Translate this page into:
Aberrant hepatic glucose levels and cellular damage reversed by Cucurbita maxima blossoms on experimental models
⁎Corresponding author at: Nutrition and Food Science Department, Faculty of Home Economics, Helwan University, Cairo, Egypt. mahaaessam82@gmail.com (Maha M. Essam El-Din)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Investigate the ameliorating effects of Pumpkin flowers (Cucurbita maxima) 10% and 20% fortified cake on cellular damage caused by alloxan-induced diabetic rats.
Methods
Forty adult male albino rats of approximately weigh about 180 ± 5 g were obtained and divided into five distinct groups to determine the effect of C. maxima flowers enriched (CMFP) cake two different concentrations (10 % and 20 %) had any beneficial impacts on rats with a single dose of 40 mg/kg alloxan to induce diabetes.
Results
The observations on the blood insulin, blood sugar, lipid profile, serum liver biomarkers, lipid peroxidation enzyme markers, and pro-oxidative mediators showed that oral administration of 10% and 20% CMFP cake lowers the blood sugar, Total Cholesterol (TC), Low-Density Lipoprotein (LDL), Very Low-Density lipoprotein (VLDL) and Triglyceride (TG). In rat treated with alloxan, the levels of alanine transaminase (ALT), alkaline phosphatase ALP), aspartate transaminase (AST) and the levels of malondialdehyde (MDA), thiobarbituric acid reactive compounds (TBARS), conjugated dienes (CD) and lipid hydroperoxides (LOOH) were significantly reduced. Moreover, blood serum insulin, high density lipoprotein (HDL), Superoxide dismutase (SOD), Catalase (CAT) and reduced glutathione (GSH) increased simultaneously; These results verified that the 20% CMFP cake was the best treatment specially in decreasing hepatic lipid peroxidation with levels between 54%, 49.5% and 75.2 % for LOOH, CD and TBARS consequently.
Conclusion
Since, pumpkin blossoms contains a wide range of phytonutrients, pumpkin blossom has the potential to be a functional food source to incorporate into diabetics’ diets to manage the disease.
Keywords
Cucurbita maxima
Cake
Diabetic rats
Lipid peroxidation
Liver biomarkers
1 Introduction
Diabetes Mellitus is a persistent, non-communicable disease caused by chronic hyperglycemia caused by abnormalities in insulin hormone secretion and function (Unnikrishnan et al., 2016). Diabetes's prevalence is rising globally today and is linked to lifestyle factors like obesity and by 2030, the World Health Organization (WHO) estimates that there are nearly 370 million people with diabetes.
The most common side effects of diabetes are dyslipidemia, which affects 40 % of people with diabetes and is cause for heart disease (Rajasekaran et al., 2006). Although many medicines are available to treat diabetes, there is a need for diabetic patients throughout the world mainly to consume antidiabetic agents as a functional food that is easy to add to the diet, cheaper, and safer. As a result, it would seem that the use of herbal products would be beneficial to administer medication to these individuals that would increase their sensitivity to insulin and blood glucose management.
By the biological actions of its phytochemicals, Pumpkin members of the Cucurbitaceae family contribute to boosting both human and animal health (Ahmad and Khan, 2019). It includes a significant antioxidant potential and pumpkin seeds rich in phenolic compounds provide a multitude of health advantages (Akomolafe et al., 2021).
Xia and Wang (2007) in their research on the hypoglycemic effects of pumpkin, streptozocin-diabetic rats were used. Due to its antidiabetic and anticancer properties, pumpkin can be used to create food products containing additional ingredients. (Dar et al., 2017).
Many studies have been conducted on various pumpkin fruit and seed variants. Pumpkin seeds and fruit contain anti-cancer and anti-diabetic effects (Xia and Wang, 2007; Hou et al., 2008). The pumpkin fruit is also high in fiber and antioxidants, it also contains bioactive substances such linoleic acid, as well as carotenoids, ascorbic acid, vitamins, minerals, dietary fibers, and vitamin E and it is a contemporary instrument for antibacterial, antihypertensive, anticancer, anti-inflammatory, and anti-hypercholesterolemia (Dar et al., 2017). Yet, there is no data available on the research on pumpkin flowers other than a report of physico-chemical characteristics described by Ghosh and Rana (2020). They reported pumpkin flowers contain the highest mineral content (Sodium, Potassium, and Calcium) and are rich in phytonutrients such as phenol, flavonoid, antioxidants, and anthocyanins. It also contains many saturated and unsaturated fatty acids.
Alloxan is a cytotoxic analogue causing diabetes that also produces reactive oxygen species (ROS) in animals. It is also absorbed that alloxan-induced liver damage is minimal depending on the strain and administration method chosen, alloxan dosages range from 50 to 200 mg/kg and from 40 to 200 mg/kg (King, 2012).
