Translate this page into:
A study on the prevalence of keratinophilic fungal biota of semi-arid region of Rajasthan, India
⁎Corresponding author. sharmaanima6@gmail.com (Anima Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Keratinophilic fungi are morphologically and physiologically allied molds that produce the keratinase enzyme which degrades the keratin materials in or on the soil. Fifty soil samples were collected from various habitats of Rajasthan namely Jaipur, Ajmer, Alwar and Sikar in India. Out of 154 isolates, a total of 31 keratinophilic fungal species of 16 genera was recovered, including Chrysosporium tropicum (11.04%), Chrysosporium indicum (9.09%), Trichophyton mentagrophytes (8.44%), Fusarium solani (7.79%), Trichophyton rubrum (7.14%), Microsporum canis (5.84%), and Aspergillus terreus (4.19%). The frequency of these keratinophilic fungi is also discussed in relation to soil pH. The most of the fungi (47.40%) were isolated from the soil samples with pH 7.00 to 7.99. Chrysosporium indicum (5.84%) reported since 8.00–8.99 pH, while Fusarium solani (5.19%), Microsporum canis (4.55%) and Trichophyton mentagrophytes (4.55%) between 7.00 and 7.99 pH. This study stipulates that the soils of Rajasthan may be major reservoirs of certain keratinophilic fungi.
Keywords
Keratinophilic fungi
Keratinase
Keratin
Chrysosporium
Trichophyton
Fungal biota
1 Introduction
Soil comprises a large portion of the earth’s land surface which is an important natural resource that affects most of the living organisms either directly or indirectly (Irum et al., 2007; Lee et al., 2011). The soil is a natural reservoir of keratinophilic fungi, which are important group of filamentous fungi, some of which typically develop on keratinized tissues of living animals (Sarmiento et al., 2016). The fungi constitute a large and diverse group of plant kingdom belonging to a large group called thallophyta (Hamza et al., 2018). This micro biota present in the forest, farmyard, park soils, waste water habitats, and oceans contained organic material (Moallaei et al., 2006; Sharma et al., 2014). The keratin degrading microorganisms are flourishing under diverse ecological conditions, and creates an extensive, ranging capacity to solubilise keratin substrates as well as other solid protein substrates. Numerous species of bacteria and fungi produce the keratinase enzyme to degrade the keratin waste like feather, hair and nail and biodegradation takes place (Soomro et al., 2007; Kansoh et al., 2009; Sharma et al., 2015a).
In recent years, keratinophilic microbiota has been receiving considerable attention throughout the world (Mercantini et al., 1980; Lee et al., 2011; Kumawat et al., 2017). Due to the strength and constancy of keratin, only few insects, bacteria, and fungi are able to break down the keratin waste and utilize them as a source of nutrition (Kunert, 2000; Sharma and Rajak, 2003; Kumawat et al., 2013; Sharma et al., 2015b; Kumawat et al., 2016). Keratinophilic fungi are generally considered as soil saprotrophs (Ajello, 1953; Ajello, 1956; Sharma et al., 2012b).
The best preference of keratinophilic fungi’s occurrence in nature are such as cattle sheds, garbage, animal burrows, sewage, bird’s nest, barber’s hair dumping area, public places like parks, schools, poultry sheds, herbivore or carnivore dung (Gupta et al., 2012). During the past years, many researchers reported about the isolation of keratinophilic fungi around the world (Brandelli and Riffel, 2005; Marcondes et al., 2008; Logaprabha and Selvi, 2010; Rizwana et al., 2012). A few investigators have reported the occurrence of keratinophilic fungi from various habitats in India (Garg, 1966; Tambekar et al., 2007; Kushwaha and Gupta, 2008; Singh and Kushwaha, 2010; Kanchana and Mesta, 2013) as well as in Rajasthan (Sharma and Sharma, 2010; Jain and Sharma, 2012; Sharma and Swati, 2014).
