Translate this page into:
Acacia nilotica polyphenol extract restores glucose homeostasis by upregulating the insulin secretion and lowering the oxidative stress through down regulation of c-Jun N-terminal kinase (JNK) signaling cascade
⁎Corresponding author at: Department of Pharmacy, University of Agriculture, Faisalabad, Pakistan.. wafa.majeed@uaf.edu.pk (Wafa Majeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
Acacia nilotica (A. nilotica) has been used in ayurvedic system of medicine for the treatment of numerous metabolic disorders from decades. Despite its wide usage in traditional medicine system, to our knowledge, little attention has been given to the mechanisms involved in therapeutic potential of A. nilotica. The pupose of our study was to understand the molecular and biochemical mechanisms involved in protective effect of A. nilotica polyphenol extract (A. nilotica PPE) on diabetes induced by alloxan monohydrate.
Methods
Total polyphenols and total flavonoids in A. nilotica PPE were determined using Folin-Ciocalteu method and Aluminiun Chloride colorimetric method respectively. HPLC analysis of A. nilotica PPE was performed to determine the phenolic contents. Antidiabetic potential of A. nilotica PPE was evaluated through biochemical (FBG, serum glucose, serum insulin, HbA1c, serum C-peptide), antioxidant defense markers, Intracellular ROS and lipid peroxidation measurements as well as histopathological analysis. qRT-PCR analysis was also executed to measure the expression level of numerous genes involved in insulin signaling cascade (Pdx-1, ngn-3, Ins-1, GLUT-4, IRS-1) and c-Jun N-terminal kinase (JNK) downstream cascade (MAPK-8, Traf-4 & Traf-6) to determine the main molecular mechanisms involved in antidiabetic potential of A. nilotica PPE.
Results
Results of the study have confirmed the presence of total polyphenolic (61.17 mg GAE/g) and total flavonoid (53.12 mg CE/g) contents in A. nilotica PPE. HPLC assay of A.nilotica PPE revealed the presence of quercitin, gallic acid, chlorogenic acid, Kaempferol and catechin. A. nilotica PPE significantly inverted the hyperglycemia by reducing the FBG, serum glucose, HbA1c levels and improving the insulin & C-peptide levels. A. nilotica PPE improved the antioxidant defense markers (SOD, CAT, GPx, GSH) and reduced the intracellular ROS and TBARS levels. Alloxan induced histological changes were also recovered in extract treated groups. A. nilotica PPE upregulated the expressions of Pdx-1, ngn-3, Ins-1, GLUT-4, IRS-1 and downregulated the expressions of MAPK-8, Traf-4 & Traf-6 genes.
Conclusion
In a nutshell, A. nilotica PPE can be used as a better therapeutic approach for the management of both type 1 and type 2 diabetes as it may improve the beta cells regeneration, biochemical indicators and reduce the cellular stress.
Keywords
A. nilotica PPE
HPLC
Insulin signaling pathway
JNK pathway
GLUT-4
ROS
1 Introduction
Diabetes mellitus (DM), one of the most recurrent metabolic disorders, has been reported as global problem and is associated with inadequate insulin secretion or its ineffectiveness (Van Doan et al., 2018; Saxena and Argal, 2018). Literature has demonstrated that diabetes mellitus influenced approximately 415 million people world widely till 2015 and this number is estimated to increase upto 642 million by 2040 (Ogurtsova et al., 2017). Chronic hyperglycemic conditions might be responsible for formation of cellular reactive oxygen species (ROS). Previous literature has demonstrated that the excessive ROS production is responsible for induction of c-Jun N-terminal kinase (JNK) signaling cascade which plays an important role in the progression of diabetes (Hassanin et al., 2018; Volpe et al., 2018). Oxidative stress activates TRAF-4 & TRAF-6 factors which results in activation of JNK downstream signaling in several tissues, including pancreatic islets. The activated JNK pathway subsequently upregulates the expression of various proapoptotic genes (Li et al., 2018). Therefore, management of ROS has been regarded as core approach for the treatment of hyperglycemia (Taha et al., 2018).
Plant based antioxidant therapies play a vital role in the management of DM. Presently, traditional and ayurvedic medicines are becoming the fundamental source for the development of newer drugs to counteract DM (Van Doan et al., 2018; Krishnaiah et al., 2011). The polyphenols in plants are considered to reduce ROS production and improve the antioxidant defense markers as well as insulin signaling cascade (Vieira et al., 2019). Insulin signaling cascade is responsible for the improvement in pancreatic beta cells regeneration, differentiation and insulin release via upregulating the expression of various genes involved in insulin signaling cascade (Gao et al., 2018).
Acacia nilotica (A. nilotica) is an umbrella shaped shrub and a multipurpose legume that is mainly found in Asia, Africa and Australia (Mukundi et al., 2015). A. nilotica is rich in polyphenols and in ayurvedia, its leaves, bark and pods are used against cancer, diabetes mellitus, inflammation, fever, menstural problems and diarrhea (Sadiq et al., 2017; Roozbeh et al., 2017). However, the biochemical and molecular mechanisms involved in antidiabetic potential of A. nilotica are still unknown. Keeping in view the high phenolic contents in A. nilotica leaves, we hypothesized that phenols extracted from A. nilotica might improve the glycemic status in alloxanized hyperglycemic rats. Alloxan is a classical diabetogenic agent and strong oxidant, widely used to induce type 1 diabetes by destroying the pancreatic beta cells via generation of reactive oxygen species (Yin et al., 2018). In the present study we determined the antioxidant, antihyperglycemic and beta cells regeneration potential of A. nilotica leaves by measuring the cellular ROS, antioxidant defense markers, biochemical indicators as well as expression levels of various genes involved in insulin signaling cascade & JNK signaling cascade.
