Translate this page into:
A vegetable oil blend administration mitigates the hyperglycemia-induced redox imbalance, renal histopathology, and function in diabetic nephropathy
⁎Corresponding authors. ma.kausar@uoh.edu.sa (Mohd. Adnan Kausar), mo.saeed@uoh.edu.sa (Mohd Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The study was designed to search the efficacy of the oil blend, composed of sesame, canola, walnut, and wheat germ oils against diabetic nephropathy (DN). In the present study, the notion that a vegetable oil blend (VOB) minimizes oxidative damage, reduces renal injury, and preserves renal architecture was tested. Male wistar rats received VOB (2 ml/ kg−1) for 56 days after diabetes induction (streptozotocin 55 mg kg−1). Biochemical evidences in serum and renal tissue had been used to evaluate the impact on kidney injury and supported with histopathological examination. A significant increase in blood glucose, blood urea nitrogen (BUN), N-acetyl-β-D-glucosaminidase (NAG), and proteinuria levels with a concomitant decrease in glomerular filtration rate (GFR) were noted in diabetic rats. Oxidative damage indices such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), transforming growth factor-beta1 (TGF-β1), malonaldehyde (MDA) and glutathione (GSH) were found higher in diabetic group with diminished glutathione reductase (GR) and superoxide dismutase (SOD) antioxidant enzymes level. Histological examination also revealed significant alterations, including glomerulosclerosis and interstitial fibrosis in diabetic group. Administration of VOB significantly modulated abnormalities in markers of renal dysfunction and tubular damage. Further oxidative damage and renal histological changes were recovered in the treatment group. In conclusion, our findings strongly suggest that renoprotective effect of VOB on nephrotic damage is attributable to its potential antidiabetic and antioxidant capabilities. There is still work to be done on a longer-term investigation or a clinical trial.
Keywords
Diabetic nephropathy
Vegetable oil blend
Oxidative stress
Nephroprotective
- DN
-
Diabetic nephropathy
- GFR
-
glomerular filtration rate
- DM
-
diabetes mellitu
- WGO
-
Wheat germ oil
- VOB
-
vegetable oil blend
- GC–MS
-
Gas chromatography-mass spectrometry
- STZ
-
Streptozotocin
- T2DM
-
Type 2 diabetes mellitus
- NAG
-
N-acetyl-β-d-glucosaminidase
Abbreviations
1 Introduction
Diabetic nephropathy (DN) is a general complication of diabetes and the leading cause of about half of all end-stage kidney diseases in the developed world. Approximately 40% of people with diabetes develop DN, diagnosed as albuminuria and/or decreased glomerular filtration rate (GFR) (Alicic et al., 2017; Saeed et al., 2021). Diabetic cases were found approximately 9.3% in 2019 globally and are predicted to be raised to 10.2% by 2030 (Saeedi et al., 2019). Even though the exact cause of renal dysfunctionality is not defined, it ultimately leads to damage to the kidney and causes severe kidney disease. DN is diagnosed by many parameters correlated with morphological and structural alterations within the kidney (Liu, 2011; Toth-Manikowski and Atta, 2015). However, hypertension and hyperglycemia have been regarded as the main mediators in the progression of DN (Khaki et al., 2010). The process of excessive production of ROS induced by persistent hyperglycemia is the main contributor underlying the pathogenesis of diabetes mellitus (DM) and its associated vascular complications, including DN (Zeng et al., 2014).
Nowadays, an unavoidable sedentary lifestyle with fast foods and no time for exercise is causing severe health problems in all age groups. Unlike allopathic drugs, which mainly use synthetic chemicals intended for specific target receptors and primarily offer symptomatic relief, the demand for natural plant-based products is growing to eradicate the root cause of the disease without adverse effects. Vegetable oils are renewable resource products gaining popularity due to their numerous health benefits (Balboa et al., 2014; Orsavova et al., 2015). Vegetable oils are the natural sources of essential fatty acids and the chief source of fat in the diet and are mainly used as the cooking medium in different food preparations (Ramesh and Murughan, 2008). Vegetable oils formulations, a complex mixture of various fatty acids, have been used prominently in the food, medical, and cosmetic industries (Dhavamani et al., 2014; Badea et al., 2015; Sarkar et al., 2017).
Several studies have found that mixing oils improved their nutritional and functional characteristics and resulted in a variety of health benefits (Reena and Lokesh, 2007; Huang and Freter, 2015). In the present study, four oils, namely sesame, canola, walnut, and wheat germ, were selected to make an oil blend, and its efficacy was evaluated to manage renal complications in the diabetic model. The richness of lignans in sesame oil, such as sesamin, sesaminol, c-tocopherol, sesamol, and other acylglycerols, was attributed to its various biological activities (Wu et al., 2019). All of these bioactive compounds likely act synergistically to contribute to the reported antioxidant, antihypertensive, antimutagenic, anti-inflammatory, antidiabetic, antithrombotic, and cardioprotective activities (Aslam et al., 2017; Wan et al., 2017**; Qin et al., 2019; Wu et al., 2019). Various beneficial effects of canola oil have been reviewed, including cardioprotective, insulin-sensitizing, antioxidant, anti-inflammatory, and anticancer (Lin et al., 2013; Amiri et al., 2019; Ramezani-Jolfaie et al.; 2020). It has been suggested that canola oil intake might improve lipid profile, hyperglycemia, and insulin sensitivity (Atefi et al., 2018).
Recently, research is focused more on walnuts (Juglans regia) than any other variety of nuts. The oil extracted from it is highly valuable and has several health benefits, including anti-inflammatory, antioxidant, cardioprotective, anti-aging, improvement of blood circulation, prevention of eczema, and stabilization of body hormones (Laubertová et al., 2015). Walnut can also help with diabetes management (Tapsell et al., 2004, Gillen et al., 2005; Zibaeenezhad et al., 2016).
Wheat germ oil (WGO), the other most crucial oil, is rich in vitamin E, policosanol, octacosanol, fat-soluble carotenoids such as lutein, zeaxanthin and β-carotene (Zou et al., 2018), has an important place in the food, medicine, and cosmetics industry (Gili et al., 2018). Previous studies reported the beneficial effects of WGO, including antioxidant, antidiabetic, and neuroprotective actions due to the presence of its active constituents (Paranich et al., 2000; Ohashi et al., 2011; El-Marasy et al., 2012; Merghani et al., 2015]. Utilization of WGO is bounded because the enzymatic activity and the level of unsaturated fatty acids are high, further stabilization techniques are needed to gain mastery over this problem. Since oils blending might overcome this problem by mixing WGO with other edible oils in countries like India, where wheat is the principal crop, oils blending may benefit a vast range of health benefits.
This study aimed to see if giving the oil mix to diabetics reduced hyperglycemia-induced oxidative damage while also restoring kidney function and histology. This study will aid new insights for future reference.