Therefore, hygienic and ecological interests have led us to study the keratinophilic mycobiota of poultry farms, roadside, public parks etc. in the soil of Rajasthan. This would help us to know the distribution and occurrence of keratinophilic fungi, which could have a role in the degradation of keratinous waste as an industrial point of view. The degraded keratinous waste can be used as the source of feed and fertilizers.
2 Material and methods
2.1 Place of work
Rajasthan is the largest state of India constituting 10.4 percent of total geographical area. The state is divided into 7 divisions, 33 districts. Rajasthan being the desert area and the atmosphere varies mainly from arid to sub-humid. The maximum temperature hovers around 40 °C to 46 °C. Sometimes, it even reaches as high a 49 °C during the summer months. The minimum temperatures sometimes fall to −2 °C in the night at some places.
2.2 Collection of soil samples
In the present study, 50 soil samples were collected from various sites of 4 districts namely Jaipur, Ajmer, Alwar and Sikar region of Rajasthan, during August 2014 to April 2015. The soil samples were collected from poultry farms, animal habitats, public parks, roadsides, slaughterhouses and the barber shop’s dump area. The soil samples were collected from the surface of soil, depth not exceeding 4–5 cm, with the help of the sterile spatula in the sterile polyethylene bags approximately 400–500 g. The polyethylene bags were tightly packed, labelled with the name of the place and brought to the laboratory for isolation of keratinophilic fungi.
2.3 Measure the soil pH
pH of each soil sample was measured after preparation of soil suspension (two gram of soil to ten ml deionized water) using a digital pH meter (Model 181, Electronics India) (Kachuei et al., 2012). The pH meter was calibrated for pH using 4.0, 7.0 and 9.0 standard pH solutions prior to the test with the sample.
2.4 Keratin substrate
Various keratin substrates such as human hair, animal hair, human nail clippings and chicken feathers were collected in plastic bags from different sources. The keratin substrates were defatted in Chloroform: Methanol solution for 24 h, after that they were washed with deionized water and oven dry at 45 °C for 2–3 h. The keratin substrates were cut into small pieces (2–3 cm) and sterilized by autoclave.
2.5 Processing of samples
The soil samples were shade dry and sieved for hair baiting technique (Vanbreuseghem, 1952). For this, about 60 g of soil samples are transferred into 90 mm. sterile petri dishes and then the small pieced keratin substances human hair, animal hair, human nail clippings, and chicken feathers were aseptically spread on top of soil sample. After that, the sterile distilled water (15–18 ml) was poured on the keratin substrate baited plates. The baited plates were incubated at 27 ± 2 °C under low light for 2–3 weeks.
2.6 Isolation and identification of keratinophilic fungi
After observing the fungal growth in baited plates, the fungal mycelia were cultured and transferred on the slants of Potato Dextrose Agar (HiMedia). The cultures were incubated at 27 ± 2 °C for 12–15 days. Morphological characterization for species identification was based on colonial morphology (macroscopic characterization) and cellular morphology (microscopic characterization) (Weitzman and Padhye, 1996).
The identification of keratinophilic fungi and their morphology was studied by Lactophenol Cotton Blue (LPCB) wet mount method. A small portion of mycelium was subjected to a few drops of LPCB on a sterile glass slide. A cover slip was placed on it and gently heated over a bunsen flame. The fungal culture was observed under low and high power magnification of the microscope (Labomed Digi-Pro Microscope and Olympus Light Microscope). The tap touch method was also used for the microscopic identification of keratinophilic fungi (Harris, 2000).
Appearance and texture of mycelium, colour of the colony, attachment of conidia and/or spores with the mycelium and shape, size of macro and micro conidia were studied to identify the species of the fungi. The cultural and morphological characteristics of fungal colonies and their identification was done by referring laboratory methods in basic mycology (Forbes et al., 2002), The genus Aphanoascus (Cano and Gurrao, 1990), A revision of Chrysosporium and allied genera (van Oorschot, 1980), Mycology of dermatomycoses (Conant et al., 1959), Descriptions of Medical Fungi (Ellis et al., 2007) and Pictorial Atlas of Soil and Seed Fungi (Watanabe, 1937).