As far as we know, no published research article investigated the effects of cake enriched with 10 % and 20 % of Cucurbita maxima powder has the potential to be considered as a health, safe and nutritious functional food to incorporate into diabetics' diets to manage the disease cellular damage and other complications, Therefore, The objective of the current study is to assess the plasma glucose, insulin levels, lipid profiling (TG, TC, LDL, HDL and VLDL), liver markers (ALT, ALP and AST), Lipid peroxidation enzymes (MDA, SOD, CAT and GSH) and by-products of lipid peroxidation (TBARS, CD and LOOH) as indicators of ameliorate effects of the cake enriched with Cucurbita maxima powder.
2 Materials and Methods
2.1 Reactive compounds
Biochemical Kits and all other chemicals were purchased from Sigma Chemicals Co. USA.
2.2 Plant material preparation
The Cucurbita maxima flowers (CMF) were freshly harvested from nearby cultivates in Cairo, Egypt. CMF were collected from stems, ordered, and rinsed in freshwater, then dried for two weeks at ambient temperature. Cucurbita maxima flower powder (CMFP) was prepared by the method adopted by Russo and Etherington (2001) with slight modifications. Grind the dried flower into powder and strain using a 0.5 mm sieve then kept them in a desiccator.
2.3 CMFP cake Preparation
The Cake was prepared using the standard technique of Penfield and Campbell (1990). The Cake was made with wheat flour (72 %) Then, the prepared cake samples were substituted with 10 % and 20 % CMFP. Fortified cake was dried at 50-60 C for 1.5 hour using vacuum oven and grounded into fine powder that added as 10% to rat basal diet.
2.4 Convergent examination of cake samples
The Convergent was checked by analyzing the moisture, ash, protein, fat and fiber of the control cake sample. Carbohydrates and nitrogen-free extract (NFE) were calculated.
2.5 Basal diet preparation
The AIN-93 protocol was used to establish the basal diet according to the procedure described by Reeves et al. (1993). It contains ingredients such as 20 %, 10 %, 5 %, 4.7 %, and 3.5 % casein, sucrose, fiber, corn oil, and salt mixture respectively, 2 % choline chloride, 1 % vitamin and the rest proportion made of corn starch.
2.6 Study's layout
The Helwan animal colony, Cairo, Egypt, provided forty adult male albino rats weighing 180 ± 5 g for the experiment. Rats were kept in acrylic cages in a controlled environment with a fresh air chamber at 24 ± 5°C, light condition (12hr light/12hr dark cycles) and relative humidity. Feed and water ad libitum were provided for adaption purposes before the trial began for one week. The entire process of handling and caring for animals was held in Faculty of Home Economics, Helwan University, Cairo, Egypt, IACUC protocol number ARC-FU-41–24, followed as per the guidelines outlined in the Declaration of Helsinki.
2.7 Initiation of diabetes in rats
Overnight fasted rats were treated with a single dose of 40 mg/kg of alloxan to induce diabetes. After the 48 h of injection, the blood sample was collected from tail vein for the analysis of blood glucose. Diabetic rats with fasting blood glucose levels ranging from 210 to 220 mg/dl were chosen for further testing, whereas those with levels less than 200 mg/dl were rejected. The rats were put into five groups of eight rats each.
Group I (normal control) received only a basal diet. Group II (diabetic control) treated with an alloxan-alone. Group III was diabetic rats fed with a basal diet combined with dried cake. Groups IV and V were diabetic-induced rats containing 10 % and 20 % CMFP-treated groups, respectively.
2.8 Biological Indices
Food intake was noted daily and body weight growth was tracked weekly. Divide the weight gain (g) by the feed intake (g) to get the feed efficiency ratio (FER). After the experimental period, anesthetized animals were sacrificed and a blood sample was taken and centrifuged to collect the serum for next determinations. Hepatic tissue slices were dissected and rinsed in ice-cold saline. (Jaishree and Badami, 2010). The tissues were fragmented and pureed in a cold buffer (pH 7.0) to yield a 20 % tissue homogenate (w/v). Centrifuge the tissue homogenate at 1200 rpm for 10 min. Then, the supernatants were carefully collected and used to measure different biochemical parameters.
2.9 Blood glucose and insulin estimation
Burrin and Price (1985) used the colorimetric approach to assess blood glucose. Clark (1999) determined that the insulin radioimmunoassay kit was utilized to detect plasma insulin.