2 Materials and methods
2.1 Experimental animals
Male Wistar rats weighing (150 ± 10 g), 7–8 weeks old were obtained from Hamdard University's Central Animal House in India and allowed to acclimate to the animal house environment for one week before the trials. Throughout the experiment, the animals were fed a conventional laboratory diet.
2.2 Preparation of vegetable oil blend (VOB)
The VOB was obtained by mixing sesame oil, canola oil, walnut oil, and wheat germ oil equally in a ratio of 1:1:1:1. It was stored in airtight glass bottles in the dark at room temperature.
2.3 Acute toxicity study
According to OECD guidelines, the acute toxicity study of the VOB was assessed using male Wistar rats weighing 140–150 g to determine the dose. The animals were fasted overnight prior to carrying out the experimental plan. A different dose of VOB was given intraperitoneally (i.p.). The LD50 was calculated according to Miller and Tainter. Even at the highest dose of VOB (20 ml/kg, p.o.), there was no mortality; as a result, the dose of VOB (2 ml/kg) was chosen for further research in animal models (Ghosh, 2005).
2.4 Gas chromatography-mass spectrometry (GC–MS) analysis
GC–MS analysis of VOB was performed on Shimadzu GCMS-QP-2010 ultra-system. The equipment has an Rtx-5 MS low bleed column with dimensions of 30 mm × 0.25 mm ID × 0.25 µm films. The carrier gas, Helium, was used at a 1.0 ml/min flow rate. The operating conditions of the column were as follows: the oven temperature was programmed from 140 to 280 °C at 5 °C min−1 increments withhold time of 5 min (280 °C) was kept for 56 min. The temperature of the injector was kept at 260 °C. The volume of injected sample was 0.3 µl with a pressure of 107.4 kPa. The total flow was 28.4 ml min−1, while column flow was 1.21 ml min−1 with linear velocity of 41.6 cm s−1 at purge flow of 3.0 ml min−1. The split ratio of ions was maintained at 230 °C temperature. The scan mass range (m/z) of 40–600 was maintained at interface line temperature of 270 °C. For GC–MS interpretation, the National Institute of Standards and Technology (NIST, New Delhi) database was used, which has approximately 62,000 patterns. The compounds present in each test solution under investigation were identified by comparing their spectra to those in the NIST library of known compounds. The components of the test solutions were identified by their names, molecular weights, and structures.
3 Experimental protocols
Diabetes was induced in rats by Streptozotocin (STZ; Cat no: 14653; in citrate buffer of pH 4.5) given intraperitoneally (i.p.). After three days of STZ administration, blood was taken from 12 h overnight fasted rats via the tail vein. The rats that manifested fasting blood glucose of more than 250 mg/dl were used in the study. A total of 32 animals were divided into four different groups. Control: given a single i.p. injection of normal saline only; diabetic: given a single dose of STZ (55 mg/kg body weight; i.p.) only; diabetic + VOB: animals were given VOB orally (200 mg/kg body weight) for 56 days after diabetes induction; Control + VOB: given VOB orally (200 mg/kg body weight) for 56 days to normal rats.
After 56 days, all groups had their fasting blood glucose (FBG) concentrations measured using a strip-operated glucometer, and 24 h urine was collected and stored to determine urinary parameters (proteinuria, N-acetyl-D-glucosaminidase (NAG), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and creatinine) to track the progression of renal damage.
3.1 Collection of sample and tissue preparation
Blood was collected, and serum was separated for analysis of various parameters. The rats were sacrificed by cervical dislocation, and the left kidney was quickly extracted, and the cortex was separated from the medulla for homogenate preparation and biochemical analysis. The other kidney (right) was taken for histopathological examination in a 10% buffered formalin solution. Homogenization of Cortical slices, approximately 0.3 g pieces, were carried out with a Polytron homogenizer (Kinematica A.G.), at 0 0.1 M of ice-cold phosphate buffer (pH 7.4). The centrifugation of the homogenate was done at 800g for 5 min at 4 °C to remove the nuclear debris and the supernatant was used for estimation of malondialdehyde (MDA). The supernatant was centrifuged (10,000g) for 20 min at 4 °C to obtain the post-mitochondrial supernatant (PMS).
3.2 Estimation of kidney dysfunctionality
Blood urea nitrogen (BUN) concentration was analyzed in serum by the urease method using a commercial kit from (Elabscience, Texas, USA; Cat no: E-BC-K183-S) and the value was expressed as mmol/l. NAG concentration was determined in urine to observe tubular damage (Pedraza-Chaverrí et al., 2005), using p-nitrophenyl-N-acetyl-β-D-glucosaminide as a substrate with the slight modification described by Price et al., (1970). One unit of NAG was equated as the amount of enzyme release per 1 µmol of p-nitrophenol in the assay medium. Creatinine concentration in urine and serum was determined using a commercial kit (Cat no: E-BC-K188-M) by sarcosine oxidase method. GFR was equated as creatinine clearance using the standard equation of Arreola-Mendoza et al. (2006). Urinary protein was measured as total protein (mg/24 h) described by the Bradford method (Bradford, 1976).
4 Assessment of oxidative stress
4.1 Estimation of urinary 8-OHdG level
Samples were centrifuged (2000g) for 20 min to measure the urine 8-OHdG level, and then dilution was used to evaluate. The urinary level of 8-OHdG was estimated using ELISA kit (Elabscience, Texas, USA; Cat no: E-EL-0028), and the value was expressed as total amounts excreted in 24 h.
4.2 Determination of TGF-β1 in the serum
TGF-β1 plays an important role in maintaining the renal architecture. Serum levels of TGF-β1 were determined using ELISA kit according to the manufacture’s protocol (Elabscience, Texas, USA; Cat no: E-EL-0162).
4.3 Analysis of oxidative damage in the renal cortex
Lipid peroxidation was equated as MDA and performed as described previously (Ohkawa et al., 1979). Value was expressed as nmol MDA formed/h/mg protein. Reduced glutathione (GSH) was determined using Gherghel method (Gherghel et al., 2005). Glutathione reductase (GR) activity was assessed using Carlberg and Mannerviek (1975) method, with slight modifications. Value was expressed as nmol NADPH/min/mg protein. Superoxide dismutase (SOD) activity was determined by the method of Marklund and Marklund (1974) and the value was expressed as units/mg protein. Total protein analysis in the homogenate and PMS was done using Bradford (1976) method.
4.4 Histopathological study
Histopathological study was performed in the kidney retained in 10% buffered formalin solution. Small kidney tissue slices were dehydrated and immersed in paraffin for post-fixation. For histological evaluation, at least three cross-sections of 3–4 μm thickness were taken and then stained with hematoxylin and eosin (H and E) and Jones periodic acid-Schiff (PAS), respectively. The tissue sections after xylene wash were mounted with DPX mountant. The slides were then taken for microscopic observation (bright field) and photography.