The percentage frequency was defined as follows (Nigam and Kushwaha, 1990).
3 Results
The results of the isolations are incorporated in Tables 1 and 2. They show the prevalence and distribution of keratinophilic fungal biota of four districts of Rajasthan. From the 50 soil samples, a total of 154 colonies of keratinophilic fungi were isolated. The fungal isolates belonged to 16 genera as follows Trichophyton (23.38%), Chrysosporium (22.73%), Fusarium (14.94%), Aspergillus (14.29%), Microsporum (6.49%), Scopulariopsis (3.25%), Aphanoascus (2.60%), Arthroderma (2.60%), Cephaliophora (2.60%), Uncinocarpus (1.95%), Emericella (1.30%), Penicillium (1.30%), Ctenomyces (0.65%), Malbranchea (0.65%), Torula (0.65%) and Trichoderma (0.65%) (Table 1). Trichophyton (23.38%) was the most predominant in this study. Chrysosporium (22.73%) was the second highest genus, followed by Fusarium (14.94%) (Table 1).
Name of fungal genus
Number
Percentage (%)
Aphanoascus
4
2.60
Arthroderma
4
2.60
Aspergillus
22
14.29
Cephaliophora
4
2.60
Chrysosporium
35
22.73
Ctenomyces
1
0.65
Emericella
2
1.30
Fusarium
23
14.94
Malbranchea
1
0.65
Microsporum
10
6.49
Penicillium
2
1.30
Scopulariopsis
5
3.25
Torula
1
0.65
Trichoderma
1
0.65
Trichophyton
36
23.38
Uncinocarpus
3
1.95
Total
154
100%
Isolated Fungi
Source of Soil Samples
Total Isolates
Frequency Percentage (%)
Poultry Farm
Animal Habitat
Road Side
Slaughter House
Barber’s Dump
Public Park
Aphanoascus arxii
–
1
1
–
1
1
4
2.60
Arthroderma multifidum
–
1
1
–
2
–
4
2.60
Aspergillus flavus
3
1
2
1
–
–
7
4.55
Aspergillus fumigatus
–
1
–
–
–
–
1
0.65
Aspergillus nidulans
–
–
1
–
–
1
2
1.30
Aspergillus niger
2
–
1
–
–
1
4
2.60
Aspergillus terreus
2
2
1
1
2
–
8
5.19
Cephaliophora irregularis
2
–
–
1
–
1
4
2.60
Chrysosporium indicum
3
1
1
4
1
4
14
9.09
Ch. queenslandicum
1
–
–
–
1
–
2
1.30
Chrysosporium tropicum
4
4
2
3
2
2
17
11.04
Chrysosporium zonatum
–
–
2
–
–
–
2
1.30
Ctenomyces serratus
–
–
–
–
1
–
1
0.65
Emericella rugulosa
–
1
–
–
1
–
2
1.30
Fusarium moniliforme
1
–
2
1
–
2
6
3.90
Fusarium oxysporum
2
–
–
1
2
–
5
3.25
Fusarium solani
2
1
2
–
1
6
12
7.79
Malbranchea saccardo
–
–
–
–
1
–
1
0.65
Microsporum audouinii
–
–
–
1
–
–
1
0.65
Microsporum canis
2
1
4
1
1
–
9
5.84
Penicillium sp.
2
–
–
–
–
–
2
1.30
Scopulariopsis brevicaulis
1
2
–
1
1
–
5
3.25
Torula sp.
–
1
–
–
–
–
1
0.65
Trichoderma sp.
