Translate this page into:
A temperature-dependent investigation of the impact of metal nanoparticles on the structural integrity of serum albumin
⁎Corresponding author at: Department of Biochemistry, College of Science, Building No. 5, P.O. Box 2455, King Saud University, Riyadh 11451, Saudi Arabia. amalik@ksu.edu.sa (Ajamaluddin Malik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nanoparticles (NPs) have distinct properties due to their small size and high surface area-to-volume ratio. These characteristics result in unique features not seen in bulk materials. Metals, semiconductors, and polymers are among the many varieties. Nanomaterials are used in various industries, including medical, electronics, and environmental cleanup. However, their potential environmental and health consequences must be carefully considered. Metal nanoparticles (MNPs) have received significant attention due to their outstanding physicochemical features and diverse applications in various sectors. MNPs are utilized in different fields, from electronics to health. Previously, the effect of various metal nanoparticles on the structure, stability, and functionality of metabolic enzymes was investigated. Copper (Cu) NPs were found to have significant negative impacts on these enzymes. The focus of the current investigation turned to serum albumin, a vital plasma protein containing oxidized cysteine residues. The findings demonstrated that NPs had a minor impact on the structure and stability of serum albumin, in contrast to the effects observed on reduced cysteine-containing enzymes.
Keywords
Metal nanoparticles
Nanotoxicity
Nanomaterial
Corona
Unfolding
Far UV CD
1 Introduction
Nanotechnology is manipulating and applying materials at the nanoscale, typically ranging from one to one hundred nanometers. Nanoparticles (NPs), the building blocks of nanomaterials, are distinguished by their small size and high surface area-to-volume ratio (Jeevanandam et al., 2018, Singh et al., 2023a, Yusuf et al., 2023). Metals, semiconductors, oxides, and polymers are all examples of NPs (Harish et al., 2022). Each type has unique qualities and behaviors that make it ideal for various functions. For example, metal nanoparticles (MNPs) frequently demonstrate improved catalytic activity, whereas quantum dots emit light of varied colors depending on their size (Tandale et al., 2021). Nanomaterials are used in a variety of fields, including medicine (drug administration and imaging), electronics (miniaturization and improved performance), and environmental remediation (water purification and pollution detection) (Liu et al., 2023, Wang et al., 2023). Their potential environmental and health consequences must be carefully evaluated.
MNPs have received considerable attention due to their outstanding physicochemical features and diverse applications in various domains (Xu et al., 2022). Due to their size and shape dependence, these nanoscale entities have distinct optical, electrical, catalytic, and magnetic capabilities (Joudeh and Linke, 2022). MNPs have numerous and significant applications. They offer increased conductivity and device miniaturization in electronics. Gold and silver NPs are used in medicine for targeted drug delivery, imaging, and diagnostic testing. Their surface plasmon resonance is also used in biosensing, where minute changes in their optical characteristics cause noticeable shifts (Klebowski et al., 2018). MNPs increasing use raises concerns about their possible toxicity (Malik et al., 2021). NPs small size allows them to pass through cellular barriers and interact with subcellular structures, potentially causing cellular malfunction. MNPs can cause oxidative stress by producing reactive oxygen species (ROS), which can cause cellular damage and inflammation. Furthermore, their distinct features may have unforeseen consequences for biological systems (Min et al., 2023).
Previously, biophysical approaches were used to analyze the effect of various MNPs on the structure, stability, and functionality of various metabolic enzymes. Copper NPs had the greatest deleterious effects on metabolic enzymes structure, stability, and activity (Malik et al., 2021). The metabolic enzymes had cysteine residues in a reduced state. This impact was attributed to the facilitation of metal-catalyzed disulfide bond formation. As a result, the current study focuses on serum albumin, an essential plasma protein that contains cysteine residues in their oxidized states. Serum albumin has remarkable properties such as higher water solubility, pH resilience, and affinity for ligand binding (Malik et al., 2013). These properties make it a versatile choice for medical applications, as it acts as a carrier for targeted drug administration and improves drug solubility. Serum albumins are often used as model proteins in biochemical and biophysical research (Mishra and Heath, 2021).