2 Materials and methods
2.1 Preparation of Acacia nilotica polyphenolic extract
Leaves of Acacia nilotica (L.) Delile were collected from the Botanical garden of University of Agriculture, Faisalabad (UAF) and were authenticated by the Botany department, UAF, Faisalabad, Pakistan. After authentication the plant material was preserved with voucher no. 21,143 in the herbarium of UAF, Faisalabad. For the preperation of polyphenolic extract 500 g of A. nilotica leaves were pulverized and extracted with 70% ethanol. After 7 days (25 °C), obtained extract was filtered and complete solvent removal was done through rotary evaporator. The obtained residue was mixed with water and extracted with petroleum ether which resulted in the formation of two separate layers. The aqueous layer was extracted using ethyl acetate along with glacial acetic acid (10 mL/L). The organic layer was evaporated to extract the polyphenols from ethyl acetate layer and dried through evaporation (Sabu et al., 2002). The A. nilotica polyphenol extract (A. nilotica PPE) was freeze dried and stored for further analysis.
2.2 Determination of total polyphenol and flavonoid contents in A. nilotica PPE
The Folin-Ciocalteu reagent method was used to calculate the total polyphenol concentration in A. nilotica PPE spectrophotometrically at 760 nm wavelength and results were demonstrated as mg GAE/g of dry weight (DW). The Aluminiun Chloride colorimetric method was employed to calculate the total flavonoid contents. The absorbance was measured at 510 nm wavelength using UV–Vis spectrophotometer and results were exhibited as mg CE/g of DW (Ahmed et al., 2014; Moonmun et al., 2017).
2.3 Determination of polyphenols in A. nilotica PPE by HPLC
HPLC analysis was performed using HPLC system (Hewlett–Packard 1100 Series sys-tem; Perlan Technologies, USA). C18 column was used as stationary phase. Combination of acetonitrile and HPLC grade water was used as mobile phase. Flow rate and injection volume were 1 mL/min and 20 μL. For the detection of bioactive compounds, UV–visible detector was used at the wavelength of 370 nm (Seal, 2016). The peaks of the bioactive compounds were confirmed by contrasting their retention times and UV spectra with corresponding standards. The reference standards used were quercitin, gallic acid, chlorogenic acid, Kaempferol and catechin.
2.4 Experimental animals
Seventy five albino wistar rats having weight 180–200 g were caged in the animal room of IPPP, University of Agriculture, Faisalabad and were acclimatized for a period of two weeks before conduct of experiment. All experimentation was organized in agreement to the guidelines of laboratory animal care approved by the GSRB (Graduate studies Research Board), UAF, Pakistan. The institutional biosafety and bioethics committee (IBC) issued ethical certificate wide letter no. 513/ORIC for the conduct of present study. Subsequent to the adaption time, rats were divided in to five groups, having 15 rats per group.
2.5 Induction of diabetes, experimental design and biological sample collection
Diabetes was induced using sole injection (i.p) of alloxan monohydrate mixed with 0.9% normal saline (150 mg/kg of body weight) in all groups excluding the group kept as normal control. Following one week of study, glucose levels from blood of all animals (tail vein method) were measured using Accu-Chek glucometer (RocheDiagnostic, Germany). A criterion of hyperglycemic rats for inclusion in study was the blood sugar level greater than 300 mg/dl. Rats were clustered into following groups (n = 15/group). Group I: Rats provided as normal control (NC) administered vehicle; Group 2: Rats provided as diabetic control (DC) administered vehicle; Group 3: Rats treated with glibenclamide (GL; 10 mg/kg); Group 4: Rats treated with A. nilotica PPE at dose rate of 250 mg/kg; Group 5: Rats treated with A. nilotica PPE at dose rate of 500 mg/kg. Fasting blood glucose levels of all rats were assessed weekly till the end of trial. After four weeks of study, overnight fasted rats were anaesthetized using 3% sodium pentobarbital (ip), then sacrificed. The obtained blood samples were centrifuged for 10 min at 4000 rpm and resultant serum was stored at −80 °C for biochemical investigations. The pancreatic rat tissues from all groups were preserved in 10% NBF solution for histopathology. Both pancreatic and hepatic tissues from NC, DC and A. nilotica PPE treated groups were also snap frozen in liquid nitrogen for gene expression analysis.
2.6 Preparation of tissue homogenate
After termination of study, the resected tissues of rats were homogenized using 10% (w/v) buffer solution (50 mM Tris-HCl & 1.15% KCl pH 7.4) and centrifuged at 9000 g for 3 min at 4 °C. The resultant supernatants were used as tissue homogenate for the biochemical investigations.
2.7 Measurement of serum biochemical assays
Fasting blood glucose (FBG; mg/dl) level was obtained by using On-Call® Ez II (SN 303S00014E09) glucometer via tail prick method. At the end of trial, serum glucose levels were assessed by rat glucose assay kit (81693, Crystal Chem, USA). Serum insulin levels were analyzed using rat insulin ELISA kit (ELR-Insulin, RayBio®, Norcross, GA, USA). Glycosylated hemoglobin (HbA1c) and serum C-peptide levels were measured using commercially available ELISA kits.