1
–
–
–
–
–
1
0.65
Trichophyton equinum
–
1
–
–
1
–
2
1.30
Trichophyton erinacei
–
–
1
–
1
–
2
1.30
Trichophyton mentagrophytes
4
2
3
1
1
2
13
8.44
Trichophyton rubrum
2
3
2
1
1
2
11
7.14
Trichophyton terrestre
2
1
1
–
–
1
5
3.25
Trichophyton verrucosum
–
–
1
–
2
–
3
1.95
Uncinocarpus queenslandicus
1
–
1
–
1
–
3
1.95
Total Isolates
37
24
29
17
24
23
154
100%
Percentage %
24.03
15.58
18.83
11.04
15.58
14.94
The data reveals that out of 50 soil samples so collected a total of 31 species of keratinophilic fungi were isolated. Chrysosporium tropicum (11.04%) was found to be the most common keratinophilic fungi isolated from all sites. Chrysosporium indicum (9.09%) was the second most prevailing fungi, followed by Trichophyton mentagrophytes (8.44%) Poultry farm soils were found most suitable for the growth of keratinophilic fungi in all habitats (Table 2).
Among all these fungal species Aphanoascus arxii (2.60%), Arthroderma multifidum (2.60%), Ctenomyces serratus (0.65%) and Uncinocarpus queenslandicus (1.95%) were isolated for the first time in Rajasthan by hair baiting technique.
During the study of soil pH, pH range varies from 6.00 to 10.00. Most of the fungi were isolated from the soil samples with pH ranges between 7.00 and 7.99 (47.40%). Chrysosporium indicum (5.84%) reported since 8.00–8.99 pH, while Fusarium solani (5.19%), Microsporum canis (4.55%) and Trichophyton mentagrophytes (4.55%) between 7.00 and 7.99 pH (Table 3).
Isolated Fungi
Soil pH
6.00–6.99
7.00–7.99
8.00–8.99
>9.00
n
%
n
%
n
%
n
%
Aphanoascus arxii
0
0.00
3
1.95
1
0.65
0
0.00
Arthroderma multifidum
0
0.00
2
1.30
2
1.30
0
0.00
Aspergillus flavus
0
0.00
4
2.60
3
1.95
0
0.00
Aspergillus fumigatus
0
0.00
0
0.00
1
0.65
0
0.00
Aspergillus nidulans
0
0.00
1
0.65
1
0.65
0
0.00
Aspergillus niger
0
0.00
2
1.30
2
1.30
0
0.00
Aspergillus terreus
0
0.00
6
3.90
2
1.30
0
0.00
Cephaliophora irregularis
0
0.00
2
1.30
2
1.30
0
0.00
Chrysosporium indicum
2
1.30
3
1.95
9
5.84
0
0.00
Chrysosporium queenslandicum
0
0.00
0
0.00
2
1.30
0
0.00
Chrysosporium tropicum
4
2.60
5
3.25
7
4.55
1
0.65
Chrysosporium zonatum
0
0.00
1
0.65
1
0.65
0
0.00
Ctenomyces serratus
0
0.00
1
0.65
0
0.00
0
0.00
Emericella rugulosa
0
0.00
1
0.65
1
0.65
0
0.00
Fusarium moniliforme
1
0.65
3
1.95
2
1.30
0
0.00
Fusarium oxysporum
0
0.00
1
0.65
4
2.60
0
0.00
Fusarium solani
0
0.00
8
5.19
4
2.60
0
0.00
Malbranchea saccardo
0
0.00
0
0.00
1
0.65
0
0.00
Microsporum audouinii
0
0.00
0
0.00
1
0.65
0
0.00
Microsporum canis
1
0.65
7
4.55
1
0.65
0
0.00
Penicillium sp.
0
0.00
1
0.65
0
0.00
1
0.65
Scopulariopsis brevicaulis
0
0.00
1
0.65
4
2.60
0
0.00
Torula sp.