2.10 Quantification of hepatic functioning and lipid concentrations
The liver enzyme biomarkers were expressed in Units/mL, AST and ALT have been detected using the Reitman and Frankel (1957) technique. The enzyme ALP was determined using King (1996) method. Serum cholesterol was tested using Allain et al. (1974) enzymatic method. Serum triglycerides were colorimetrically evaluated using Wahlefeld's (1974) approach. The level of High density lipoprotein (HDL) was determined using the method of Albers et al. (1983). Very low density lipoprotein (VLDL) concentration and Low density lipoprotein (LDL) were estimated using Friedewald et al. (1972) equations.
2.11 Non-enzymatic antioxidant indicators in hepatic tissue and lipid peroxidation
MDA and antioxidant biomarkers (SOD, CAT and GSH) were all determined using liver homogenates. Lipid peroxidation (LPO) was measured using estimations of malondialdehyde (MDA) (Rudnicki et al. (2007); reduced glutathione (GSH) (Ellman, 1959); superoxide dismutase (SOD) (Spitz and Oberley 1989); catalase (CAT) (Sinha, 1972). Pro-oxidative markers like Thiobarbituric acid reactive substances (TBARS) (Yagi, 1987); conjugated dienes (CD) (Rao and Recknagel, 1968); lipid hydroperoxides (LOOH) (Jiang et al., 1992).
2.12 Statistical analysis
All the data were analyzed using one-way analysis of variance (ANOVA), followed by Duncan's Multiple Range Test (DMRT) using SPSS version 11 (SPSS, Chicago, IL), P 0.05 was chosen as the upper limit of statistical significance.
3 Results
The convergent composition of the C. maxima flower powder fortified cake (10 % and 20 %) and the control cake is presented in Table 1. The percent of crude protein, moisture, ash, calcium, fiber and phosphorus in the control cake made with 100 % wheat flour are 13.33, 10.67, 12.03, 1.92, 0.79, 1.18, 0.11 and 59.97 respectively. However, the 10 % and 20 % CMFP added cake contains more moisture, protein, ash, fiber, calcium and phosphorus compared to the control cake. The results disclosed that 20 % CMFP fortified cake comprises an increase of 2.91, 0.64, 0.89, 0.04, 0.02, 0.02, 0.01 and a decline of 3.53 percent in the moisture, protein, ash, fiber, calcium and phosphorus respectively compared to 10 % CMFP added cake. Values are expressed as mean ± SD, n = 5.
Parameters
Control cake
Values in %
CMFP fortified cake
10 %
20 %
Moisture
13.33
15.22
18.13
Crude Protein
10.67
11.43
12.07
Ether extract
12.03
12.82
13.7
Ash
1.92
1.99
2.03
Crude Fiber
0.79
0.88
0.9
Calcium
1.18
1.22
1.24
Phosphorus
0.11
0.14
0.15
Nitrogen free extract
59.97
56.3
52.77
Table 2 displays the result of CMFP added cake on plasma glucose and insulin levels in diabetic rats. The induction of alloxan in the rats caused a rise in the blood glucose level up to 212.39 mg/dL and a decrease in insulin level to 7.11 µU/mL. However, noticeable differences in the glucose and insulin levels were observed between diabetic and untreated rats. The 20 % CMFP fortified cake-treated alloxan-induced diabetic rats showed the highest decrease in glucose level of 93.17 mg/dL and an increase in insulin level of 15.14 µU/mL compared to other treatments and close similar to normal rats significantly. Values are expressed as mean ± S.D. n = 8 rats/group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT).
Groups
Glucose (mg/dL)
Insulin (μU/mL)
G1- Normal control
90.46 ± 4.43c
15.96 ± 1.02a
G2- Diabetic control
212.39 ± 5.25a
7.11 ± 0.78c
G3- Control cake + Alloxan
128.10 ± 11.37b
10.28 ± 1.04b
G4- CMFP 10 % + Alloxan
102.31 ± 7.33 bc
13.88 ± 1.07ab
G5- CMFP 20 % + Alloxan
93.17 ± 5.28c
15.14 ± 1.03a
Further, the effect of CMFP-fortified cake on the Feed consumption rate, body weight gain and Feed efficiency ratio (FER) of diabetic rats are revealed in Table 3. Food intake and body weight of the diabetic control group showed that food intake, body weight and FER increased compared with the normal control group. However, the body weight is not controlled even in the 10 and 20 % CMFP-fortified cake-treated diabetic rats (40.25 and 43.51) that showed higher than normal control (28.35). Values not sharing a common superscript differ significantly at p < 0.05 (DMRT).