2.8 Measurement of antioxidant defense markers
The pancreatic & hepatic tissue homogenates of rats of all groups were accessed for Superoxide dismutase (SOD) activity (Eslami et al., 2015). Catalase (CAT) enzyme concentration was measured and exhibited as μmol of H2O2 decomposed/min/mg of protein (Takahara et al., 1960). Glutathione peroxidase (GPx) enzyme level (nmol of glutathione oxidized/min/mg of protein) was analyzed (Rotruck et al., 1973). Reduced glutathione (GSH) enzyme activity (mg/dL of tissue) was also examined (Ellman, 1959).
2.9 Determination of ROS and lipid per oxidation
Intracellular ROS (reactive oxygen species) concentration was measured using 2,7-dichlorofluorescein diacetate (DCF-DA) as a probe (Rashid et al., 2012). The progression of DCF was measured using a fluorescence spectrometer at 488–510 nm wavelength for 10 min. Thiobarbituric acid reactive substances (TBARS) assay kit was employed to determine the lipid peroxidation in the form of MDA (malondialdehyde) at 532 nm wavelength and results were demonstrated as nmol MDA/mg protein (Ohkawa et al., 1979).
2.10 Histological analysis
Pancreatic tissue samples were fixed in NBF (10%), embedded in paraffin, serial sections were obtained with the help of microtome and stained with H&E dye for photographic investigations (Bancroft and Gamble, 2007).
2.11 qRT-PCR assay for selected genes
RNA extraction was performed using TRIZOL (ThermoFisher Scientific,Massachusetts, USA) method. After extraction quantification of RNA samples was performed using nanodrop. RevertAid cDNA synthesis kit (ThermoFisher Scientific) was used for cDNA synthesis in accordance with manufacturer’s guidelines.
qRT-PCR (Quantitative real-time PCR) analysis was executed using iQ5 Bio-Rad machine (Maxima SYBR Green/ROX Master Mix; ThermoFisher). Expressions of Pdx-1, ngn-3, Ins-1, MapK-8, Traf-6, Traf-4, GLUT-4 & IRS-1 were studied. Beta actin was used as housekeeping gene. During PCR, denaturation of template was done at 95 °C for fifteen seconds. Annealing temperature was 58 °C for 30 sec and extension time was 20 sec at 72 °C. Finally the qRT-PCR data were analysed using 2*(-ΔΔct) method. The below mentioned oligonucleotides (Primers) sequence was used for the amplification of genes.
Pdx-1F TCCCGAATGGAACCGAGACT
Pdx-1R TTCATCCACGGGAAAGGGAG
Ngn-3F TGCAGCCACATCAAACTCTC
Ngn-3R GGTCACCCTGGAAAAAGTGA
Ins-1F AGGCTCTGTACCTGGTGTGTG
Ins-1R CGGGTCCTCCACTTCACGAC
Mapk-8F CTCAGCATCCGGTCTCTTCG
Mapk-8R CTGCTGTCTGTATCCGAGGC
Traf-6F GCGCCTAGTAAGACAGGACC
Traf-6R CACATGCATGCTCTGCGTTT
Traf-4F CGACTACAAGTTCCTGGAGAAGC
Traf-4R AGGTGTCGCAGAAGCGGTG
GLUT-4F ACTCTTGCCACACAGGCTCT
GLUT-4R AATGGAGACTGATGCGCTCT
IRS-1F GATACCGATGGCTTCTCAGACG
IRS-1R TCGTTCTCATAATACTCCAGGCG
Beta actinF CGAGTACAACCTTCTTGCAGC
Beta actinR TATCGTCATCCATGGCGAACTG
2.12 Statistical analysis
All the results were demonstrated as mean ± standard error (mean ± SE). Statistically data were assessed using one-way analysis of variance (ANOVA) followed by DMR test. Results were observed statistically significant with P ≤ 0.05.
3 Results
3.1 Assessment of total polyphenolic, flavonoid contents and identification of phenolic compounds
Significant amounts of total polyphenolic (61.17; mg GAE/g of sample) and total flavonoid (53.12; mg CE/g of sample) contents were seen in A. nilotica PPE. HPLC assay revealed the presence of different phenolics in A. nilotica PPE, i.e. quercitin, gallic acid, chlorogenic acid, Kaempferol and catechin (Table 1 and Fig. 1)
Compound
Retention time
Concentration (ppm)
Quercitin
3.127
17.05
Gallic acid
5.213
12.66
Chlorogenic acid
17.373
8.15
Kaempferol
22.927
9.85
Catechin
26.333
8.01
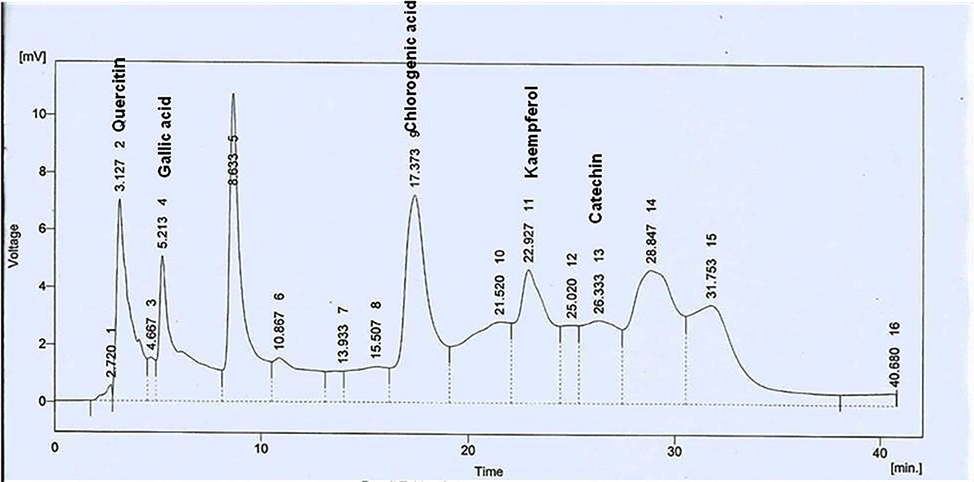
HPLC chromatogram of A. nilotica PPE.