0
0.00
0
0.00
1
0.65
0
0.00
Trichoderma sp.
0
0.00
1
0.65
0
0.00
0
0.00
Trichophyton equinum
0
0.00
1
0.65
1
0.65
0
0.00
Trichophyton erinacei
0
0.00
1
0.65
1
0.65
0
0.00
Trichophyton mentagrophytes
2
1.30
7
4.55
4
2.60
0
0.00
Trichophyton rubrum
0
0.00
6
3.90
5
3.25
0
0.00
Trichophyton terrestre
1
0.65
4
2.60
0
0.00
0
0.00
Trichophyton verrucosum
0
0.00
1
0.65
2
1.30
0
0.00
Uncinocarpus queenslandicus
1
0.65
0
0.00
2
1.30
0
0.00
Total
12
7.79%
73
47.40%
67
43.51%
2
1.30%
4 Discussion
The present study clearly specifies the diverse distribution frequency of keratinophilic fungi in soil samples of Rajasthan. The climatic conditions (temperature exceeds 46 °C in summer and high humidity during monsoon season) are favourable for the higher incidence of keratinophilic fungi. The keratinolytic activity of keratinophilic fungi is important for the environment and has attracted attention of researchers around the globe (Gugnani et al., 2012). Keratinophilic fungi play an important role in the natural degradation of keratin substrates, i.e. chicken feathers, hooves, horns, hair etc. in and on the soil (Moallaei et al., 2006; Rizwana et al., 2012). These fungi are ecologically important and are present in the environment with variable distribution patterns (Deshmukh and Verekar, 2006). Researchers from several countries have reported on the incidence of keratinophilic fungi, including, dermatophytes in soils of various habitats (Soomro et al., 2007; Yazdanparast et al., 2013). McAleer (1980) have isolated 271 keratinophilic fungi from 299 soil samples collected from home gardens, parks and animal yards, Perth Metropolitan area, Australia. Pakshir et al. (2013) isolated 411 colonies of keratinophilic fungi from 196 soil samples collected from 43 public parks in Shiraz, Iran. Subsequently, Awad and Kraume (2011) have reported 46 fungal species belonging to 21 genera were isolated from 72 soil samples collected from activated sludge from wastewater treatment plants with MBR in Berlin, Germany. Hamza et al. (2018) isolated keratinophilic fungi from Murtala Amusement Park, Minna from 360 soil samples during dry and rainy seasons. Above authors identified a total of 542 isolates from eleven genera were i.e. Aspergillus, Candida, Fusarium, Paecilomyces, Mucor, Chrysosporium, Alternaria, Penicillium, Trichoderma, Microsporum, and Rhizopus.
From Indian plains, Randhawa and Sandhu (1965) studied that 45.9% keratinophilic fungi were isolated from 485 soil samples in Delhi. Deshmukh et al. (2010) reported that 58 isolates of keratinophilic fungi were isolated from 138 soil samples from various locations in Laddakh. In history of Rajasthan, Singh et al. (1994) studied 60 soil samples from different localities and large number of keratinophilic biota was observed in a survey of soils of Ghana Birds’ Sanctuary, Bharatpur district.
The most frequently isolated keratinophilic fungi in this study have been Chrysosporium tropicum, Chrysosporium indicum, Trichophyton mentagrophytes, Fusarium solani, Trichophyton rubrum and Microsporum canis. The present study revealed the occurrence of 154 isolates belonging to 16 genera and 31 species in the soil of Rajasthan.
