sabarathanam et al., 2002; Kanaporn et al., 2020).
The SND-1 formulation challenged with pathogen significantly enhanced the histochemical defense markers such as lignin, H2O2 and phenol compared with other treatments. Milan et al. (2021) reported that increased depositions of lignin and H2O2 in leaf epidermis of the pathogen P. viticola infected Vitis vinifera L. plants treated with talcum formulation of Trichoderma harzianum and showed potentially up to 82.9% protection from the downy mildew disease. In their study, the histochemical depositions in the Trichoderma harzianum treated Vitis vinifera L. plants exhibited gradual increase at early hours (0, 4, 12 and 24 h) of the of the pathogen infection and decreased at later time intervals (at 36, 48 and 72 h). Similarly, the rhizobacteria treated Vigna radiata (L.) R. Wilczeck plants effectively conferred the M. phaseolina infection through instigating maximum histochemical depositions (Boubakri et al., 2012; Javed et al., 2021). Lignin, H2O2 and phenol accumulations can acts as mechanical barrier, signal molecule, and synthesis of specific substrates that alters modifications of defense enzymes that decrease the diffusion of toxins by pathogen. Lignin (aromatic polymer) deposition increases the strength and imperviousness, perturbations and high level of lignin biosynthesis induce phenylpropanoid pathway and the corresponding defense related enzymes results in controlling the invasion of the pathogen. The H2O2 acts as stress tolerance and higher concentrations leads to oxidation of cell components such as lipids, proteins and nucleic acids which shows enhancement in defense state in pathogen affected cells (Milan et al., 2021). Another function of H2O2 in plant defense is it involves in the mechanism like superoxide dismutation (SOD) and it's higher accumulation leads to the increased and regeneration of enzymatic activity such as catalase and Glutathione reductase (GSH reductase) which significantly involved in the upregulation of antioxidant and oxidation reactions during the pathogen suppression. Similarly phenol accumulations at pathogen infected sites slowdown the pathogen proliferation through the mechanisms like synthesis of stress related simple phenols, phytoalexins and hydoxycinnamic acids etc. (Lee et al., 2019).
The upregulation of defense enzymes in SND-1 formulation with the inoculation of Cercospora pathogen exhibited maximum induced protection by the associated PAL, POX and GLU antioxidant enzyme activities. The similar study reported the enhanced activity of PAL, GLU and POX in T. virens-Tv4 treated mung bean plants exhibited maximum protection against R. solani (Alfi et al., 2020). The accelerated activity of these defense enzymes can counteract the pathogen at the time of infection and plays a significant role in induction of resistance patterns in plants through the biosynthesis of cell wall thickenings such as lignin, H2O2 and phenol which play vital role as defense barriers. PAL initiates primary enzyme metabolism (phenylpropanoids) which leads to increased synthesis of defense related phenols and lignins. The GLU is the polysaccharide that counteracts the pathogen cell wall and hydrolyzes the chitin a major component of the fungal cell wall (Javed et al., 2021). POX is the oxidoreductive enzyme which directly correlates with the suppression of pathogen through cell wall cross-linking, phytoalexin synthesis, suberization, auxin metabolism and phenol oxidation (Manjula et al., 2015).
The PAL, GLU and POX acts as key enzymes that regulates synthesis of phenolic compounds, flavonoids, and hydrocinnamtes also plays a significant role in lignin synthesis, creates oxidative stress, and ROS during pathogen infection and significant impact on plant physiology and expression of defense genes (Solekha et al., 2019; Rudrappa et al., 2022; Hammerschmidt et al., 1982). These enzymes play a vital in biochemical defense mechanism through increasing the cells wall deposition by activating the genes related to synthesis of lignin, H2O2 , callose, phenol and suberin. A maximum phenolic content in the plants treated with SND-1 formulation and pathogen in comparison with other treatments. In previous report by Alfi et al. (2020) and Umar et al. (2019), where significant defense response was observed through maximum accumulation of phenolic content after R. solani and MYMV pathogen infection in mung bean plants. Increased phenolic content correlated with resistance and also proper functioning of plant tissues (Sidra et al., 2017). The several studies reported that antifungal action of different flavonoids against phytopathogens such as F.oxysporum, S. sclerotium, A. flavus and R. solani (Morkunas et al., 2005; Katsumata et al., 2018). The accelerated phenolic and flavonoid content significantly involves in the synthesis of estres, lignin, tannins and flavonoids are chelating molecules of metal stresses (Ullah et al., 2017).
The maximum chlorophyll content in plants treated with SND-1 bioformulation and challenged with Cercospora pathogen showed the survival and adaptation of mung bean plants in stressful (Pathogen infestation) conditions. However, the exogenous application of bioformulations can induce the accelerated activity of physiological conditions (Enzymatic levels) that strengthen the synthesis of chlorophyll in leaves (Li et al., 2018). Djebaili et al. (2021) reported there was a significant increase of chlorophyll content in Triticum durum by the application of different actinomycetes formulations. As the chlorophyll increases, the plants get proper photosynthesis process and high survival capability during the biotic stresses (Umar et al., 2019).