3.2 Effect of A. Nilotica PPE on FBG, serum glucose, serum insulin, HbA1c and C-Peptide levels
In alloxan induced diabetic rats FBG level significantly (P ≤ 0.05) raised from week 0 to week 4. Oral administration of GL and A. nilotica PPE from week 1 to week 4 significantly (P ≤ 0.05) reduced the hyperglycemia in treated groups as shown in Fig. 2. Serum glucose and HbA1c levels were drastically (P ≤ 0.05) increased in all diabetic rats in comparison to NC group. Instead, GL and graded doses of A. nilotica PPE notably (P ≤ 0.05) decreased the serum glucose and HbA1c levels compared to DC group. The levels of serum insulin and C-peptide were appreciably (P ≤ 0.05) raised in GL and A. nilotica PPE treated rats compared to DC group rats (Table 2).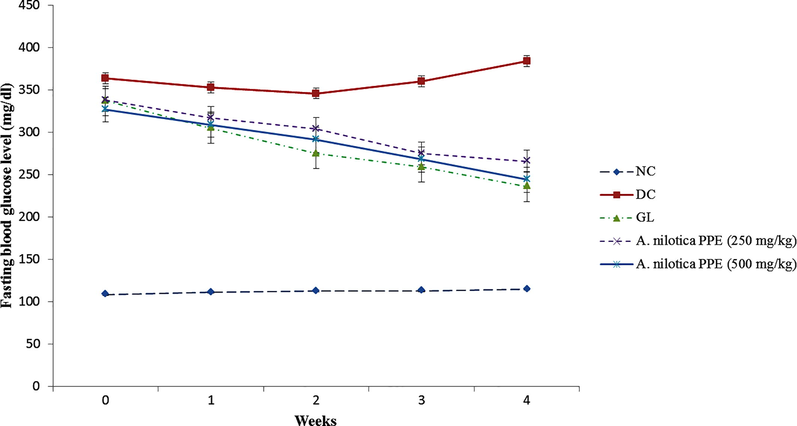
Effect of A. nilotica PPE on FBG level in alloxanized diabetic rats from week 0 to week 4.
Parameter
Groups
NC
DC
GL
A. nilotica PPE(250 mg/kg)
A. nilotica PPE(500 mg/kg)
Serum glucose (mg/dL)
113.51 ± 2.88D
491 ± 3.99a
123 ± 1.89C
221.71 ± 2.51B
136.16 ± 2.14BC
Serum insulin (mg/dL)
18.95 ± 0.53A
7.17 ± 0.95D
16.31 ± 0.61B
12.98 ± 0.51BC
15.91 ± 0.65B
HbA1c (%)
5.18 ± 0.03E
10.95 ± 0.13A
5.93 ± 0.09D
8.14 ± 0.06C
6.01 ± 0.05D
Serum C-peptide
1.49 ± 0.03A
0.52 ± 0.05E
1.12 ± 0.12B
0.95 ± 0.07CD
1.03 ± 0.06BC
3.3 Effect of A. nilotica PPE on antioxidant defense markers and lipid peroxidation
Levels of catalase, GPx, SOD and GSH were drastically (P ≤ 0.05) declined after induction of hyperglycemia. However, administration of GL and A. nilotica PPE (250 mg/kg & 500 mg/kg) considerably (P ≤ 0.05) restored the antioxidant enzyme levels. Moreover, levels of ROS and TBARS were significantly raised in hyperglycemic rats. We found significant decline in ROS and TBARS levels in GL and A. nilotica PPE treated groups (Tables 3 and 4).