4.5 Statistical analysis
Statistical analysis of data was done using one-way ANOVA to compare the groups followed by Tukey–Kramer test post-analysis test for multiple comparisons. The statistical software SPSS 23 was used to perform all analysis and data were expressed as mean ± S.E.M. P-value <0.05 were considered statistically significant.
5 Results
5.1 Phytochemical analysis
The GC–MS analysis of VOB showed total of 38 phytoconstituent presents in the mixture of oils (Indian variety). The active compounds with their retention time (RT), and concentration (peak area %) are presented in Table 1. The chromatogram is presented in Fig. 1. The retention times ranged between 4.29 and 44.13 min. The most abundant compounds were γ-Sitosterol (9.02%), Phenol, 2,4-bis(1,1-dimethyethyl) (8.17%), vitamin E (7.40%), heneicosane (3.63%) and γ-tocopherol (3.53%).
Sl.no
Name
R.Time
Area%
1
TETRADECANE
4.297
2.14
2
Heneicosane
5.984
3.63
3
PHENOL, 2,4-BIS(1,1-DIMETHYLETHYL)-
6.644
8.17
4
Hexadecane
8.739
2.74
5
HEXADECANE, 2,6,10,14-TETRAMETHYL-
11.215
3.00
6
Eicosane
12.369
1.18
7
Heptadecane
13.951
1.04
8
2-Bromotetradecane
15.730
0.25
9
Tetracosane, 1-iodo-
16.448
0.40
10
Nonane, 5-methyl-5-propyl-
16.710
0.39
11
Triethanolamine triacetate
20.767
0.10
12
Tetracontane
20.852
0.39
13
9,12-Octadecadienoic acid, methyl ester
21.134
2.29
14
9-Octadecenoic acid, methyl ester, (E)-
21.295
1.37
15
1,3,12-Nonadecatriene
22.654
1.82
16
Ethyl Oleate
22.795
0.64
17
TRIACONTANE
25.432
0.53
18
1-Cyclohexyldimethylsilyloxy-3,5-dimethylbenzene
25.928
1.44
19
O O'-BIPHENOL, 4,4′,6,6′-TETRA-T-BUTYL-
26.636
0.52
20
PHYTAN
30.289
2.49
21
PHYTANE
31.082
1.02
22
2-Methyltriacontane
33.495
1.86
23
Tetrapentacontane
34.089
1.61
24
1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester
34.222
0.90
25
Squalene
35.364
3.29
26
Dipivefrine, N.O-bis(pentafluoropropionyl)-
37.107
0.88
27
HEXATRIACONTANE
37.617
0.55
28
.delta.-Tocopherol
37.696
0.72
29
TETRACOSANE, 2,6,10,15,19,23-HEXAMETHYL-
38.297
0.27
30
(3S,8S,9S,10R,13R,14S,17R)-17-((2R,5R)-5,6-Dimethylhe
38.387
0.55
31
.gamma.-Tocopherol
39.382
3.53
32
STIGMAST-5-EN-3-OL, OLEAT
39.810
2.19
33
Phytonadione
40.450
2.60
34
Vitamin E
40.619
7.40
35
Octadecanoic acid, 2,2,3,3,4,4,4-heptafluorobutyl ester
41.095
1.48
36
ERGOST-5-EN-3-OL, (3.BETA.,24R)-
42.307
3.22
37
STIGMASTA-5,22-DIEN-3-OL, (3.BETA.,22E)-
42.837
2.53
38
.gamma.-Sitosterol
44.135
9.02
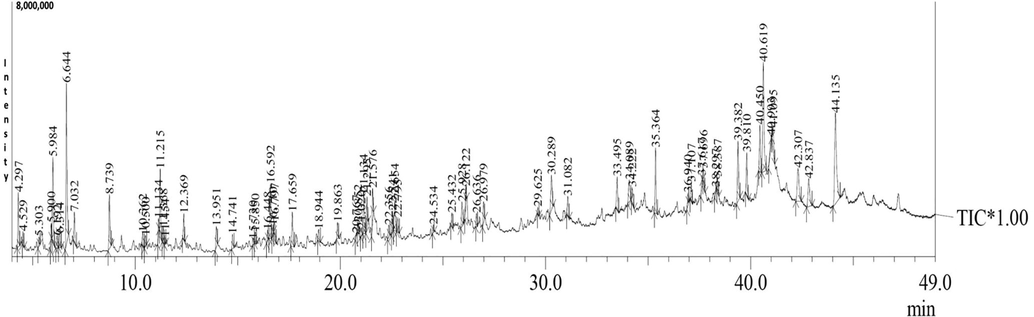
GC–MS chromatogram of VOB.
5.2 VOB supplementation reduced hyperglycemia
Hyperglycemia is the main consequence of diabetes progression. Blood glucose level was measured in all groups. A sharp increase in the blood glucose level was detected in diabetic rats (Table 2). As depicted from the results, the administration of VOB in the treatment group showed significantly lower blood glucose levels when compared to diabetic rats. Values were expressed as mean ± S.E.M. (n = 8). Diabetic group showed a significant increase in FBG and BUN. VOB treatment preserved the marker levels significantly. (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
Control
Diabetic
Diabetic + VOB
Control + VOB
FBG (mg/dl)
88.76 ± 3.53
375.75 ± 8.24* (+323.33%)
212.54 ± 5.6**
(−112.54%)102.27 ± 4.58 (+15.22%)
BUN (mmol/l)
20.29 ± 1.8
59.95 ± 2.1*
(+195.46%)30.34 ± 1.7**
(−49.39%)23.44 ± 1.9
(+15.52%)
5.3 Effect of VOB on markers of renal function
5.3.1 VOB supplementation attenuated proximal tubular damage
The function of proximal tubules is to reabsorb organic molecules, especially glucose. Persistent hyperglycemia carries out an alteration in tubular function, ultimately affecting the kidney's excretory function. NAG excretion in urine is considered a sensitive indicator of tubular damage occurring with the progression of DN. NAG level was estimated to evaluate the efficacy of VOB treatment on hyperglycemia-induced tubular derangements (Fig. 2). Diabetic rats showed increased loss of NAG concentration in urine due to severe tubular damage. The data conclusively depicted that the supplementation of VOB showed a significant (P < 0.05) restoration of NAG level as compared to the diabetic group. While administration of VOB in control rats did not show any significant changes when compared to the control group.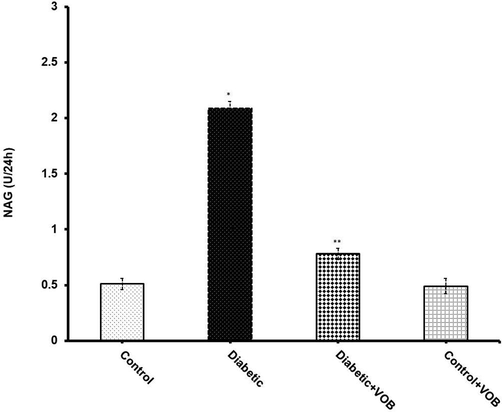