Groups
Food consumption (g / day)*
Body weight gain ratio**
FER**
G1- Normal control
20.53
28.35 ± 1.32d
0.067 ± 0.001c
G2- Diabetic control
26.86
54.72 ± 1.21a
0.031 ± 0.002a
G3- Control cake + Alloxan
26.17
51.83 ± 0.99b
0.059 ± 0.001b
G4- CMFP 10 % + Alloxan
23.51
43.51 ± 1.11bc
0.061 ± 0.002c
G5- CMFP 20 % + Alloxan
21.73
40.25 ± 1.15bc
0.064 ± 0.002c
The effect of CMFP-fortified cake on the different lipid parameters of diabetic rats is depicted in Figs. 1a & 1b. Compared to the normal control and the diabetic (untreated) group showed a highly significant rise in total lipid level of 4.25 g/L. Also compared to the diabetic control group and treated groups fed with CMFP enriched cakes showed a highly significant drop in total lipid level similar to normal control rats (Fig. 1a). In serum total cholesterol level, the diabetic rats (untreated) group showed a maximum of 168.26 mg/dl compared to normal control rats (96.73 mg/dl). However, in the treated group, a maximum reduction of 100.13 mg/dl was observed in 20 % CMFP-fortified cake-treated alloxan-induced diabetic rats. The 20 % CMFP-fortified cake resulted in a statistically noteworthy reduction in the levels of Total lipids, TG, TC, LDL, and VLD in diabetic rats (Fig. 1b). However, there is no noticeable difference in total lipid profiles (TL, TC, TG, LDL, Total lipids, and VLDL) between the treatment group, which consumed 20 % and 10 % CMFP fortified cake treated and normal control group. However, in HDL levels in CMFP-fortified cake treated group was compared to the diabetic group. There is a significant similarity between the increase in 20 % CMFP fortified cake and the normal control group HDL level.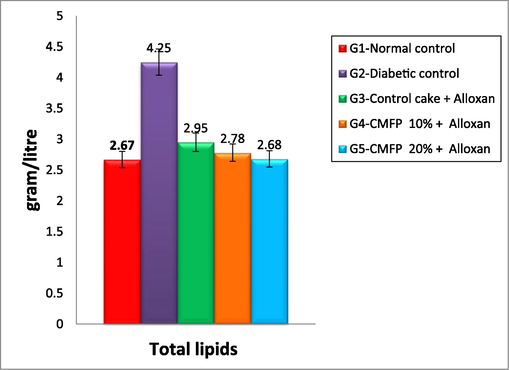
Effect of CMFP fortified cake on total lipids of Alloxan induced diabetic rats.
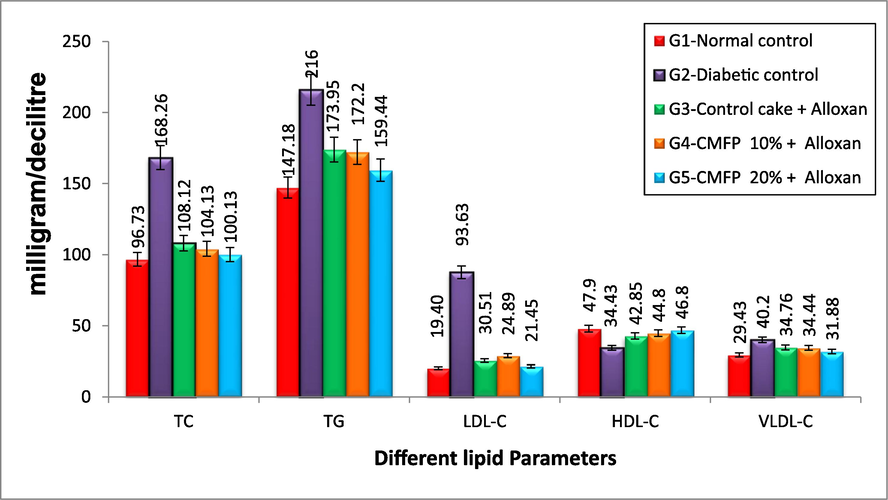
Effect of CMFP fortified cake on Total TC, TG, LDL-c, HDL-c and VLDL-c of Alloxan induced diabetic rats.