Parameter
Groups
NC
DC
GL
A. nilotica PPE(250 mg/kg)
A. nilotica PPE(500 mg/kg)
Catalase (μmol H2O2/min/mg protein)
54±2.32A
28.95±1.26CD
46.91±1.69B
37±1.95BC
48.6±1.64B
GPx (nmol/min/mg protein)
25±0.96A
13.75±0.85C
21.13±1.29AB
18.25±1.14B
20.96±1.44AB
SOD (U/mg proteins)
6.87±0.98A
3.71±1.11C
6.09±1.03AB
4.89±0.89B
5.97±1.05AB
GSH (mg/dL tissue)
36±1.56A
23±1.33C
31.75±1.21AB
28.87±1.74B
33.15±1.38AB
ROS (%)
97±3.12CD
186±3.73A
104±1.93C
135±1.98B
108±2.23C
TBARS (nmol MDA/mg protein)
2.37±1.12C
5.76±1.03A
3.35±0.61BC
4.25±0.91B
3.07±0.81BC
Parameter
Groups
NC
DC
GL
A. nilotica PPE(250 mg/kg)
A. nilotica PPE(500 mg/kg)
Catalase (CAT) (μmol H2O2/min/mg protein)
36 ± 2.1A
17.35 ± 0.99C
33.27 ± 1.12AB
26.25 ± 2.07B
31.67 ± 1.92AB
GPx (nmol/min/mg protein)
18.6 ± 0.77A
10.15 ± 1.05C
15.59 ± 0.93AB
13.48 ± 0.98B
16.17 ± 0.89AB
SOD (U/mg proteins)
5.11 ± 1.17A
2.73 ± 0.74C
4.41 ± 0.84AB
4.05 ± 0.98B
4.77 ± 0.94AB
GSH (mg/dL tissue)
29.21 ± 1.08A
15.88 ± 0.99D
23.95 ± 0.87B
20.15 ± 1.02C
25.25 ± 0.97B
TBARS (nmol MDA/mg protein)
2.11 ± 0.88C
5.06 ± 0.91A
2.69 ± 1.13C
3.12 ± 1.11B
2.46 ± 1.07C
3.4 A. nilotica PPE alleviated the alloxan induced histological changes in pancreatic tissues
As shown in Fig. 3a, normal histoarchitecture of pancreatic cells was observed in NC group rats. Though, pancreatic tissues of DC group rats demonstrated minute islets of langerhans with devastation of pancreatic β cells (Fig. 3b). In group 3, normal histoarchitecture of pancreatic beta cells was restored along with increased population of cells (Fig. 3c). In group 4, rats showed noticeable recovery in cellular damage as obvious from partial restoration of islets of langerhans & pancreatic β cells (Fig. 3d). In group 5, notable recovery was observed in pancreatic parenchyma and active β cells region which was comparable to GL (Fig. 3e).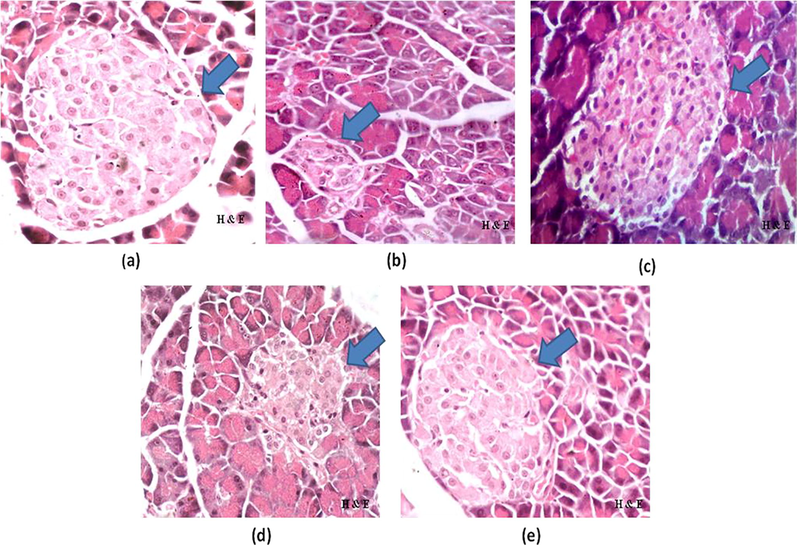
a–e: Photomicrographs of pancreas of (a) NC, (b) DC, (c) GL, (d) A. nilotica PPE (250 mg/kg), (e) A. nilotica PPE (500 mg/kg) (H&E × 40).
3.5 Effect of A. nilotica PPE on relative expression of MAPK-8/JNK1 singnaling cascade genes
In DC group MAPK-8, Traf-4 and Traf-6 expressions (MAPK-8/JNK1 singnaling cascade) were considerably (P ≤ 0.001) augmented due to alloxan induced ROS production in comparison to NC group rats. Though, oral administration of A. nilotica PPE appreciably (##P ≤ 0.01; ###P ≤ 0.001) lessen the expressions of MAPK8, TRAF-4 and TRAF-6 genes in comparison to DC group rats (Fig. 4a–c).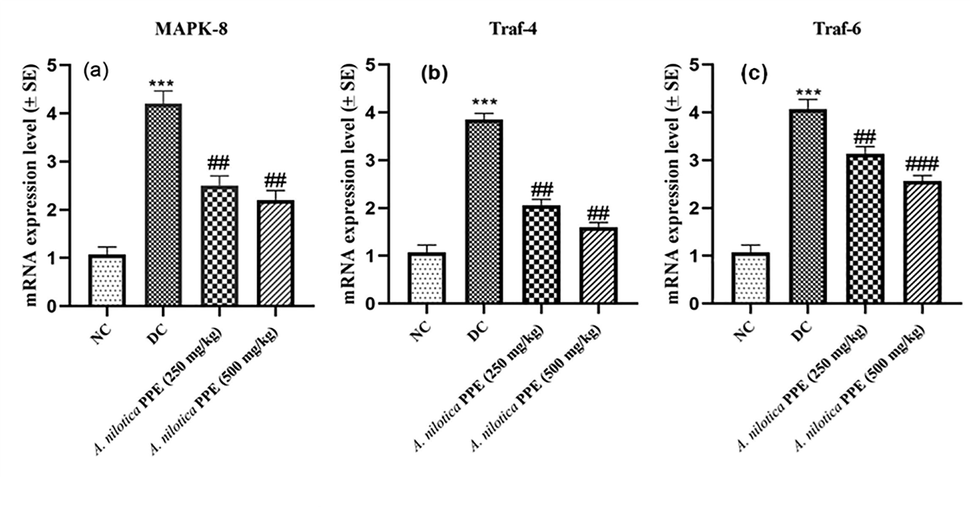
a–c: Expression profiles of a) MAPK-8 gene b) TRAF-4 gene c) TRAF-6 gene.