Effect of VOB supplementation on NAG level in experimental groups. Diabetic group showed a significant increase in NAG level in serum. VOB administration preserved the level significantly. Values were expressed as mean ± S.E.M. (n = 8). (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
5.3.2 VOB supplementation ameliorated GFR
Results showed an abrupt increase in BUN levels in diabetic animals when compared to control rats. The data conclusively manifested that supplementation with VOB significantly reduced BUN levels in the diabetic-treated group. It is well known in clinical and experimental studies that GFR was diminished with the development of DN. The diabetic group exhibited a sharp increase in creatinine level with a steep decrease in creatinine clearance, indicating low values of GFR compared with the control group as equated by creatinine clearance (Fig. 3). Supplementation with VOB in diabetic animals restored these altered low values of GFR compared with the diabetic group. Excretion of protein in urine is an index of glomerular damage. Proteinuria was elevated in the diabetic group, which was blunted significantly in VOB treated group (Fig. 4). Thus, treatment with VOB was not limited to proximal tubules but also broadened to glomeruli. Our results indicate that the depletion in GFR may be due to glomerular damage supported by the histological analysis in diabetic rats associated with early stages of DN.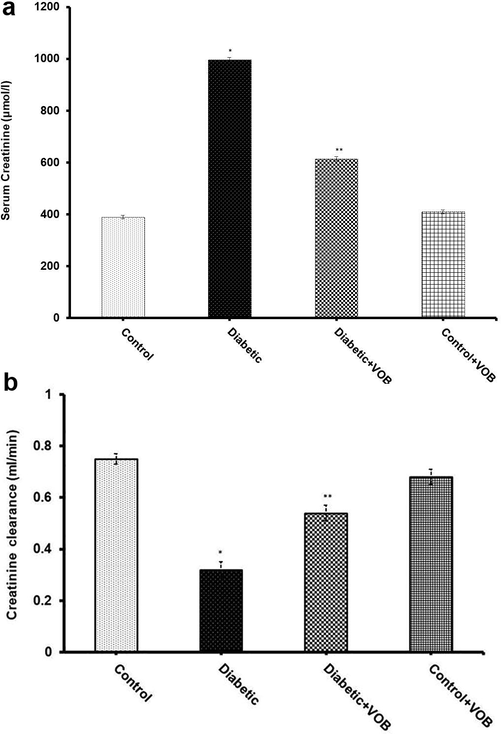
(A) Effect of VOB supplementation on serum creatinine level in experimental groups. (B) Effect of VOB administration on creatinine clearance in experimental groups. Values expressed as mean ± S.E.M. (n = 8). (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
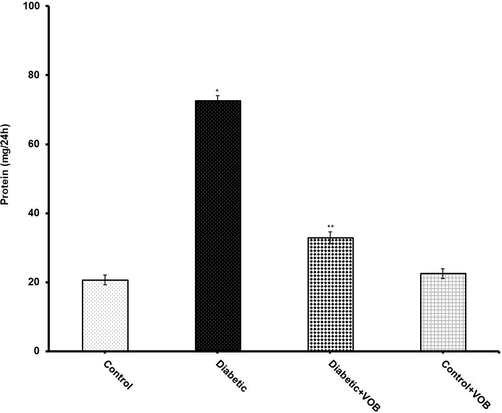
Effect of VOB supplementation on excretion of urinary protein in experimental groups. Values were expressed as mean ± S.E.M. (n = 8). (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
5.3.3 Effect on parameters of oxidative stress
It is well established that hyperglycemia-induced oxidative stress contributes to renal damage in STZ treated diabetic rats. The oxidative damage parameters measured in this study indicate that VOB has nephroprotective effects in the STZ-induced diabetic model.
5.3.4 VOB supplementation modulated 8-OHdG level
As 8-OHdG is considered one of the overriding patterns of ROS-induced oxidative DNA damage, it has been suggested as a sensitive indicator of oxidative stress. An abruptly elevated urinary 8-OHdG was observed in diabetic rats versus the control group (Fig. 5). Administration of VOB for 56 days was enough to significantly diminish 8-OHdG concentration in the treated group compared to the untreated diabetic group.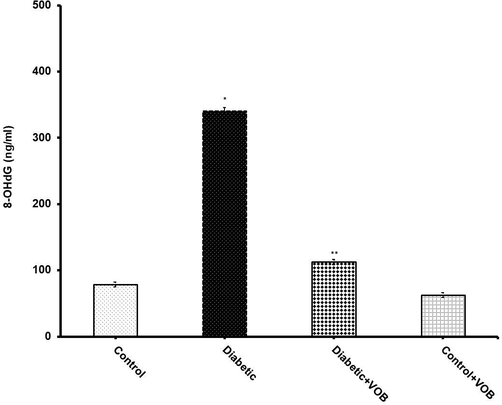
Effect of VOB administration on urinary 8-OHdG excretion in experimental groups. Diabetic group showed a significant increase in 8-OHdG levels in urine samples. VOB treatment significantly decreased its level in Diabetic + VOB group. Values are expressed as mean ± S.E.M. (n = 8). (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
5.3.5 VOB supplementation restored TGF-β1 concentration
TGF-β1 is a well-established fibrogenic cytokine and it is suggested as the central mediator to carry out the hypertrophic alterations associated with the progression of DN. TGF-β1 values were significantly elevated in the diabetic group compared to the control group (Fig. 6). Treatment with VOB restored a significant level of TGF-β1 when compared to the diabetic group.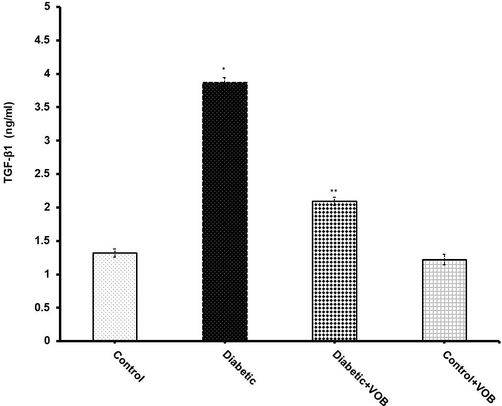
Effect of VOB administration on TGF-β1 level in experimental groups. Diabetic group showed a significant increase in TGF-β1 level. While administration with VOB significantly augmented its level in Diabetic + VOB group. Values were expressed as mean ± S.E.M. (n = 8). (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
5.3.6 VOB supplementation decreased MDA content
It was noted that MDA contents were found higher in the diabetic group when compared to the control group. In the VOB supplemented group, MDA content diminished significantly compared to the untreated diabetic group (Table 3). Values were expressed as mean ± S.E.M. (n = 8). Diabetic group showed a significant increase in MDA level with a decreased GSH, GR, and SOD activity in kidney tissue. VOB supplementation restored all these changes. (*P < 0.05 Diabetic group vs. Control OR Control + VOB group; **P < 0.05 Diabetic + VOB group vs. Diabetic group).