Among isolated keratinophiles, Chrysosporium tropicum (11.04%) was most predominant fungal species recovered most frequently. Members of Chrysosporium genus are common soil saprobes, which have keratinophilic nature and involved in the breakdown of keratinous substrates (Roilides et al., 1999). The frequent occurrence of Chrysosporium as a geophilic keratinophilic fungus in this study is in agreement with those studies who also recorded the soil keratinophilic fungi in other countries (Rizwana et al., 2012, Gora et al., 2017). This observation agrees with the study of Jain and Sharma (2012) who also reported that Chrysosporium tropicum was the predominant species in soils of Jaipur. Another study in Jaipur (India) showed that Chrysosporium tropicum (26%) was most prevailing fungi in the schools and college playground soils of Jaipur (Sharma and Sharma, 2010).
In our study Chrysosporium indicum (9.09%) was the second dominant fungal species. Chrysosporium indicum was most predominant species in salt pans at Mumbai (India) and soils of the Gir Forest National Park, Wildlife Sanctuary at Gujarat (Deshmukh, 2004; Deshmukh and Verekar, 2014). Rizwana et al. (2012) studied that Chrysosporium indicum (33.75%) was the most predominant species isolated followed by C. tropicum (26.25%) in soils of public parks and playgrounds of Riyadh, Saudi Arabia.
Trichophyton mentagrophytes (8.44%) was third dominant species in the present study. This species was isolated from all habitat’s soils screened. In India, Shukla (2014) have reported that Trichophyton mentagrophytes (11.33%) was most dominating fungal species from 150 soils samples. Anbu et al. (2004) observed Trichophyton mentagrophytes (68.2%) as second dominant species isolated from poultry farm and feather dumping soil in Tamil Nadu. Fusarium species were isolated in this study. Fusarium solani (7.79%) was predominant in Fusarium genera. Fusarium moniliforme (3.90%) was isolated from poultry farm, road side, slaughter house and public park’s soil, whereas Fusarium oxysporum (3.25%) was isolated from poultry farm, slaughter house and barber’s dump soil. Kaul and Sumbali (2000) isolated some Fusarium species from poultry farm soils of Jammu, India. Trichophyton rubrum was 7.14% in present distribution and isolated from all habitat’s soils. The other geophiles belonging to Trichophyton genera were Trichophyton terrestre (3.25%), Trichophyton verrucosum (1.95%), Trichophyton equinum (1.30%) and Trichophyton erinacei (1.30%) followed in decreasing order.
Microsporum canis (5.84%), and Microsporum audouinii (0.65%) were isolated in the present study. Ali-Shtayeh et al. (1988) reported Microsporum audouinii on the hairs of goats from the west bank of Jorden. Sharma et al. (2012a) stated that Microsporum sp. was isolated on keratin substrates such as human hair, human nail and chicken feather at variable environmental conditions of temperature, pH and metal ions was elucidated.
Aspergillus terreus (5.19%) was the most dominant species in Aspergillus genera and isolated from each type of soil except public park’s soil. The other Aspergillus species isolated were Aspergillus flavus (4.55%), Aspergillus niger (2.60%), Aspergillus nidulans (1.30%) and Aspergillus fumigatus (0.65%) in the present investigation. Altayyar et al. (2016) screened keratinophilic fungi in soil of different area in South of Libya and reported that Aspergillus species were the highest isolated 58.9% keratinophilic fungi. Soomro et al. (2007) isolated Aspergillus niger (19.78%), A. flavus (14.97%), A. candidus (06.95%), A. wentii (06.04%) and A. fumigatus (18.71%) from the sludge by Hair Bait Technique in Khairpur, Sindh, Pakistan. In another research in Pakistan by Irum et al. (2007) reported that Aspergillus niger (31.59%), A. flavus (21.40%), A. fumigatus (2.82%), A. candidus (11.55%), A. ustus (2.35%), A. wentii (5.26%) and A. nidulans (2.05%) were isolated by the soil dilution plate method and baiting techniques.
Scopulariopsis brevicaulis (3.25%) was isolated from poultry farm, animal habitat, slaughter house and barber’s shop dump soils in our study. This species showed the morphological expression of human hair and animal nail invasion in vitro (Marchisio et al., 2000). Cephaliophora irregularis (2.60%) was isolated from poultry farm, slaughter house and public parks whereas Chrysosporium zonatum (1.30%) was screened from roadside soils.