Fig. 2 depicts the data on the effect of CMFP-supplemented cake on serum liver biomarkers in diabetic rats. Changes in AST, ALT and ALP levels were detected in all treated groups when compared to the normal control group. The level of AST, ALT and ALP levels were decreased to 70.20 U/L, 32.20 U/L and 37.46 IU/L respectively in the rats fed with 20 % CMFP-fortified cake. Compared to the normal control group the AST was slightly higher, the ALT level slightly lower and the ALP level was on par with the rats fed with 20 % CMFP-fortified cake. However, the AST, ALT and ALP enzymes increase drastically in diabetic control rats by recording a value of 89.92 U/L, 59.23 U/L and 51.32 IU/L respectively.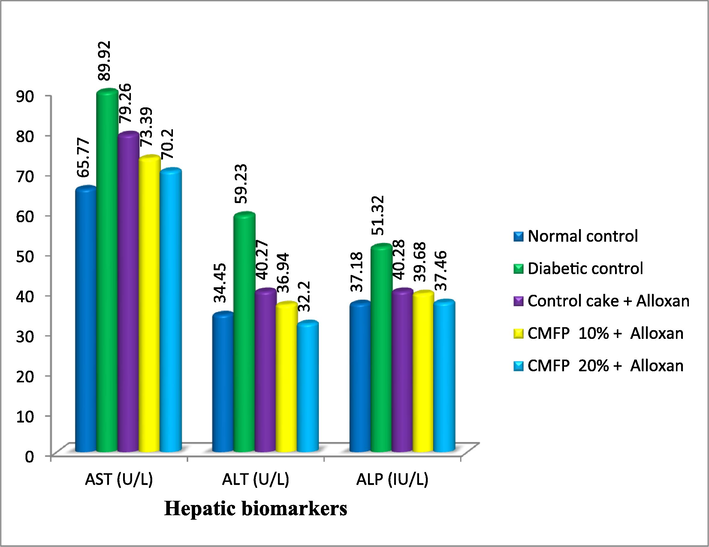
Effect of CMFP fortified cake on hepatic biomarkers of Alloxan induced diabetic rats.
Fig. 3a, Fig. 3b demonstrates variations in the lipid peroxidation, MDA, SOD, CAT, and GSH in diabetic-induced rats. The lipid peroxidation enzyme MDA (6.12 µmol/mg protein) is significantly (p < 0.05) higher and lower enzymes levels such as recorded GSH (12.55 mmol /min/mg protein), SOD (30.98 U /mg protein) and CAT (0.139 U /mg protein) in diabetic (untreated) rats compared to normal control and other treatments. Diabetic rats fed a 20 % CMFP supplemented cake had a substantial effect on MDA by reducing it to 3.23 µmol/mg protein and increase in GSH (22.84 mmol/min/mg protein), SOD (54.36 U /mg protein) and CAT (0.180 U /mg protein) which is as alike to the normal control.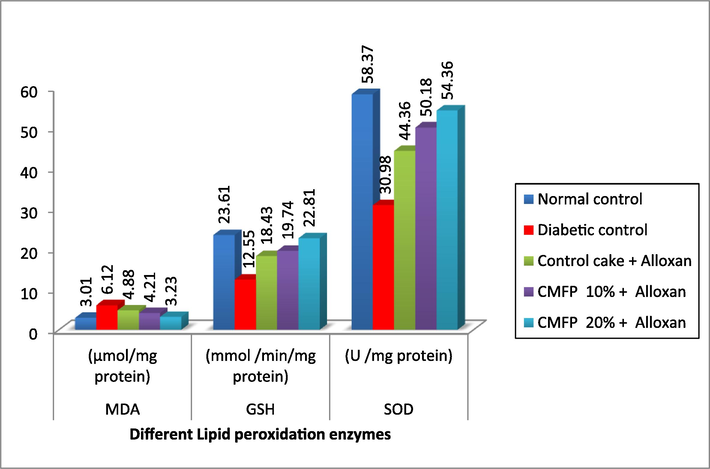
Effect of CMFP fortified cake on different lipid peroxidation enzyme level in Alloxan induced diabetic rats.
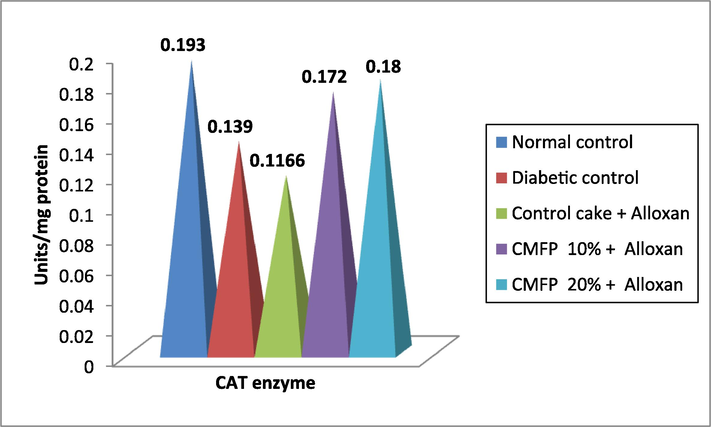
Effect of CMFP fortified cake on lipid peroxidation enzyme- CAT level in Alloxan induced diabetic rats.