5 Conclusion
In the present study, the application of Amycolatopsis sp. SND-1 alginate bioformulation significantly triggered the growth patterns and histochemical and biochemical defense patterns in the Cercospora infected in mung bean plants. Further, the induction of systemic resistance study unveiled the involvement of SND-1 formulation in the acceleration of histochemical depositions such as lignin and H2O2 and phenol with decreased cell death and biochemical defense-related enzymatic levels (PAL, GLU, and POX) that resulted in significant defense patterns in mung bean plants. An increased phenolic, flavonoid, and chlorophyll content in SND-1 formulation treated mung bean plants showed the higher levels of the physiological and survival potential. Finally, we concluded that Amycolatopsis sp. SND-1 formulation has the ability to counteract the Cercospora pathogen stress in mung bean plants through the enhancement of cellular and enzymatic defense processes that render significant adaptability under pathogen stress in mung bean plants.
Funding
This research was funded by Researchers Supporting Project (Project Number- RSP2023R231), King Saud University, Riyadh, Saudi Arabia.
Availability of data and materials
The datasets presented made available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Dhanyakumara Shivapoojar Basavarajappa: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Raju Suresh Kumar: Software, Writing – review & editing. Muthuraj Rudrappa: Methodology, Writing – review & editing. Halaswamy Hiremath: Methodology, Writing – review & editing. Abdulrahman I. Almansour: Funding acquisition, Writing - review & editing. Karthikeyan Perumal: Writing – review & editing. Gireesh Babu Kantli: Writing – review & editing. Sreenivasa Nayaka: Conceptualization, Resources, Supervision, Writing – review & editing.
Acknowledgements
The authors grateful to the P. G. Department of Studies in Botany for granting access to laboratory and intrumentation facility. The University Scientific Instrumentation Centre (USIC) and Sophisticated Analytical Instrumentation Facility (SAIF), Karnatak University, Dharwad, Karnataka, India for providing instrumentation facilities. The project was funded by Researcher Supporting Project number (RSP2023R231) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plant growth promotion and suppression of Phytophthora drechsleri damping-off in cucumber by cellulase-producing Streptomyces. Bio. Control.. 2017;62:805-819.
- [CrossRef] [Google Scholar]
- Trichoderma virens-Tv4 enhances growth promoter and plant defense-related enzymes of mungbean (Vigna radiata) against soil-borne pathogen Rhizoctonia solani. Biodivers.. 2020;21:2410-2419.
- [CrossRef] [Google Scholar]
- Effects of a powder formulation of Streptomyces cameroonensis on growth and resistance of two cocoa hybrids from cameroon against Phytophthora megakarya (Causal Agent of Black Pod Disease) J. Microbiol. Biotechno.l. 2022;32:160-169.
- [CrossRef] [Google Scholar]
- Formulation-based antagonistic endophyte Amycolatopsis sp. SND-1 triggers defense response in Vigna radiata (L.) R. Wilczek. (Mung bean) against Cercospora leaf spot disease. Arch. Microbiol.. 2023;205(77)
- [CrossRef] [Google Scholar]
- A swamp forest Streptomyces sp. strain KF15 with broad spectrum antifungal activity against chilli pathogens exhibits anticancer activity on HeLa cells. Arch. Microbiol.. 2022;204:540.
- [CrossRef] [Google Scholar]
- Thiamine induced resistance to Plasmopara viticola in grapevine and elicited host–defense responses, including HR like-cell death. Plant. Physiol. Biochem.. 2012;57:120-133.
- [CrossRef] [Google Scholar]
- Bioprospection and secondary metabolites profiling of marine Streptomyces levis strain KS46. Saudi. J. Biol. Sci.. 2022;29:667-679.
- [CrossRef] [Google Scholar]
- Actinomycetes: an unexplored microorganisms for plant growth promotion and biocontrol in vegetable crops. World. J. Microbiol. Biotechnol.. 2018;34:132.
- [CrossRef] [Google Scholar]
- Characterization of plant growth-promoting traits and inoculation effects on Triticum durum of actinomycetes isolates under salt stress conditions. Soil. Syst.. 2021;5:2-26.
- [CrossRef] [Google Scholar]
- Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol.. 2009;106:13-26.
- [CrossRef] [Google Scholar]
- Exploiting plant growth-promoting Amycolatopsis sp. for bio-control of charcoal rot of sorghum (Sorghum bicolor L.) caused by Macrophomina phaseolina (Tassi) Goid. Archiv. Phytopathol. Plant. Protec.. 2019;52:543-559.
- [CrossRef] [Google Scholar]
- Association of enhanced peroxidase activity with induced systemic resistance of cucumber of Colletotrichum lagenarium. Physiol. Plantarum Pathol.. 1982;20:73-82.
- [Google Scholar]
- Structure of 8Sα globulin, the major seed storage protein of mung bean. Acta. Crystallogr. D. Biol. Crystallogr.. 2006;62:824-832.