2 Materials and methods
2.1 Materials
The bovine serum albumin was purchased from Sigma-Aldrich. The following bare metal nanoparticles were purchased from US Research Nanomaterials, Inc.: 50–80 nm Ag NPs (Ag, 99.99 %; Stock#: US1028), 15 nm Au NPs (Au, >99.95+%; Stock #: US1054), 70 nm Cu NPs (Cu, 99.9 %; Stock #: US1089), 800 nm Mg NPs (Mg, 99 %;Stock#: US1061), and 15 nm Pt NPs (Pt, 99.95 %; Stock#: US1808).
2.2 Treatment of serum albumin with MNPs
The working 20 mM phosphate buffer solution pH 7.5 was prepared by dilution of 0.5 M phosphate buffer pH 7.5 sterilized stock solution. Serum albumin stock solutions of 0.5 mg ml−1 were made in a pH 7.5 phosphate buffer solution (20 mM). The serum albumin concentration was then calculated using a molar extinction coefficient of 42925 M−1 cm−1 and adjusted to 0.4 mg ml−1. The effects of five different MNPs (Ag, Au, Cu, Mg, and Pt NPs) on the structure and stability of serum albumin were studied. MNPs stock solutions (1 mg ml−1) were prepared using deionized water and homogenized by ultrasonication before being treated with albumin. A 1:1 vol ratio of NPs to albumin was combined and incubated for 48 h at room temperature. The final serum albumin concentration was 0.2 mg ml−1 in both the presence and absence of various NPs. Albumin structure and stability changes by MNPs were investigated using Far UV CD spectroscopy and a spectrofluorometer. These measurements were conducted at varying temperatures.
2.3 Temperature-dependent effects of MNPs on the secondary structure of serum albumin
Serum albumin (0.2 mg ml−1) was treated with or without MNPs, and subsequent far-UV CD spectra were recorded to assess secondary structural alterations using Chirascan Spectropolarimeter (Applied Photophysics Ltd, UK). CD spectra were collected in the far-UV range of 190–250 nm, using cuvettes with 1.0 mm path length and a 1.0 nm bandwidth, and Far UV CD data were collected at 0.5 s per point. We recorded spectra in triplicate from 20 and 90 °C in a CD spectropolarimeter with a Peltier temperature controller. The samples were allowed to equilibrate at their respective temperatures for 5 min before the measurements were made. MNPs spectra were obtained and subtracted from their corresponding protein-MNPs spectra. The secondary structure content of serum albumin with and without MNPs treatment was determined using K2D2 online tool.
2.4 Temperature-dependent effects of MNPs on the tertiary structure of serum albumin
The tertiary structure of serum albumin was evaluated using a Cary Eclipse fluorescence spectrofluorometer (Agilent Technologies, USA) with and without MNPs treatments. The intrinsic fluorescence spectra of the materials were recorded in a quartz cell with a path length of 10 mm. Protein samples (0.1 mg ml−1) were incubated at 20 and 90 °C. The samples were excited at 280 nm, and emissions were recorded between 300 and 450 nm (excitation and emission slit = 5 nm). The samples were left at their designated temperatures for 5 min to achieve thermal equilibrium.
2.5 Effects of MNPs on the thermal stability of serum albumin
The thermal stability of serum albumin with and without MNPs treatment was determined using Far UV CD at 222 nm in a Chirascan Spectropolarimeter. All serum albumin samples (0.2 mg ml−1) were gradually heated from 20 to 90 °C at a rate of 1 °C min−1 using an internal temperature probe to monitor changes in the secondary structure. The measurements were taken at 222 nm.