3.6 Effect of A. nilotica PPE on relative expression of insulin signaling cascade genes
Expressions of Pdx-1, Ins-1 and ngn-3 genes were remarkably (**P ≤ 0.01; ***P ≤ 0.001) reduced in pancreatic tissues of DC group rats in comparison to NC group. Instead, A. nilotica PPE notably (##P ≤ 0.01; ###P ≤ 0.001) raised the Pdx-1, Ins-1 and ngn-3 expressions compared to DC group dose dependently (Fig. 5a–c).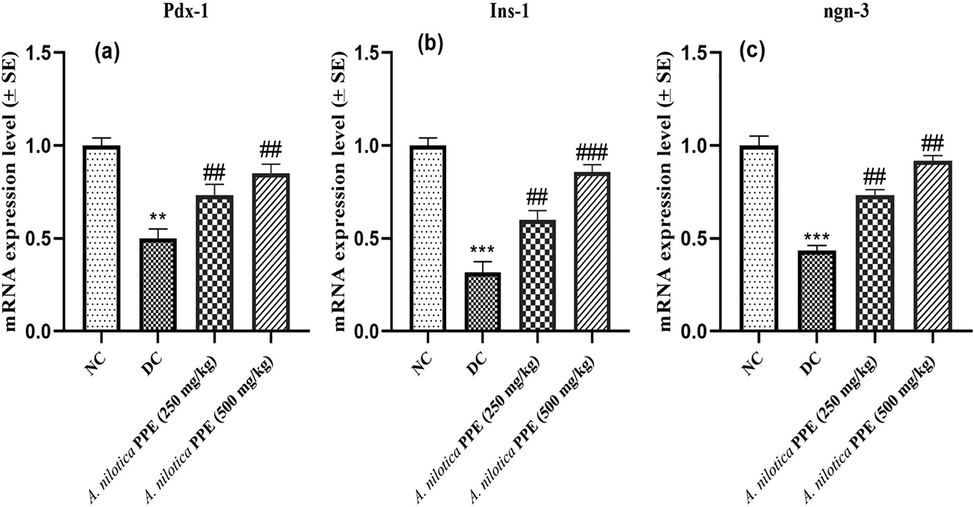
a–c: Expression profiles of a) Pdx-1 gene b) Ins-1 gene c) ngn-3 gene.
3.7 Effects of A. nilotica PPE on relative expression of Glut-4 & IRS-1 genes
IRS-1 and Glut-4 expressions were drastically (≤0.001) reduced in DC group rats compared to NC group. However, administration of A. nilotica PPE (250 mg/kg and 500 mg/kg) notably (##P ≤ 0.01) upregulated the IRS-1 and GLUT-4 expressions in groups 3 and 4 in comparison to DC group (Fig. 6a-b).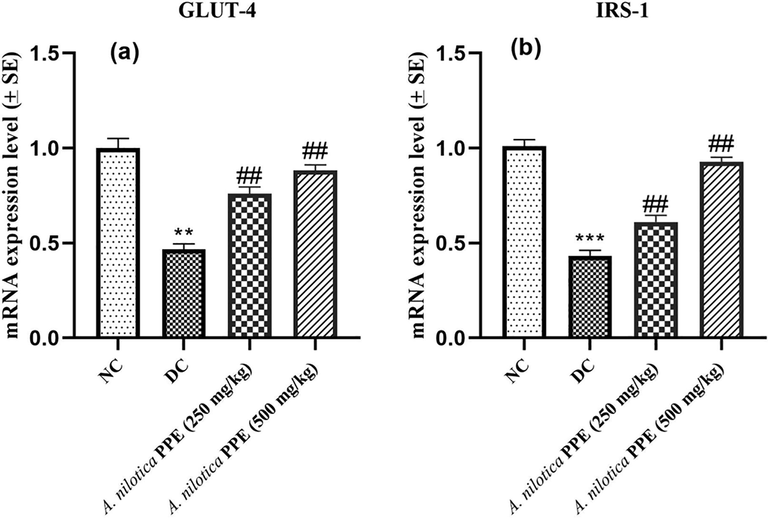
a–b: Expression profiles of a) GLUT-4 gene b) IRS-1 gene.