- [CrossRef] [Google Scholar]
- Effect of necrotrophic fungus and PGPR on the comparative histochemistry of Vigna radiata by using multiple microscopic techniques. Microsc. Res. Tech.. 2021;84:2737-2748.
- [CrossRef] [Google Scholar]
- Streptomyces palmae CMU-AB204T, an antifungal producing-actinomycete, as a potential biocontrol agent to protect palm oil producing trees from basal stem rot disease fungus, Ganoderma boninense. Biol. Control.. 2020;148:1-12.
- [CrossRef] [Google Scholar]
- Xylosylated Detoxification of the rice flavonoid phytoalexin sakuranetin by the rice sheath blight fungus Rhizoctonia solani. Molecules. 2018;23:276.
- [CrossRef] [Google Scholar]
- Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J.. 2019;2(23)
- [CrossRef] [Google Scholar]
- Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol.. 2018;6(64)
- [CrossRef] [Google Scholar]
- Non-specific lipid transfer proteins (ns-LTPs) from maize induce resistance in pearl millet against downy mildew disease. Phytoparasitica. 2015;43:437-447.
- [CrossRef] [Google Scholar]
- Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021
- [CrossRef] [Google Scholar]
- Development of formulations based on Streptomyces rochei strain PTL2 spores for biocontrol of Rhizoctonia solani damping-off of tomato seedlings. Biocontrol. Sci. Tech.. 2017;27:723-738.
- [CrossRef] [Google Scholar]
- Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant. Physiol. Biochem.. 2005;43:363-373.
- [CrossRef] [Google Scholar]
- Biotic and abiotic constraints in mungbean production progress in genetic improvement. Front. Plant. Sci.. 2019;10:1340.
- [CrossRef] [Google Scholar]
- Structure and function of bark and wood chloroplasts in a drought-tolerant tree (Fraxinus ornus L.) Tree. Physiol.. 2023;1–16
- [CrossRef] [Google Scholar]
- Amycolatopsis pittospori sp. nov., an endophytic actinobacterium isolated from native apricot tree and genome mining revealed the biosynthesis potential as antibiotic producer and plant growth promoter. Anton. Van. Leeuwenh.. 2021;114:365-377.
- [CrossRef] [Google Scholar]
- Towards development of climate-smart mungbean: challenges and opportunities. In: Kole C., ed. Genomic Designing of Climate Smart Pulse Crops. New York: Springer Nature; 2019.
- [Google Scholar]
- Bio-fabrication of Zinc Oxide nanoparticles to rescue Mung Bean against Cercospora leaf spot disease. Front. Plant. Sci.. 2022;13:1052984.
- [CrossRef] [Google Scholar]
- Bioproduction, purification and physicochemical characterization of melanin from Streptomyces sp. strain MR28. Microbiol. Res.. 2022;263
- [CrossRef] [Google Scholar]
- Mechanism of antagonism by Streptomyces griseocarneus(strain Di944) against fungal pathogens of greenhouse-grown tomato transplants. Can. J. Plant. Pathol.. 2015;37:197-211.
- [CrossRef] [Google Scholar]
- Formulation of a Streptomyces biocontrol agent for the suppression of rhizoctonia damping-off in tomato transplants. Biol. Control.. 2002;23:245-253.
- [CrossRef] [Google Scholar]
- Screening of mung bean varieties for resistant against Macrophomina phaseolina causing dry root rot. Int. J. Trop. Agri.. 2018;36:147-149.
- [Google Scholar]
- Biochemical changes in the leaves of mungbean (Vigna radiata) plants infected by phytoplasma. Turk. J. Biochem.. 2017;42:591-599.
- [CrossRef] [Google Scholar]
- Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant. Comm.. 2021;2:1-17.
- [CrossRef] [Google Scholar]
- Phenylalanine ammonia-lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J. Plant. Pathol.. 2019;102:359-365.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles using Paenibacillus sp. in-vitro and their antibacterial, anticancer activity assessment against human colon tumour cell line. J. Environ. Biol.. 2020;42:118-127.
- [CrossRef] [Google Scholar]
- Exogenous priming of chitosan induces upregulation of phytohormones and resistance against cucumber powdery mildew disease is correlated with localized biosynthesis of defense enzymes. Int. J. Biol. Macromol.. 2020;162:1825-1838.
- [CrossRef] [Google Scholar]
- Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease. J. Sci. Food. Agri.. 2017;97:1149-1157.
- [Google Scholar]
- Flavan-3-ols are an effective chemical defense against rust infection. Plant. Physiol.. 2017;175:1560-1578.
- [Google Scholar]
- Enhancing resistance level against Mungbean Yellow Mosaic virus by inducing defense related enzymes in Mung bean. Pakistan. J. Agric. Res.. 2019;32:241-251.
- [CrossRef] [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Am. J. EnoL. Vitic.. 1993;25:119.
- [Google Scholar]