3 Results
Proteins are dynamic biomolecules found in biological fluids. When NPs enter cells or organisms, they interact with proteins. The interaction promotes the formation of a unique structure known as the “protein corona.” The present study seeks to understand the effects of the interaction of MNPs with an extracellular protein containing oxidized cysteine residues. This study focuses on the impact of five discrete MNPs (Ag, Au, Cu, Mg, and Pt NPs) on the structural alterations of serum albumin, including secondary and tertiary conformations. This study also looks into the influence of these MNPs on the thermostability of serum albumin.
3.1 Effects of MNPs on the secondary structure of serum albumin
We used Far UV-CD measurements to investigate the impact of five different MNPs on the secondary structural configuration of serum albumin. Circular dichroism allows the examination of differences in protein secondary structures under a wide range of experimental conditions [26]. Furthermore, CD is a widely used method for studying protein folding pathways and interactions between proteins and ligands. At a temperature of 20 °C, serum albumin has a high content of alpha-helical structure (Fig. 1A). Our findings revealed that the interaction of serum albumin with NPs decreased its secondary structure composition. Adding Au NPs caused a considerable decrease in alpha-helical content, which decreased from 54 % to 26 % (Table 1). Other NPs species showed a moderate loss in secondary structure.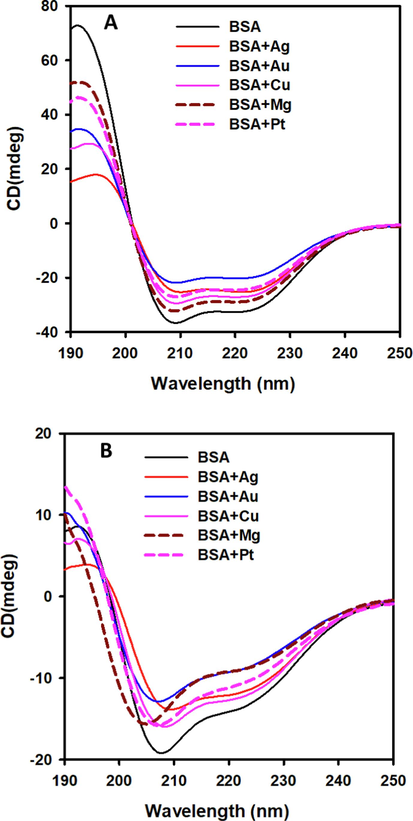
Albumin exposed to Ag, Au, Cu, Mg, and Pt NPs (A) at 20 °C and (B) at 90 °C. Albumin samples (0.2 mg/ml) were measured in 1-mm path-length cuvettes and recorded as shown by CD spectroscopy in the far-UV range.
20 °C
90 °C
alpha-helix
beta-sheet
alpha-helix
beta-sheet
BSA
54.54
7.42
19.69
31.52
BSA+Ag
43.29
10.79
20.46
29.8
BSA+Au
26.47
19.07
16.33
34.68
BSA+Cu
45.53
10.37
20.46
29.8
BSA+Mg
49.94
10.12
16.33
34.68
BSA+Pt
43.29
10.79
20.46
29.8
When the temperature was raised to 90 °C, serum albumin underwent a structural shift (Fig. 1B). The addition of NPs enhanced the decrease in secondary structural content. Fig. 1B showed that the 208 nm peak shifted towards shorter wavelengths in the presence of Au and Mg NPs, indicating an increased formation of random coil structures in serum albumin.
A previous study examining the impact of five MNPs on metabolic enzymes revealed significant modifications to their secondary structure (Malik et al., 2021). Cu NPs caused a partial to total loss of secondary structure in metabolic enzymes, whereas other NPs caused moderate changes in the secondary configurations of the enzymes (Malik et al., 2021). Comparable experiments with insulin, lysozyme, fibrinogen, γ-globulin, cytochrome C, and histone H3 have demonstrated NPs ability to interact with proteins and elicit secondary structural changes. The extent to which NPs influence protein secondary structure is determined by physiochemical properties such as NPs size, shape, surface properties, and chemical composition (Bhogale et al., 2014, Aghili et al., 2016).