Table 4 additionally illustrates the result of CMFP-supplemented cake levels on by-products of lipid peroxidation (TBARS, CD and LOOH) in hepatic tissue of diabetic rats. It revealed that the diabetic rats fed with 20 % CMFP enriched cake recorded a minimum TBARS value of 0.84 mM/100 g of wet tissue which is on par with normal control (0.77 mM/100 g of wet tissue). Similarly, the same treatment showed a minimum value of CD of 57.22 mM/100 g of wet tissue and LOOH of 70.19 mM/100 g of wet tissue and proved on par with the normal control. The diabetic-induced rats indicated the maximum value of 3.39, 113.23 and 125.30 mM/100 g of wet tissue. Values are expressed as mean ± S.D. n = 8 rats/group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT).
Groups
TBARS (mmol/100 g wet tissue)
CD (mmol/100 g wet tissue)
LOOH (mmol/100 g wet tissue)
G1- Normal control
0.77 ± 0.04d
54.35 ± 1.42d
69.36 ± 1.88c
G2- Diabetic control
3.39 ± 0.12a
113.23 ± 1.33a
125.30 ± 2.11a
G3- Control cake + Alloxan
1.26 ± 0.06c
89.27 ± 1.25c
91.21 ± 2.17b
G4- CMFP 10 % + Alloxan
0.93 ± 0.03cd
68.29 ± 1.39cd
76.27 ± 1.93c
G5- CMFP 20 % + Alloxan
0.84 ± 0.04d
57.22 ± 1.53d
70.19 ± 1.99c
4 Discussion
Pumpkin (C. maxima) flowers may offer several health benefits and are highly nutritious. It is a great source of antioxidants, compounds that may neutralize harmful free radicals and reduce inflammation in your body. Besides numerous types of antioxidants, pumpkin flower is rich in carotenoids, anthocyanins, carotene, flavonoids and phenols. However, there is inadequate research on the effects of pumpkin flowers. Therefore, the current study was planned to evaluate the effect of Cake fortified with C. maxima flower in improving antioxidant, and hepatic glucose and ameliorating lipid abnormalitiess in Alloxan intoxicated diabetic rat model.
King (2012) pointed that alloxan can precisely block insulin production that is produced by glucose and can be utilized to chemically induce diabetes mellitus after the aqueous solution of alloxan was given at a dose of 40 mg/kg body weight to rats with fasting blood glucose levels ranging from 210-220 mg/dl.
The present experiment explored the convergent composition of 10 % and 20 % CMFP-enhanced cakes, which included high moisture, crude protein, ash, fiber, calcium and phosphorus than the control cake. It is stated that moisture is a resource for blood and oxygen transport and that the presence of crude fiber may aid in digestion. Also, the Ash represents high mineral content which is mandatory for proper physiological functioning of the body. Hence, the convergent analysis of the cake fortified with 10 % and 20 % CMFP revealed that CMFP is a valuable source of nutrients.
As a result, this research addressed the influence of CMFP-fortified cake on plasma glucose and insulin levels in diabetic rats. Induction of alloxan resulted in a rise in blood sugar amounts vice versa insulin levels in rats. However, the treatment with 20 % CMFP fortified cake significantly (p < 0.05) resulted in the highest decrease in glucose level (93.17 mg/dL) and increase in insulin level (15.14 µU/mL) than diabetic control rats. It should be emphasized that the rats fed with 20 % CMFP fortified cake indicated normal insulin and glucose levels as showed by normal control rats. These results are reliable with those of Ashiq et al. (2022), detected a decrease in blood glucose levels in groups of diabetic rats fed with 15 g of pumpkin seed.
The alterations in insulin and glucose levels demonstrated the onset of diabetes in alloxan-treated rats. The differences in insulin and glucose levels caused by various doses of 10 and 20 % CMFP are related to the impact on the regulation of glucose metabolism in diabetic rats. According to Ashok et al. (2013), the effect may also be attributable to the exertion of hypoglycemic effects by increasing either insulin secretion or peripheral glucose consumption.
Furthermore, the consequence of CMFP-supplemented cake on food intake rate, body weight gain and Feed efficiency ratio (FER) of diabetic rats was examined in the current study. Various patterns in dietary consumption parameters were observed, demonstrating that body weight is not regulated even in diabetic rats fed cake enriched with 10 % and 20 % CMFP. Conversely, feed consumption rate and Feed efficiency ratio (FER) were reduced which were significantly (p < 0.05) on par with normal control rats. Grover et al. (2002) discovered that Aegle marmelose leaf extract helped restore body weight and normal blood glucose levels.