Control
Diabetic
Diabetic + VOB
Control + VOB
MDA
(nmol MDA formed/h/mg protein)0.51 ± 0.15
1.82 ± 0.26*
(+256.86%)0.61 ± 0.18**
(−66.48%)0.45 ± 0.21
(−11.76%)
GSH (nmol/mg protein)
5.38 ± 1.1
2.03 ± 0.82*
(−62.26%)3.86 ± 0.92**
(+90.14%)6.14 ± 1.2
(+14.12%)
GR
(nmol NADPH/min/mg protein)9.63 ± 1.2
5.04 ± 2.1*
(−47.66%)7.89 ± 3.3**
(+56.54%)10.03 ± 2.8
(4.15%)
SOD (U/mg protein)
7.04 ± 0.94
2.44 ± 0.72*
(−65.34%)4.87 ± 1.2**
(+99.59%)6.78 ± 0.98
(−3.69%)
5.3.7 VOB supplementation improves GSH level
Conventionally, GSH is a main unit of the endogenous antioxidant defence network and contributes as a mediator in many free radical scavenging reactions, especially hydrogen peroxide in our body system. The estimation of GSH level was performed to evaluate the effect of VOB on the internal antioxidant defence array during DN.
5.3.8 VOB supplementation increased GR and SOD activity
GR and SOD values of different groups of animals during the experimental period were recorded in (Table 3). Accordingly, the diabetic group exhibited significantly decreased levels of GR and SOD in renal tissue compared with the normal control rats. Supplementation of VOB showed significant restoration of these parameters.
5.3.9 Effect of VOB supplementation on renal morphology
Figs. 7 & 8 show the photomicrographs of H&E and PAS stain of renal cortical sections of control and experimental groups of rats. H& E stained renal cortical sections of the control group demonstrated well-preserved renal parenchyma with normal glomeruli surrounded by Bowman’s capsule and renal tubules. Renal sections from the diabetic group exhibited atrophy of glomeruli with focally hyaline deposit in renal tubules and edema, vacuolation, and necrosis in renal tubular epithelial cells. Administration of VOB in the treated group restored most of the degenerative changes with well-retained renal parenchyma. Only minor degenerative changes were seen in isolated areas, with no inflammatory lesions. After 56 days of experimental study, Fig. 8 shows the histopathological observations of renal sections using PAS staining. Multifocal areas of glomerular basement membrane thickening were seen in the diabetic group. Sclerotic changes in glomeruli, accumulation of plasma proteins between the glomerular endothelium and glomerular basement membrane (i.e., fibrin cap) and fibrinous deposits in the renal interstitium were also seen focally, compared with the control group. Significantly, treatment with VOB modulated the glomerulosclerosis condition and preserved renal parenchyma.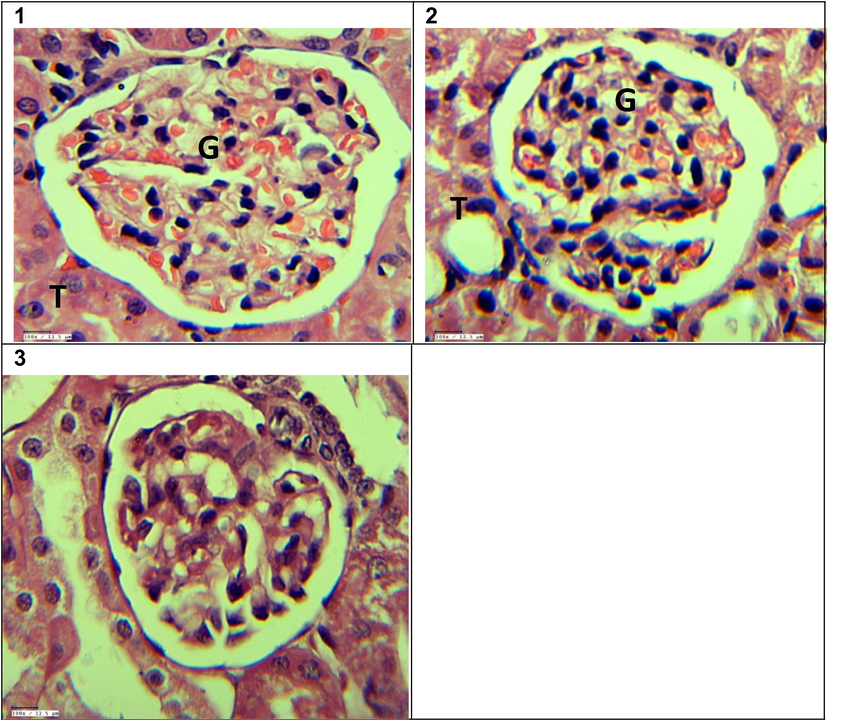
H&E stained sections of Kidney showing 1) Control Kidney – Apparently normal glomerulus (G) and renal tubules (T) 40×. 2) Diabetic Kidney –Atrophied glomerulus (G) and mild degenerative changes in the surrounding renal tubular epithelial cells (T) 40×. 3) Diabetic + VOB treated Kidney–Apparently normal glomerulus (G) and renal tubules (T) 40×.