3.2 Effects of MNPs on the tertiary structure of serum albumin
The study used intrinsic fluorescence spectroscopy to analyze changes in the tertiary structure of proteins after exposure to different MNPs. After being excited at 280 nm, the intrinsic fluorescence spectra of both untreated and MNPs-treated albumin samples were obtained in the 300 to 400 nm range (Fig. 2). All samples were fixed at 0.1 mg ml−1 in a 20 mM phosphate buffer, pH 7.5. The interaction of NPs and proteins resulted in changes in intrinsic fluorescence due to the localization of tryptophan residues in a different microenvironment (Malik et al., 2015). This investigation focused on the interactions between serum albumin and Mg and Cu NPs, which exhibited fluorescence quenching at temperatures ranging from 20 to 90 °C (Fig. 2A and B). MNPs interacted with serum albumin. Cu NPs demonstrated the most significant quenching ability on serum albumin, acting under ambient (20 °C) and elevated temperature (90 °C) conditions. Pt and Au NPs, on the other hand, produced relatively weak quenching effects. These findings show that serum albumin has various impacts on different tertiary structures.
Intrinsic tryptophan fluorescence of serum albumin treated with MNPs. Five NPs (Ag, Au, Cu, Mg, and Pt) were incubated with serum albumin at (A) 20 °C and (B) 90 °C. Total intrinsic fluorescence spectra of the serum albumin (0.1 mg/ml) samples were recorded by exciting at 280 nm, and spectra were recorded from 300 to 400 nm. In both panels, the control sample (untreated albumin) was shown in black, albumin + Ag NPs in red, albumin + Au NPs in blue, albumin + Cu NPs in pink, albumin + Mg NPs in the dashed brown, and albumin + Pt NPs in dashed pink.
3.3 Effects of MNPs on the thermostability of serum albumin
Far-UV CD quantifies protein secondary structure. Thermal unfolding compromises the structural integrity of proteins. Changes in secondary structure due to thermal stress have frequently been used to determine by far-UV CD (Bai et al., 2019). Untreated and MNPs-treated serum albumin samples were subjected to a controlled and gradual temperature increase ranging from 20 to 90 °C to obtain the melting temperature curves. As shown in Fig. 3, the temperature was raised at a consistent rate of 1 °C per minute. The changes in ellipticity at 222 nm were collected as a temperature function. The thermal spectral profiles of serum albumin samples treated with Ag, Au, Cu, Mg, and Pt NPs were quite similar to those of the untreated albumin sample. This finding implies that the presence of these MNPs has little effect on albumin thermostability. Based on our findings, the interactions between Pt and Ag NPs and serum albumin significantly improved albumin thermal stability, ranging from 3 to 4 °C (Table 2).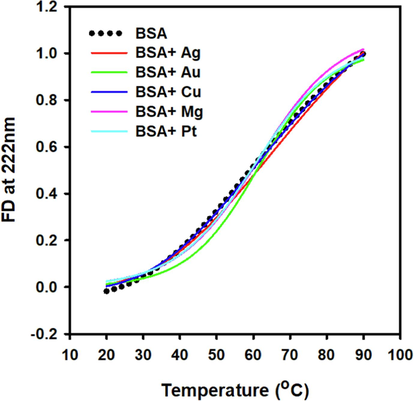
Far UV CD was used to measure the thermal stability of NP-treated albumin. Albumin was incubated with five NPs (Ag, Au, Cu, Mg, and Pt). The thermal stabilities of NP-treated albumin were measured in 1.0 mm cuvettes (0.2 mg/ml albumin). Samples were gradually heated from 20C to 90 °C at a rate of 1 °C min−1, and variations in ellipticity at 222 nm were shown as a function of temperature.