The implications of CMFP-fortified cake on the essential lipid parameters of diabetic rats demonstrated a significant increase in total lipid level of 4.25 g/L in the diabetic (untreated) rat group. Elevated levels of total cholesterol, particularly LDL-cholesterol in the blood is route cause for coronary heart disease (Hannan et al. 2003). However, a maximum reduction of serum total cholesterol level (100.13 mg/dL) and in the levels of TC, TG, LDL and VLDL was noticed in diabetic rats given a 20 % CMFP-supplemented cake. Interestingly, observations revealed a substantial resemblance in HDL-c level between the 20 % CMFP fortified cake and the normal control group, as per the report of Ashok Sharma et al. (2013), an alcoholic extract of Cucurbita maxima significantly (p < 0.05) decreased TC, LDL, VLDL, and TG levels while significantly (p < 0.05) increasing HDL levels. The considerable reduction in the total cholesterol and increase in HDL is a highly desired biochemical state for the prevention of atherosclerosis and ischemic diseases (Sachdeva and Khemani, 2003).
The serum examination of the enzymes ALP, AST, and ALT is a useful reference for hepatotoxicity evaluation (Abirami et al. 2015). In this study, the serum liver biomarkers of diabetes induced rats were considerably affected by CMFP-fortified cake treatment as revealed that rats fed 20 % CMFP-fortified cake had lower levels of ALT, AST, and ALP enzymes. However, these enzymes increase drastically in diabetic control rats. Similar outcomes were noted in the findings by Abirami et al. (2015) and the elevated level of these enzymes in the control group suggested that the liver had been injured and lost structural integrity.
The alterations in GSH, MDA, SOD, and CAT enzyme concentrations in diabetes rats were observed by presenting increased MDA and lesser GSH, SOD, and CAT activity. According to Abirami et al. (2015), an upsurge in liver MDA indicates greater lipid peroxidation, which damages tissue and impairs the mechanism of antioxidant defense by failing to avert excessive free radical generation. The negative changes in the activity of the enzymes CAT, SOD and GSH could represent the harmful consequences of toxic substances producing reactive oxygen species. Nonetheless, in the studies we conducted, diabetic rats fed a 20 % CMFP-fortified cake had a positive influence on suppressing MDA and boosting GSH, SOD, and CAT levels in comparison to the normal control. According to Renata et al. (2022), those consequences may be attributable to the existence of several established antioxidant and antibacterial capabilities, as well as the presence of many natural bioactive substances such as flavonoids, phenolic acids, and anthocyanins. Many authors proved the antioxidant and scavenging effect of pumpkin flowers and many other flowers that were used as functional food (Li et al., 2014; Benvenuti et al., 2016; Pires et al., 2018).
The production of CD and LOOH signifies the beginning and progression of the lipid peroxidation process respectively and the formation of TBARS represents the decomposition of LOOH, furthermore, diabetic rats fed 20 % CMFP-enriched cake had the lowest levels of TBARS, CD and LOOH. This result could be attributed to its antioxidant action, which inhibits the formation of these reactive compounds. Jayaraman and Namasivayam (2011) reported that the administration of naringenin decreased the level of bilirubin, ALP, LDH, TBARS, LOOH and CD in Male albino rats. Popovic et al. (2019) stated that anthocyanins from bilberry fruit extract resulted in a substantial decline in the lipid peroxidation by products.
5 Conclusion
The results of this study certainly showed cake enriched with 20 % CMFP- lowered blood glucose and increased insulin levels in alloxan induced diabetic animals. Besides, it decreases the lipid peroxidation level, pro-oxidative markers and antioxidant-regulating enzymes. As the Pumpkin flower contains medically important many phytonutrients (phenol, flavonoid antioxidants and anthocyanins) this could be acts as a prospective alternate source of functional food to be added to the diet of diabetic patients for the control of diabetes and its related diseases in humans.
CRediT authorship contribution statement
Mohamed Farouk Elsadek: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Tse-Wei Chen: Validation, Software, Resources, Methodology. Khalid S. Al-Numair: Supervision, Resources, Project administration, Funding acquisition. Maha M. Essam El-Din: Writing – review & editing, Methodology, Formal analysis, Data curation.
Acknowledgment
The authors extend their appreciation to the Researchers supporting project number (RSP2024R349) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Human Wellness. 2015;4(1):35-41.
- [Google Scholar]
- Pumpkin: horticultural importance and its roles in various forms; a review. International J. of Horti and Agri.. 2019;4(1):1-6.
- [Google Scholar]
- Diets supplemented with raw and roasted pumpkin (Cucurbita pepo L.) seeds improved some biochemical parameters associated with erectile function in rats. J. of Food Biochemistry.. 2021;45(2):13629.
- [Google Scholar]
- Enzymatic determination of high density lipoprotein cholesterol: Selected methods. Clin Chem.. 1983;10:91-99.