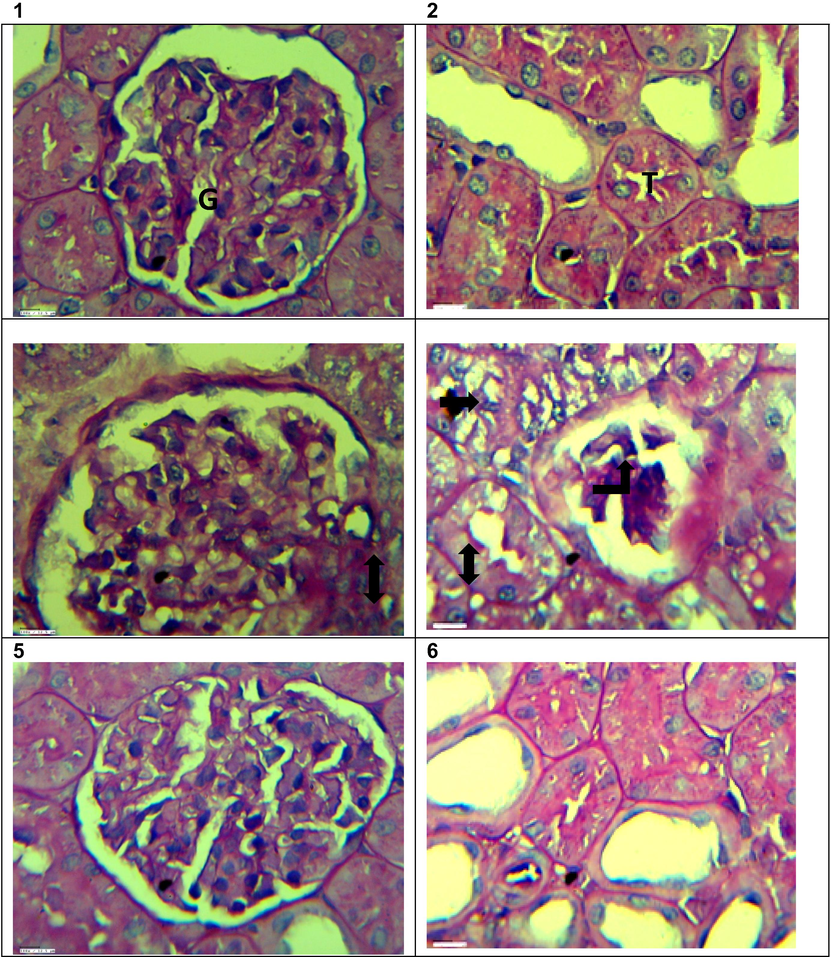
PAS stained sections of Kidney showing 1) Control Kidney – Apparently normal glomerulus (G) and renal tubules 40×. 2) Control Kidney – Apparently normal renal tubules (T) 40×. 3) Diabetic Kidney – Diffuse thickening of glomerular basement membrane (double arrow) 40×. 4) Diabetic Kidney- Moderately thickened glomerular basement membrane (double arrow), sclerotic changes in glomeruli (upward arrow) and fibrin cap (right arrow) 40×. 5) Diabetic + VOB treated Kidney – Apparently normal glomerulus (G) and renal tubules (T) 40× 6) Diabetic + VOB treated Kidney – Apparently normal renal tubules (T) 40×.
6 Discussion
In the present study, we evaluated that STZ-induced type 2 diabetes mellitus (T2DM) causes hyperglycemia, renal dysfunctionality followed by renal oxidative damage in rats similar to previous research (Clozel et al., 2006). Administration of VOB was effective to revert these alterations significantly due to their antidiabetic and antioxidant potential. Additionally, we also found that VOB treatment was effective to restore histological alteration against STZ induced T2DM.
Diabetes prevalence is one of the most prominent challenges globally, as the mortality rate is rising about 1.6 million worldwide and is considered the third strong risk factor for worldwide premature mortality due to hyperglycemia and its consequence oxidative stress (Oguntibeju, 2019). Synergistic strategy is always supported by nature due to multitarget synergistic mode. Nowadays, scientific studies have supported the use of natural compounds from plant resources because of the doubts raising the adverse reactions of synthetic agents (Kumar et al., 2021). Natural products are also a great drift of novel therapeutic agents for many medical conditions, including metabolic disorders. Oils are one of the most nutritional products present in our diet and dietary manipulation has been implicated in managing various medical conditions. The present study used a mixture of four oils, namely, sesame, canola, walnut, and wheat germ, for making VOB. This study was focused on evaluating the therapeutic efficacy of this VOB to prevent oxidative renal damage and improve renal function and histological changes in DN animal models. Remarkably, we found that treatment with VOB ameliorated oxidative stress and restored renal architecture and function in STZ-induced DN.
The GC–MS analysis of VOB revealed the presence of bioactive components e.g. phenolics, sterols, esters, vitamins, tocopherols, alcohols, acetate and fatty acids. These diverse groups of phytochemicals have gained much attention as potential natural antidiabetic agents and antioxidants. Major compounds were identified as γ-sitosterol, phenol, 2,4-bis (1,1-dimethylethyl), vitamin E and γ-tocopherol. Previous studies have suggested that phenol 2,4-bis (1,1-dimethylethyl) and vitamin E as potent free radical scavengers, γ-sitosterol as insulin secretagogues by regulating ATP sensitive K+ channel and γ-tocopherol as insulin sensitizers mediated through PPARγ mechanism (Chang et al., 2007; Balamurugan et al., 2011; Gray et al., 2011).
Our study revealed that the diabetic group showed a significant increase in blood glucose level, while administration of VOB significantly improved the glucose level in the treated group. The protective effect of VOB may be due to the presence of enormous constituents of different oils, which decreased hyperglycemia in the diabetic group by improving glucose uptake, correcting insulin action/insulin sensitivity and retaining islet architecture similar to previous studies (Mitra 2007; Sankar et al., 2011; Merghani et al., 2015; Zibaeenezhad et al., 2016; Atefi et al., 2018; Qin et al., 2019;).
Diabetes is well known for impairing kidney function and making it difficult to properly filter blood and produce urine due to altered cell membrane permeability and functional integrity (Abtahi-Evari et al., 2017). One of the indicators of renal damage is altered GFR, evaluated by estimating creatinine and BUN levels. The present study exhibited increased creatinine and BUN levels, whereas diminished creatinine clearance in the diabetic group is similar to previous research (Zeng et al., 2014). We examined that administration of VOB significantly ameliorated GFR by improving creatinine clearance in the treatment group due to its antioxidant potential (Paranich et al., 2000; Fukuda et al., 2003; Wan et al., 2015; Atefi et al., 2018) coinciding with the previous studies (Sharma, Kulkarni, Chopra, 2006). Excretion of proteins in urine is marked by tubular and glomerular damage during DN. In the present study, proteinuria was increased in diabetic rats due to altered GFR, similar to previous studies (Chiarelli et al., 1995; Vedel et al., 1996). While the supplementation of VOB reduced the excretion of total protein in the urine, indicating the renoprotective role of VOB. One possible reason to decrease the degree of proteinuria in the treatment group may be attenuating hyperglycemia and retaining tubular damage (Clark et al., 2000).
N-acetyl-β-d-glucosaminidase (NAG), an enzyme, is found in the proximal tubular part of the kidney. Previous reports have shown the secretion of NAG in urine increased following tubular damage in the early stages of DN (Fu et al., 2012). Eventually, a strong specific marker has been suggested to examine the interstitial tubule damage during the development of DN (Fu et al., 2012; Gluhovschi et al., 2016). In the present study, we showed that NAG level was found to be increased in diabetic rats, whereas supplementation with VOB modulated the loss of NAG in the urine, suggesting the tubular protective function of VOB. Further decline in tubular damage may occur due to reduced renal inflammation caused by hyperglycemia, consistent with previous studies (Takao et al., 2011; Parveen et al., 2019).
Substantial evidence has proposed that persistent hyperglycemia is the main causal factor in the advancement of early stages of DN, and augmented oxidative stress has been suggested as the main determinant in the pathological changes that occurred during diabetes and diabetes-associated renal complications (Forbes et al., 2008). The generation of ROS directly or indirectly (glycolysis, polyol pathway, PKC, NAD(P)H oxidase, etc.) is a consequence of hyperglycemia. Accumulating evidence has shown that control of oxidative stress is necessary to reduce the severity of kidney damage; that’s why antioxidant therapy is preferred (Sharma et al., 2006; Ramesh et al., 2005; Aslam et al., 2017).