Ctenomyces serratus (0.65%) was isolated from barber’s dump soil. C. serratus is the telomorphic state of Myceltophthora vellerea keratinophilic fungi (Abdullah et al., 1997). Deshmukh and Verekar (2011) isolated Ctenomyces serratus (5.66%) from the soils of Vedanthangal Water Bird Sanctuary. In present study Emericella rugulosa (1.30%), Penicillium sp. (1.30%), Malbranchea saccardo (0.65%), Torula sp. (0.65%) and Trichoderma sp. (0.65%) were also isolated in low frequency. Penicillium species were isolated by Gherbawy et al. (2006) from Human Hairs and Nails at Four Governorates in Upper Egypt.
Among all these fungal species Aphanoascus arxii (2.60%), Arthroderma multifidum (2.60%), Ctenomyces serratus (0.65%) and Uncinocarpus queenslandicus (1.95%) were isolated in Rajasthan by hair baiting technique. Singh and Jain (2015) reported that Arthroderma multifidum (35%) was isolated from soil of Ujjain, India. In another research by Otcenasek et al. (1967) who isolated Arthroderma multifidum from nests of birds in Czechoslovakia.
The present study investigated the relationship between the occurrence of fungi and the pH of soil. In the current study, all the 154 keratinophilic fungi were isolated from the soils with pH between 6.00 and 9.00. Most of the fungi were isolated from the soil samples with pH between 7.00 and 7.99 (47.40%). 43.51% keratinophilic fungi were isolated between pH 8.00–8.99. Only 7.79% keratinophilic fungi were seen in pH 6.00–6.99 as shown in Table 3 and Fig. 1. These findings have been confirmed by other studies as well.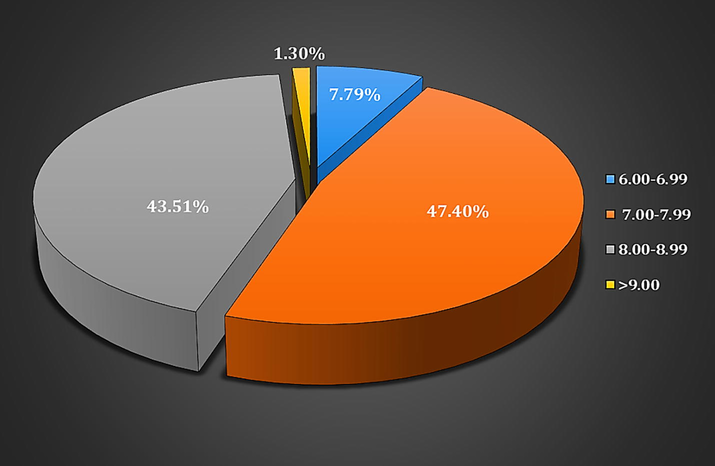
Frequency of keratinophilic fungi isolated from soils of Rajasthan at different pH values.
Bohme and Ziegler (1965) reported the effect of the soil pH on the presence of keratinophilic fungi for the first time. Our present findings are agreement with the study of Kachuei et al. (2012) who also reported that 45.7% of keratinophilic fungi were screened between pH 7.01–8.00, 42.3% in 8.01–9.00 and 12% in 6.00–7.00 pH of soil samples. Pakshir et al. (2013) observed that 66.42%, 32.6%, and 0.97% keratinophilic fungi were isolated from the soil samples with pH of 7.01–8, 8.01–9, and 6–7, respectively. Shukla (2014) reported that each soil samples have different pH that is ranging from 6.5 to 10.5 and organic materials in Chhattisgarh. In our study, the most isolated Chrysosporium tropicum species (10.56%) were isolated from the soil with pH 8.00–8.99.