- [Google Scholar]
- Ashiq, H., Tusneem, K., Muhammad Abdullah, J., Saima, N., Khansa, I., Ayesha, R., Muhammad Azhar, I., Muhammad Abid, M., Muhammad Yousaf, Q., Jawed, A., Atif, A., 2022. In Vitro Role of Pumpkin Parts as Pharma-Foods: Antihyperglycemic and Antihyperlipidemic Activities of Pumpkin Peel, Flesh, and Seed Powders, in Alloxan-Induced Diabetic Rats. International Journal of Food Science. 10 pages.
- Antidiabetic and antihyperlipidemic activity of cucurbita maxima duchense (Pumpkin) seeds on streptozotocin induced diabetic rats. J. Pharmacognosy and Phytochemistry.. 2013;1(6):108.
- [Google Scholar]
- Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci Hortic.. 2016;199:170-177.
- [Google Scholar]
- Assays for insulin, proinsulin (s) and C-peptide. Ann. Clin. Biochem.. 1999;36(5):541-564.
- [Google Scholar]
- Pumpkin the functional and therapeutic ingredient: a review. Int. J. Food Sci. Nutr.. 2017;2(6):165-170.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem.. 1972;18(6):499-502.
- [Google Scholar]
- Medicinal plants of India with antidiabetic potential. J. Ethnopharmacol.. 2002;81:100.
- [Google Scholar]
- Effect of soluble dietary fibre fraction of Trigonella foenum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type-2 diabetic model rats. J. Ethnopharmacol.. 2003;88:73-77.
- [Google Scholar]
- Atomic resolution structure of cucurmosin, a novel type 1 ribosome-inactivating protein from the sarcocarp of Cucurbita moschata. J. Struct. Biol.. 2008;164(1):81-87.
- [Google Scholar]
- Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J. Ethnopharmacol.. 2010;130(1):103-106.
- [Google Scholar]
- Naringenin modulates circulatory lipid peroxidation, anti-oxidant status and hepatic alcohol metabolizing enzymes in rats with ethanol induced liver injury. Fundam Clin Pharmacol.. 2011;25(6):682-689.
- [Google Scholar]
- Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem.. 1992;202(2):384-389.
- [Google Scholar]
- The hydrolases-acid and alkaline phosphatases. In: Practical Clinical Enzymology. London: Nostrand Company Limited; 1996. p. :191-208.
- [Google Scholar]
- The use of animal models in diabetes research. Br J Pharmacol.. 2012;166(3):877-894.
- [Google Scholar]
- Total phenolic contents and antioxidant capacities of 51 edible and wild fowers. J Funct Foods.. 2014;6:319-330.
- [Google Scholar]
- Evaluating food by objective methods. In: Experimental Food Science (3rd ed.). San Diego, CA: Academic Press, Inc.; 1990. p. :23-45.
- [Google Scholar]
- Edible flowers as sources of phenolic compounds with bioactive potential. Food Res Int.. 2018;105:580-588.
- [Google Scholar]
- Anthocyanins protect hepatocytes against CCl4-induced acute liver injury in rats by inhibiting pro-inflammatory mediators, polyamine catabolism, lipocalin-2, and excessive proliferation of kupffer cells. Antioxidants (Basel). 2019;4(8(10)):451.
- [Google Scholar]
- Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol.. 2006;33:232-237.
- [Google Scholar]
- Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Experimental and Molecular Pathol.. 1968;9(2):271-278.
- [Google Scholar]
- Reeves, P.G., Nielsen, F.H., Fahey, Jr. G.C., 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet.
- A colorimetric method for the determination of serum glutamate oxaloacetate transaminase. Am J. Clin. Pathol.. 1957;28:53-56.
- [Google Scholar]
- Nutritional Value and Antioxidant Activity of Fresh Pumpkin Flowers (Cucurbita sp.) Grown in Poland. Appl. Sci.. 2022;12(13):6673.
- [Google Scholar]
- Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem.. 2007;100:719-724.
- [Google Scholar]
- Effect of Hibiscus rosa sinensis Linn. Ethanol on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J. Ethnopharmacol.. 2003;89:61-66.
- [Google Scholar]
- An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem.. 1989;179:8-18.
- [Google Scholar]
- Diabetes mellitus and its complications in India. Nat. Rev Endocrinol.. 2016;12(6):357-370.
- [Google Scholar]
- Enzymatic Determination of triglycerides- methods of enzymatic analysis. HU. Bergmeyer, Ed. Academic Press, New York.. 1974;5:1831-1835.
- [Google Scholar]
- Hypoglycaemic role of Cucurbita ficifolia (Cucurbitaceae) fruit extract in streptozotocin-induced diabetic rats. J. Sci. Food Agric.. 2007;87(9):1753-1757.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103452.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