Our findings confirmed that the increase in oxidative DNA damage marker 8-OHdG, elevated MDA content and the fibrotic cytokine TGF-β1 level was found in diabetic animals. Accompanying with the beneficial effects of VOB, in the present study, we noticed a steep augmentation of these elevated oxidative stress markers (8-OHdG, MDA) and TGF-β1 levels in the treatment group. We also evaluated the efficacy of VOB on the endogenous antioxidant system as one of the mechanisms to control diabetes-induced oxidative renal damage. Diabetic animals supplemented with VOB showed increased GSH, GR, and SOD in the treated group, similar to previous studies (Osawa, 1999; Paranich et al., 2000; Ramesh et al., 2005; Laubertová, et al., 2015). Thus, the results revealed that the antioxidant potential of VOB mitigates oxidative stress-prompted renal damage in the early stage of diabetes in a rat model of experimental DN. The protective effect of VOB is attributed due to the presence of enormous levels of phytoconstituents that act synergistically to preserve renal damage during oxidative stress, probably via attenuation of NADPH oxidase and inducible NOS (iNOS) activity and superoxide radicals formation (An and Zhang, 2010; Lei et al., 2012; Acharya and Talahalli, 2019). Earlier canola oil, one of the parts of VOB, also has shown a renoprotective effect by downregulating the expression of collagen VI, CD68 and TGF-β1 (Garman et al., 2009). Other mechanisms may be inhibition of lipid peroxidation reaction by various antioxidants, and vitamin E in VOB improves overall pancreatic β-cell function, which might attenuate protein glycation, lipid oxidation, and insulin sensitivity. Our results were also added with improved histopathological features. Significant structural alterations were notified in the renal glomerulus and tubules of diabetic rats. All severities like glomerulosclerosis and tubulointerstitial fibrosis were restored after VOB supplementation, suggesting the reno-protective efficacy of VOB in DN (Clark et al., 2000; Garman et al., 2009).
7 Conclusion
On the basis of the above findings, it could be concluded that VOB, a combination of four plant oils, employs a remarkable nephroprotective effect through modulation of renal functions, restoring redox imbalance and maintaining the renal architecture. This might be due to the presence of enormous types of bioactive compounds in VOB with potent antioxidant and inflammatory properties. Therefore, VOB may be used as the source of nutrition and may be helpful in renal diseases and associated complications. Further research is required to explore the benefits of VOB with more windows and duration response preclinical studies.
8 Data availability
The data used to support the findings of this study are included in the article.
Ethical approval
All guidelines for handling animals were discussed and authorized by Institutional Animal Ethics Committee, Jamia Hamdard, New Delhi, India.
Acknowledgement
The Scientific Research Deanship, University of Hail, Saudi Arabia funded this research, with project number RG-20 042.
Conflicts of interest
The authors declare that they do not have any conflict of interest.
References
- Protective effect of Galega officinalis Extract on streptozotocin-induced kidney damage and biochemical factor in diabetic rats. Crescent J. Med. Biol. Sci.. 2017;4:108-114.
- [Google Scholar]
- Aging and hyperglycemia intensify dyslipidemia-induced oxidative stress and inflammation in rats: assessment of restorative potentials of ALA and EPA + DHA. Inflammation. 2019;42(3):946-952.
- [CrossRef] [Google Scholar]
- Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol.. 2017;12(12):2032-2045.
- [CrossRef] [Google Scholar]
- The effect of canola oil compared with sesame and sesame-canola oil on cardio-metabolic biomarkers in patients with type 2 diabetes: design and research protocol of a randomized, triple-blind, three-way, crossover clinical trial. ARYA Atheroscler.. 2019;15(4):168-178.
- [CrossRef] [Google Scholar]
- Effects of sesamin on lipid metabolism in hyperlipidemia rats. J. Xi’an Jiaotong. Univ. (Med. Sci).. 2010;31:67-70.
- [Google Scholar]
- Alpha-tocopherol protects against the renal damage caused by potassium dichromate. Toxicologist. 2006;218:237-246.
- [CrossRef] [Google Scholar]
- Evaluation of White sesame seed oil on glucose control and biomarkers of hepatic, cardiac, and renal functions in male Sprague-Dawley rats with chemically induced diabetes. J. Med. Food. 2017;20(5):448-457.
- [CrossRef] [Google Scholar]
- The effects of canola and olive oils on insulin resistance, inflammation and oxidative stress in women with type 2 diabetes: a randomized and controlled trial. J. Diabetes Metab. Disord.. 2018;17(2):85-91.
- [CrossRef] [Google Scholar]
- Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod.. 2015;67:18-24.
- [CrossRef] [Google Scholar]
- Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol.. 2011;667(1–3):410-418.
- [CrossRef] [Google Scholar]
- Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crops Prod.. 2014;58:104-110.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72(1–2):248-254.
- [CrossRef] [Google Scholar]
- Glutathione reductase levels in rat brain. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud.. 2007;48:407-417.
- [Google Scholar]
- Glomerular hyperfiltration increases the risk of developing microalbuminuria children. Pediatr. Nephrol.. 1995;9(2):154-158.
- [CrossRef] [Google Scholar]
- A novel treatment for lupus nephritis: lignan precursor derived from flax. Lupus. 2000;9(6):429-436.
- [CrossRef] [Google Scholar]
- The urotensin-II receptor antagonist palosuran improves pancreatic and renal function in diabetic rats. J. Pharmacol. Exp. Ther.. 2006;316(3):1115-1121.
- [CrossRef] [Google Scholar]
- Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo: a photochemiluminescence based analysis. Food Chem.. 2014;164:551-555.
- [CrossRef] [Google Scholar]
- Effect of Nigella sativa and wheat germ oils on scopolamine-induced memory impairment in rats. Bull. Fac. Pharm. Cairo Univ.. 2012;50(2):81-88.
- [Google Scholar]
- Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446-1454.
- [CrossRef] [Google Scholar]
- Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012;41(1):82-88.
- [CrossRef] [Google Scholar]
- Antioxidative polyphenols from walnuts (Juglans regia L.) Phytochemistry. 2003;63(7):795-801.
- [CrossRef] [Google Scholar]
- Omega-3 fatty acid rich diet prevents diabetic renal disease. Am. J. Physiol. Ren. Physiol.. 2009;296(2):F306-F316.
- [CrossRef] [Google Scholar]
- Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci.. 2005;46(3):877-883.
- [CrossRef] [Google Scholar]
- Fundamentals of experimental pharmacology (3rd ed. Available from:). Kolkata, India: Hilton Publishing; 2005.
- Wheat germ thermal treatment in fluidised bed. Experimental study and mathematical modelling of the heat and mass transfer. J. Food Eng.. 2018;221:11-19.