Samples
Tm (°C)
BSA
61.25
BSA+Ag
65.93
BSA+Au
61.21
BSA+Cu
62.46
BSA+Mg
61.31
BSA+Pt
64.14
4 Discussion
The remarkable properties of NPs continue to excite researchers, medical professionals, and consumers (Singh et al., 2023b, Zhang et al., 2023). Nanomaterials, including MNPs, are gaining popularity in various biological and medical fields. For example, plasmon resonant MNPs (Au, Ag, Pt, and Pd), in which free electrons oscillate at the metal surface, have recently been harnessed in medical imaging (Cui et al., 2023). Numerous MNPs showed promise as antibacterial, antiviral, antifungal, and antifouling agents. The physicochemical properties of NPs differ significantly from those of their bulk counterparts (Sharmin et al., 2021). This uniqueness confers novel properties on NPs, bringing opportunities and challenges. While NPs provide new avenues for use, they can harm organs and tissues. These effects are caused by the different physicochemical properties of NPs (Ajdary et al., 2018).
The dissolution and oxidation of MNPs raise significant concerns regarding their potential toxicity. Dissolution refers to the release of ions by MNPs into the environment, influenced by size, shape, and surface chemistry. MNPs can dissolve under physiological conditions, releasing metal ions. Additionally, MNPs can undergo oxidation, worsening their toxicity in biological systems, as they react with reactive oxygen species (ROS) generated within cells. Oxidative stress from MNP-ROS interactions can damage cells through lipid peroxidation, DNA damage, and protein oxidation (Malik et al., 2022). The molecular mechanisms of MNPs entail complex interactions with cellular components, resulting in various adverse effects like oxidative stress, inflammation, genotoxicity, and mitochondrial dysfunction (Min et al., 2023). Understanding these mechanisms is crucial for assessing MNPs safety and devising strategies to mitigate their potential risks. MNPs can infiltrate cells via endocytosis, phagocytosis, or membrane penetration, interacting with organelles and biomolecules internally. Reactive oxygen species (ROS) play a pivotal role in MNP toxicity, particularly with transition metals like silver, copper, or iron, which can trigger ROS production through Fenton or Haber-Weiss reactions (Abdal Dayem et al., 2017). The oxidative stress damages lipids, proteins, and DNA, inducing cell death. MNPs also activate immune cells, leading to inflammation through Toll-like receptors (TLRs) or pro-inflammatory cytokine release like IL-1β and TNF-α (Wojcik et al., 2021). Some MNPs induce DNA damage, promoting genomic instability and increasing the risk of mutations, cancer, and long-term health issues. Additionally, MNPs disrupt mitochondrial function, resulting in energy depletion, ROS production, and apoptosis, which is associated with cancer and neurological disorders (Zhao et al., 2023). Furthermore, MNPs alter protein structure and function, disrupting essential cellular processes and signaling pathways, thereby perturbing cell homeostasis and function (Malik et al., 2021).
Most studies on NP-protein interactions have focused on nonmetals, carbon nanomaterials, and silica. This study focuses on the effects of several MNPs on the structural characteristics (secondary and tertiary) and thermostability of serum albumin. When nanoparticles encounter biomolecules, they rapidly transform, forming a “protein corona” around their surface through interactions with proteins. This dynamic protein corona significantly influences the behavior of nanoparticles within biological environments. Proteins with higher affinity form a “hard” corona, which has a tighter attachment to the NPs surface than proteins with lower affinity, which form a “soft” protein corona (Bashiri et al., 2023, Mahmoudi et al., 2023). Early in protein corona development, numerous proteins readily adsorb on NPs. Stronger-affinity ones gradually replace these proteins. (Kopac, 2021). The formation of the protein corona is intricate, influenced by factors like electrostatic forces, hydrogen bonding, hydrophobic interactions, and protein-nanoparticle affinities in biological fluids. Its composition depends on nanoparticle properties (size, shape, surface charge), protein type/concentration, and exposure duration (Bashiri et al., 2023). The corona bridges nanoparticles and cells, impacting their recognition, uptake, and biological responses. It masks nanoparticle surfaces, altering cellular uptake—some proteins enhance internalization, while others hinder it. Moreover, the corona modulates cellular interactions, transport, and signaling pathways, affecting nanoparticle-induced cytotoxicity or inflammation (Rampado et al., 2020). Despite recent efforts to untangle the complexity of protein corona formation, a complete knowledge of its composition in the complex biological context, as well as the ramifications of its development, remains challenging.