4 Discussion
Remedial therapies for the management of diabetes are linked with adverse actions. So, natural resources are becoming potential alternatives to cure diabetes mellitus and other metabolic disorders (Sharma et al., 2016). In the present study we determined the mechanisms involved in antihyperglycemic potential of A. nilotica PPE in diabetic rats. Phytochemical assay revealed the presence of appreciable amounts of phenolic and flavonoid contents in A. nilotica PPE. In accordance with HPLC analysis, the concentrations (ppm) of phenolic compounds were as: Quercitin > Gallic acid > Kaempferol > Chlorogenic acid > catechin (Table 1). Gallic acid improves insulin release and glucose translocation as well as acts as a defensive agent against oxidative stress (Kade et al., 2014; Kahkeshani et al., 2019). Chlorogenic acid is also found to improve glucose homeostasis and oxidative stress markers (Prince and Roy, 2013). Indeed, phenolic compounds in A. nilotica PPE are responsible for therapeutic effectiveness against diabetes due to their antioxidant and antihyperglycemic potential.
In the present study we used alloxan monohydrate to induce diabetes which produced type 1 diabetes via selective destruction of pancreatic beta cells and ultimately resulted in hyperglycemia. In hyperglycemic condition; there is impairment of glucose homeostasis which results in enhanced glucose, HbA1c and reduced insulin levels. FBG level is an index of hyperglycemia and it was appreciably increased in diabetic rats. The International Diabetes Federation (IDF) has regarded the HbA1c as reliable diagnostic biochemical marker for diabetes. However, reduction in FBG, serum glucose, HbA1c as well as increase in insulin & C-peptide levels have demonstrated the antihyperglycemic properties of A. nilotica PPE. These results are in accordance with preceding research studies (Narasimhan et al., 2015).
According to the results, A. nilotica PPE remarkably (P ≤ 0.05) improved the pancreatic and hepatic antioxidant markers and notably decreased the TBARS level (Table 3 & 4). In alloxanized rats significant raise in intracellular ROS was observed. Growing evidence has correlated the raised ROS levels with autooxidation of glucose (Petersen et al., 2016). The antihyperglycemic potential of A. nilotica PPE is attributed to its powerful antioxidant defense activity due to the presence of excessive amounts of polyphenols. No previous study has reported the molecular and biochemical mechanisms involved in antioxidant and hypoglycemic activities of polyphenols from A. nilotica.
Histopathological analysis has revealed that A. nilotica PPE substantially regularized the histoarchitecture of pancreatic tissues. A significant rise in population of pancreatic beta cells and reduction in necrotic tissues was observed in A. nilotica PPE treated rats dose dependently. Preceding research studies have demonstrated that the polyphenols present in traditional plants might be responsible for the restoration of pancreatic β-cells by reducing the oxidative stress induced apoptosis (Gandhi and Sasikumar, 2012; Agwaya and Nandutu, 2016). Keeping in view the biochemical & histopathological findings, it was observed that the GL and higher dose of A. nilotica PPE showed comparable antihyperglycemic activity.
Recent evidence demonstrated that ROS production stimulates the TNF receptor mediated factors (Traf-4; Traf-6) which results in activation of JNK signaling cascade and upregulation of various proapoptotic genes (Li et al., 2018; Gao et al., 2018). In our study, A. nilotica PPE remarkably decreased the expressions of Traf-4 and Traf-6 genes as well as reduced the phosphorylation of MapK-8/Jnk1 gene and resisted the JNK pathway activation in dose dependent manner. In order to demonstrate the pancreatic beta cells regenerative potential of A. nilotica PPE, we explored the effect of A. nilotica PPE on expressions of Pdx-1, Ins-1 and ngn-3 genes (Insulin signaling cascade). The Pdx-1 is a crucial gene in proper working of beta cells and it acts as a key regulator in the transcription of glucose-stimulated insulin gene (Ins-1) (Gao et al., 2018). The ngn-3 is regarded as key gene for pancreatic beta cells differentiation (Zhu et al., 2017). We found that treatment with A. nilotica PPE (250 mg/kg; 500 mg/kg) significantly improved the expressions of Pdx-1, Ins-1 and ngn-3 genes towards normal.
Furthermore, our results showed that A. nilotica PPE enhanced the IRS-1 protien phosphorylation and GLUT-4 translocation resulting in upregulation of glucose transport as well as lipid metabolism (Li et al., 2019; Wu et al., 2016). Due to some technical problems, we could not perform FTIR analysis of extract to determine the major functional groups responsible for antioxidant and hypoglycemic activities. However, we quantified the different polyphenols present in plant extract using HPLC-UV analysis. The results of present study have suggested the antioxidant, antihyperglycemic and pancreatic beta cells regenerative properties of polyphenols present in A. nilotica.
5 Conclusion
From our results, it was concluded that polyphenols from A. nilotica were effective in the reversal of hyperglycemia via modulation of glucose homeostasis indicators and oxidative stress markers. Furthermore, A. nilotica PPE downregulated the ROS induced JNK signaling pathway and improved the antioxidant defense activities. A. nilotica PPE also showed strong regenerative pancreatic beta cells potential through upregulating the insulin signaling cascade. Therefore, the clinical use of A. nilotica PPE for the treatment of both type 1 and type 2 diabetes mellitus has the scientific significance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hypoglycemic activity of aqueous root bark extract Zanthoxylum chalybeum in alloxan-induced diabetic rats. J. Diabetes Res.. 2016;1–5
- [CrossRef] [Google Scholar]
- Phenolic and flavonoid contents anti-oxidative potential of epicarp and mesocarp of Lageneriasiceraria fruit: a comparative study. Asian Pac. J. Trop. Med.. 2014;7:249-255.