- [CrossRef] [Google Scholar]
- Structured dietary advice incorporating walnuts achieves optimal fat and energy balance in patients with type 2 diabetes mellitus. J. Am. Diet. Assoc.. 2005;105(7):1087-1096.
- [CrossRef] [Google Scholar]
- Urinary biomarkers in the assessment of early diabetic nephropathy. J Diabetes Res.. 2016;2016:4626125.
- [CrossRef] [Google Scholar]
- Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Rev.. 2011;69(3):155-161.
- [CrossRef] [Google Scholar]
- Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci.. 2015;16(1):924-949.
- [CrossRef] [Google Scholar]
- Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother. Res.. 2010;24(9):1285-1291.
- [CrossRef] [Google Scholar]
- Herbal medicines for diabetes management and its secondary complications. Curr. Diabetes Rev.. 2021;17(4):437-456.
- [CrossRef] [Google Scholar]
- Effect of walnut oil on hyperglycemia-induced oxidative stress and pro-inflammatory cytokines production. Eur. J. Nutr.. 2015;54(2):291-299.
- [CrossRef] [Google Scholar]
- Effects of sesamin on streptozotocin (STZ)-induced NIT-1 pancreatic B-cell damage. Int. J. Mol. Sci.. 2012;13(12):16961-16970.
- [CrossRef] [Google Scholar]
- Evidence of health benefits of canola oil. Nutr. Rev.. 2013;71(6):370-385.
- [CrossRef] [Google Scholar]
- Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol.. 2011;7(12):684-696.
- [CrossRef] [Google Scholar]
- Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [CrossRef] [Google Scholar]
- Protective role of wheat germ oil against hyperglycemia and hyperlipidemia in streptozotocin induced diabetic rats. Asian J. Anim. Vet. Adv.. 2015;10(12):852-864.
- [CrossRef] [Google Scholar]
- Study on the benefits of sesame oil over coconut oil in patients of insulin resistance syndrome, notably type 2 diabetes and dyslipidaemia. J. Hum. Ecol.. 2007;22(1):61-66.
- [CrossRef] [Google Scholar]
- Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45-63. PMID 31333808.
- Octacosanol ameliorates hyperlipidemia and oxidative stress in KKAy mice with type 2 diabetes. J. Anal. Biol. Sci.. 2011;34:223-233.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci.. 2015;16(6):12871-12890.
- [CrossRef] [Google Scholar]
- Protective role of dietary polyphenols in oxidative stress. Mech. Ageing Dev.. 1999;111(2–3):133-139.
- [CrossRef] [Google Scholar]
- Paranich VA, Cherevko OI, Frolova NA, Paranich AV. The effect of wheat germ oil on the antioxidant system of animals. Lik Sprava. 2000;2(2):40-4. PMID 10862473.
- Evaluation of vegetables and fish oils for the attenuation of diabetes complications. Cell Mol. Biol. (Noisy-le-grand). 2019;65(7):38-45.
- [CrossRef] [Google Scholar]
- Time course study of oxidative and nitrosative stress and antioxidant enzymes in K2Cr2O7-induced nephrotoxicity. BMC Nephrol.. 2005;6:4.
- [CrossRef] [Google Scholar]
- The excretion of N-acetyl-β-glucosaminidase and β-galactosidase following surgery to the kidney. Clin. Chim. Acta. 1970;27(1):65-72.
- [CrossRef] [Google Scholar]
- Sesamol intervention ameliorates obesity-associated metabolic disorders by regulating hepatic lipid metabolism in high-fat diet-induced obese mice. Food Nutr. Res.. 2019;63
- [CrossRef] [Google Scholar]
- Influence of sesame oil on blood glucose, lipid peroxidation, and antioxidant status in streptozotocin diabetic rats. J. Med. Food. 2005;8(3):377-381.
- [CrossRef] [Google Scholar]
- The combined effects of cholesteryl ester transfer protein (CETP) TaqIB gene polymorphism and canola, sesame and sesame-canola oils consumption on metabolic response in patients with diabetes and healthy people. J. Cardiovasc. Thorac. Res.. 2020;12(3):185-194.
- [Google Scholar]
- Hypolipidemic effect of oils with balanced amounts of fatty acids obtained by blending and interesterification of coconut oil with rice bran oil or sesame oil. J. Agric. Food Chem.. 2007;55(25):10461-10469.
- [CrossRef] [Google Scholar]
- Assessment of antidiabetic activity of the shikonin by allosteric inhibition of protein-tyrosine phosphatase 1B (PTP1B) using state of art: an in silico and in vitro tactics. Molecules. 2021;26(13):3996.
- [CrossRef] [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract.. 2019;157:107843.
- [Google Scholar]
- Sesame oil exhibits synergistic effect with antidiabetic medication in patients with type 2 diabetes mellitus. Clin. Nutr.. 2011;30(3):351-358.
- [CrossRef] [Google Scholar]
- Use of vegetable oils in dermatology: an overview. Int. J. Dermatol.. 2017;56(11):1080-1086.
- [CrossRef] [Google Scholar]
- Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol.. 2006;33(10):940-945.
- [CrossRef] [Google Scholar]
- Possible involvement of intracellular angiotensin II receptor in high-glucose-induced damage in renal proximal tubular cells. J. Nephrol.. 2011;24(2):218-224.
- [CrossRef] [Google Scholar]
- Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27(12):2777-2783.
- [CrossRef] [Google Scholar]
- Diabetic kidney disease: pathophysiology and therapeutic targets. J. Diabetes Res.. 2015;2015:1-16.
- [Google Scholar]
- Glomerular hyperfiltration in microalbuminuric NIDDM patients. Diabetologia. 1996;39(12):1584-1589.
- [CrossRef] [Google Scholar]
- The relationship of antioxidant components and antioxidant activity of sesame seed oil. J. Sci. Food Agric.. 2015;95(13):2571-2578.
- [CrossRef] [Google Scholar]
- Wu MS, Aquino LBB, Barbaza MYU, Hsieh CL, Castro-Cruz KA, Yang LL, Tsai PW. Anti-inflammatory and Anticancer Properties of Bioactive compounds from Sesamum indicum L.-A Review. Molecules. 2019;24(24):4426. doi: 10.3390/molecules24244426.
- Zeng CC, Liu X, Chen GR, Wu QJ, Liu WW, Luo HY, Cheng JG. The molecular mechanism of rhein in diabetic nephropathy. Evid Based Complement Alternat Med. 2014;2014:487097. doi: 10.1155/2014/487097.
- The effect of walnut oil consumption on blood sugar in patients with diabetes mellitus Type 2. Int. J. Endocrinol. Metab.. 2016;14(3):e34889
- [CrossRef] [Google Scholar]
- Effect of roasting on physico-chemical properties, antioxidant capacity, andoxidative stability of wheat germ oil. Lebensm. Wiss. Technol.. 2018;90:246-253.
- [CrossRef] [Google Scholar]