This study aimed to understand more about the interaction between serum albumin and NPs and the possible impact on structural properties and protein stability. First, we used far-UV circular dichroism (CD) spectroscopy to assess the influence of these NPs on the secondary structure of albumin. This is an excellent technique for monitoring changes in the secondary structure of proteins (Malik et al., 2017, Khan et al., 2018). Our investigation demonstrated that adding Au NPs to the albumin milieu reduced its secondary structure to a more significant extent comparatively than other MNPs (Fig. 1). Moreover, the presence of Ag NPs resulted in a partial reduction of the secondary structure of albumin (Fig. 1). The sequence of MNPs effects on albumin's secondary structure at 20 °C was following: the most influential were Au NPs, followed by Ag and Pt NPs, Cu NPs, and finally Mg NPs. Notably, earlier research has shown that NPs can alter the secondary structure of a variety of proteins, including but not limited to gamma-globulin, insulin, lysozyme, histone H3, fibrinogen, and cytochrome C. Variables such as NPs size, shape, surface charge, and chemical composition influence the degree of disruption to the protein's secondary structure (Bhogale et al., 2014, Aghili et al., 2016). This comprehensive work adds to our understanding of how NPs characteristics can alter protein molecular topologies by elucidating the intricate interplay between NPs and albumin's secondary structure.
Intrinsic fluorescence spectroscopy appears to be a preferred method for characterizing changes in protein tertiary structure under varied environmental conditions. Because of its high sensitivity, this method requires a relatively low amount of protein while providing information on changes in a protein's tertiary structure under various stress conditions (Malik et al., 2016). The existence of an indole fluorophore within Trp residues, which undergoes a dual isoenergetic transition, is an intriguing feature. However, tyrosine side chains experience only a single transition in electronic states. This distinction adds to tryptophan's higher fluorescence and microenvironment sensitivity over tyrosine (Ghisaidoobe and Chung, 2014, Malik et al., 2016). Changes in protein conformation invariably result in changes in the polarity of fluorophores in the microenvironment. As a result, changes in fluorescence wavelength maxima (ƛmax) and/or fluorescence intensity (Imax) occur as indicators of tertiary structural changes within the protein. Fig. 2 displays the tertiary structures of albumin after NPs exposure at 20 °C and 90 °C. Cu NPs were obviously the dominant cause of fluorescence quenching in albumin at both temperatures. Mg NPs, on the other hand, produced a partial loss of tertiary structure. AuNPs boosted the molecular chaperone function of human eye lens alpha-crystallin. Through fluorescence and Far UV CD methods, it was observed that AuNPs interact with alpha-crystallin through H-bonding and van der Waals interactions without notably altering its secondary structure (Sharma et al., 2022). AgNPs interact with monoclonal antibodies (mAb), altering plasmonic absorption. Upon interaction, a protein corona develops around AgNPs, changing and broadening their surface plasmon resonance (SPR) band from 400 nm to 410 nm. Further analyses involving circular dichroism and fluorescence indicate that AgNPs impact the secondary and three-dimensional structures of mAb (Perez Medina Martinez et al., 2018).
A recent study that looked at the effects of five different MNPs on the structure, stability, and enzymatic activities of four metabolic enzymes revealed that Cu NPs caused partially to complete loss in secondary and tertiary structure, thermal stability, and enzymatic activity (Malik et al., 2021). The protein chemistry metabolic enzymes, which are classified as cytosolic proteins, differ from the secretory proteins such as albumins. With many cysteine residues, the latter promotes stable disulfide bond formation. Naturally, disulfide bonds promote stability by decreasing conformational entropy. Except in a few archaea, cytosolic proteins typically prevent stable disulfide bond formation due to a significantly higher ratio of reduced glutathione to oxidized glutathione (estimated to range from 30:1 to 100:1) in the cytoplasm. This high ratio adopts a reducing environment, preventing stable disulfide bonds (Bechtel and Weerapana, 2017, Robinson and Bulleid, 2020).