- [CrossRef] [Google Scholar]
- Bancroft, J.D., Gamble, M. 2007. Theory and Practice of Histological Techniques. Philadelphia, Churchill, Livingstone, pp. 131–133.
- Eslami, H., Batavani, R.A., Asr, S. 2015. Changes of stress oxidative enzymes in rat mammary tissue, blood and milk after experimental mastitis induced by E. coli lipopolysaccharide. In Veterinary Research Forum, 6 (2) 131. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
- Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed.. 2012;2:281-286.
- [CrossRef] [Google Scholar]
- NEFA-induced ROS impaired insulin signalling through the JNK and p38MAPK pathways in non-alcoholic steatohepatitis. J. Cell. Mol. Med.. 2018;22(7):3408-3422.
- [Google Scholar]
- Balanites aegyptiaca ameliorates insulin secretion and decreases pancreatic apoptosis in diabetic rats: Role of SAPK/JNK pathway. Biomed. Pharmacother.. 2018;102:1084-1091.
- [Google Scholar]
- Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic. Med. Sci.. 2019;22(3):225.
- [Google Scholar]
- Influence of gallic acid on oxidative stress-linked streptozotocininduced pancreatic dysfunction in diabetic rats. J. Basic. Clin. Physiol. Pharmacol.. 2014;25(1):35-45.
- [Google Scholar]
- A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process.. 2011;89:217-233.
- [CrossRef] [Google Scholar]
- Intermittent high glucose exacerbates A-FABP activation and inflammatory response through TLR4-JNK signaling in THP-1 cells. J. Immunol. Res.. 2018;2018:1-9.
- [Google Scholar]
- Effects and underlying mechanisms of bioactive compounds on type 2 diabetes mellitus and Alzheimer’s disease. Oxid. Med. Cell. Longev. 2019;2019:25.
- [Google Scholar]
- Quantitative phytochemical estimation and evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of Heliconia rostrata. Indian J. Pharm. Sci.. 2017;79:79-90.
- [CrossRef] [Google Scholar]
- Antidiabetic effects of aqueous leaf extracts of acacia nilotica in alloxan induced diabetic mice. J. Diabetes Metab.. 2015;6:1-6.
- [Google Scholar]
- Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab.. 2015;40(8):769-781.
- [Google Scholar]
- I.D.F. diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract.. 2017;128:40-50.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Brief communication: Plasma lipid oxidation predicts atherosclerotic status better than cholesterol in diabetic apolipoprotein E deficient mice. Exp. Biol. Med.. 2016;242(1):88-91.
- [Google Scholar]
- p-Coumaricacidattenuatesapoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int. J. Cardiol.. 2013;168(4):3259-3266.
- [Google Scholar]
- Protective role of Dsaccharic acid-1, 4- lactone in alloxan induced oxidative stress in the spleen tissue of diabetic rats is mediated by suppressing mitochondria dependent apoptotic pathway. Free Radic. Res.. 2012;46(3):240-252.
- [Google Scholar]
- Hypoglycemic effects of Acacia nilotica in type II diabetes: a research proposal. BMC. Res. Notes.. 2017;10(1):1-5.
- [Google Scholar]
- Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Anti-diabetic activityof green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol.. 2002;83(1–2):109-116.
- [Google Scholar]
- In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC. Compl. Alternative. Med.. 2017;17(1):372.
- [Google Scholar]
- Evaluation of antidiabetic activity of a herbal formulation. EcPharmacol. Toxicol. 2018:349-355.
- [Google Scholar]
- Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharma. Sci.. 2016;6:157-166.
- [CrossRef] [Google Scholar]
- Sharma, B., Joshi, S.C., Jasuja, N.D., Singh, S.K., Sharma, G. 2016. Evaluation of anti-diabetic and heapatoprotectivity of coriandrum sativum in alloxan induced experimental animals: a histopathology study. Int. J. Pharm. Sci. Res. 7, 4510-4515. https://dx.doi.org/10.13040/IJPSR.0975-8232.7(11).1000-06.
- Effect of Pseuduvaria macrophylla in attenuating hyperglycemia mediated oxidative stress and inflammatory response in STZ-nicotinamide induced diabetic rats by upregulating insulin secretion and glucose transporter-1, 2 and 4 proteins expression. J. Appl. Biomed.. 2018;16(4):263-273.
- [Google Scholar]
- Hypocatalasemia: a new genetic carrier state. J. Clin. Investig.. 1960;39(4):610-619.
- [Google Scholar]
- Antidiabetic activity, glucose uptake stimulation and α-glucosidase inhibitory effect of Chrysophyllum cainito L. stem bark extract. BMC Complement Altern. Med.. 2018;18:267.
- [CrossRef] [Google Scholar]
- Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis.. 2018;9(2):119.
- [Google Scholar]
- Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome—Review of classical and new compounds: Part-I. Pharmaceuticals. 2019;12(4):152.
- [Google Scholar]
- Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt–GLUT4, GSK3β and mTOR–S6K1. J. Nutr. Biochem.. 2016;34:126-135.
- [Google Scholar]
- Hypoglycemic effects in alloxan-induced diabetic rats of the phenolic extract from mongolian oak cups enriched in ellagic acid, kaempferol and their derivatives. Molecules. 2018;23(5):1046.
- [Google Scholar]
- PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem. Cell. Res. Ther.. 2017;8(1):1-7.
- [Google Scholar]