This research highlights the significant impact of MNPs on biological systems. It stresses the importance of careful design and application of MNPs in biomedicine. Researchers should thoroughly investigate MNPs interactions with biological molecules like enzymes and proteins to mitigate negative effects. This understanding can guide the development of MNPs with reduced toxicity and improved compatibility. Strategies such as surface modification and coating with biocompatible materials should be utilized. Targeted delivery systems can enhance efficacy and minimize off-target effects. Comprehensive biological compatibility testing of MNPs is crucial before their use in biomedical applications, assessing enzyme activity and protein stability for safety and effectiveness.
5 Conclusion
MNPs have unique physicochemical features allowing various uses in electronics, medicine, and other fields. Their interactions with biomolecules, particularly proteins, provide options for targeted biofunctionalization. However, the potential toxicity of MNPs emphasizes the significance of comprehensive safety review and responsible design in their creation and use. Protein corona formation is a complex process in which NPs become coated with biomolecules upon entry into biological systems, altering protein-NP interactions. The findings of our study demonstrated that MNPs had a mild impact on the structure and stability of serum albumin. Notably, unlike metabolic enzymes characterized by cysteine residues in their reduced forms, adding MNPs resulted in a slight increase in serum albumin stability. Our investigation highlights the various consequences of MNPs-protein interactions, emphasizing the need to exploit their potential while considering their diverse effects on biological systems. This information expands our understanding of how NPs might shape protein structures and is a critical guide for designing applications that maximize benefits while minimizing dangers.
CRediT authorship contribution statement
Ajamaluddin Malik: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Abdulaziz Alamri: Resources, Formal analysis. Javed Masood Khan: . Nojood Altwaijry: Resources, Investigation, Formal analysis. Abir Alamro: . Abdullah S. Alhomida: Supervision, Resources, Conceptualization. Hamza Odeibat: Data curation. Mohammad Shamsul Ola: Investigation, Formal analysis.
Acknowledgments
The authors are grateful to the Researchers Supporting Project Number (RSPD2024R727), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18(1)
- [Google Scholar]
- Investigating the interaction of fe nanoparticles with lysozyme by biophysical and molecular docking studies. PLoS. One. 2016;11(10):e0164878.
- [Google Scholar]
- Health concerns of various nanoparticles: a review of their in vitro and in vivo toxicity. Nanomaterials. (basel). 2018;8(9)
- [Google Scholar]
- Isothermal analysis of ThermoFluor data can readily provide quantitative binding affinities. Sci. Rep. 2019;9(1):2650.
- [Google Scholar]
- Nanoparticle protein corona: from structure and function to therapeutic targeting. Lab. Chip. 2023;23(6):1432-1466.
- [Google Scholar]
- From structure to redox: The diverse functional roles of disulfides and implications in disease. Proteomics. 2017;17(6)
- [Google Scholar]
- Comprehensive studies on the interaction of copper nanoparticles with bovine serum albumin using various spectroscopies. Colloids. Surf. B. Biointerfaces. 2014;113:276-284.
- [Google Scholar]
- Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023;123(11):6891-6952.
- [Google Scholar]
- Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Forster resonance energy transfer techniques. Int. J. Mol. Sci. 2014;15(12):22518-22538.
- [Google Scholar]
- Review on nanoparticles and nanostructured materials: bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials. (basel). 2022;12(3)
- [Google Scholar]
- Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein. J. Nanotechnol. 2018;9:1050-1074.
- [Google Scholar]
- Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnology. 2022;20(1):262.
- [Google Scholar]
- An intermittent amyloid phase found in gemini (G5 and G6) surfactant induced β-sheet to α-helix transition in concanavalin A protein. J. Mol. Liquids. 2018;269:796-804.
- [Google Scholar]
- Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018;19(12)
- [Google Scholar]
- Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021;169:290-301.
- [Google Scholar]
- Plasmonic sensor based on offset-splicing and waist-expanded taper using multicore fiber for detection of Aflatoxins B1 in critical sectors. Opt. Express. 2023;31(3):4783-4802.
- [Google Scholar]
- The protein corona from nanomedicine to environmental science. Nat. Rev. Mater 2023:1-17.
- [Google Scholar]
- Isolation and characterization of serum albumin from Camelus dromedarius. Exp. Ther. Med. 2013;6(2):519-524.
- [Google Scholar]
- Spectroscopic and thermodynamic properties of recombinant heat shock protein A6 from Camelus dromedarius. Eur. Biophys. J. 2015;44(1–2):17-26.
- [Google Scholar]
- Structural and thermodynamic properties of kappa class glutathione transferase from Camelus dromedarius. Int. J. Biol. Macromol. 2016;88:313-319.
- [Google Scholar]
- Spectral and thermal properties of novel eye lens zeta-crystallin. Int. J. Biol. Macromol. 2017;102:1052-1058.
- [Google Scholar]
- Impact of metal nanoparticles on the structure and function of metabolic enzymes. Int. J. Biol. Macromol. 2021;188:576-585.
- [Google Scholar]
- Metal nanoparticles: biomedical applications and their molecular mechanisms of toxicity. Chem. Papers. 2022;76:6073-6095.
- [Google Scholar]
- Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants. (basel). 2023;12(3)
- [Google Scholar]
- Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int. J. Mol. Sci. 2021;22(16)
- [Google Scholar]
- Nanoparticles for protein sensing in primary containers: interaction analysis and application. AAPS. PharmSciTech. 2018;19(4):1672-1680.
- [Google Scholar]
- Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020;8:166.
- [Google Scholar]
- Mechanisms of Disulfide Bond Formation in Nascent Polypeptides Entering the Secretory Pathway. Cells. 2020;9(9)
- [Google Scholar]
- Enhancement in chaperone activity of human alphaA-crystallin by nanochaperone gold nanoparticles: Multispectroscopic studies on their molecular interactions. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022;279:121344
- [Google Scholar]
- Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon. 2021;7(3):e06456.
- [Google Scholar]
- Alanine aminotransferase detection using TIT assisted four tapered fiber structure-based LSPR sensor: From healthcare to marine life. Biosens. Bioelectron. 2023;236:115424
- [Google Scholar]
- Humanoid-shaped WaveFlex biosensor for the detection of food contamination. Biomed. Opt. Express. 2023;14(9):4660-4676.
- [Google Scholar]
- Fluorescent quantum dots: An insight on synthesis and potential biological application as drug carrier in cancer. Biochem. Biophys. Rep. 2021;26:100962
- [Google Scholar]
- Excitation of multiple Fano resonances on all-dielectric nanoparticle arrays. Opt. Express. 2023;31(6):10805-10819.
- [Google Scholar]
- Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021;22(2)
- [Google Scholar]
- Metal nanoparticles as a promising technology in targeted cancer treatment. Drug. Deliv. 2022;29(1):664-678.
- [Google Scholar]
- Nanoparticles as drug delivery systems: a review of the implication of nanoparticles' physicochemical properties on responses in biological systems. Polymers. (basel). 2023;15(7)
- [Google Scholar]
- Humanoid shaped optical fiber plasmon biosensor functionalized with graphene oxide/multi-walled carbon nanotubes for histamine detection. Opt. Express. 2023;31(7):11788-11803.
- [Google Scholar]
- Cancer metabolism: the role of ROS in DNA damage and induction of apoptosis in cancer cells. Metabolites. 2023;13(7)
- [Google Scholar]