It is comprehensible from our outcomes that the soils of poultry farms, animal habitats, public parks, roadsides, slaughterhouses and the barber shop’s dump area are the perfect environment for the growth and occurrence of keratinophilic fungi including geophilic dermatophytes. These areas can serve as a habitat that promotes the fungal growth. The growth of keratinophilic fungi is accredited on keratin substrates like hair and feathers as well as organic debris in these soils. Presence of organic substance and keratin substrates in or on soils are major factors affecting the presence of keratinophilic fungi in soils. The present study also showed the influence of ecological factors (pH) on keratinophilic fungi in the soil.
5 Conclusion
The present study states that the soils of Rajasthan state, India may be major reservoirs as suitable for the growth and existence of keratinophilic fungi which is emblematic of its very hot, humid and semi-arid environment. The results of this study indicated that soil pH significantly affected the incidence of keratinophilic fungi. Neutral to alkaline soils provided favourable conditions for the growth of keratinophilic fungi whereas acidic soils were found to be unfavourable. Keratinophilic fungi may have a significant role in keratin degradation in the environment. Keratinolytic potential of isolated keratinophilic fungi was usually considered in regard to both, keratinolysis in the growth environment and the activity of keratinases against keratin substrates. According to the present study, the abundance of keratinolytic biota in Rajasthan (India), which degraded the keratinous substrate in vitro condition states about the potential approach of keratinous waste management and their bioconversion into valuable products i.e. animal feed and fertilizers.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
We thank to Director, School of Sciences for encouragement and Head, Department of Biotechnology, JECRC University, Jaipur for providing the laboratory facilities.
References
- The dermatophytes, Microsporum gypseum as a saprophyte and parasite. J. Invest. Dermat.. 1953;21(3):157-171.
- [Google Scholar]
- Soil as a natural reservoir for human pathogenic fungi. Science. 1956;123(3203):876-879.
- [Google Scholar]
- Keratinophilic fungi on the hair of goats from the West Bank of Jordan. Mycopathologia. 1988;104(2):103-108.
- [Google Scholar]
- Isolation and identification of soil keratinophilic fungi from different area in south of Libya. Int. J. Appl. Med. Biol. Res.. 2016;1(1):27-32.
- [Google Scholar]
- Keratinophilic fungi of poultry farm and feather dumping soil in Tamil Nadu, India. Mycopathologia. 2004;158(3):303-309.
- [Google Scholar]
- Keratinophilic fungi in activated sludge of wastewater treatment plants with MBR in Berlin, Germany. Mycology. 2011;2(4):276-282.
- [Google Scholar]
- Verbreitung und keratinophilie von anixiopsis stercoraria (hansen) hansen. Archiv Klinische Exp. Dermatol.. 1965;223(4):422-428.
- [Google Scholar]
- Production of an extracellular keratinase from Chryseobacterium sp. growing on raw feathers. Electr. J. Biotechnol.. 2005;8(1):35-42.
- [Google Scholar]
- Mycology of dermatomycoses. In: Manual of Clinical Mycology. Philadelphia: W.A. Saunders Company; 1959. p. :329-352.
- [Google Scholar]
- Isolation of dermatophytes and other keratinophilic fungi from the vicinity of salt pan soils of Mumbai, India. Mycopathologia. 2004;157(3):265-267.
- [Google Scholar]
- The occurrence of dermatophytes and other keratinophilic fungi from the soils of Himachal Pradesh (India) Czech. Mycol.. 2006;58(1–2):117-124.
- [Google Scholar]
- Incidence of keratinophilic fungi from the soils of Vedanthangal Water Bird Sanctuary (India) Mycoses. 2011;54(6):487-490.
- [Google Scholar]
- Isolation of keratinophilic fungi from selected soils of The Gir Forest National Park and Wildlife Sanctuary, Gujarat, (India) Kavaka. 2014;43:6-10.
- [Google Scholar]
- The occurrence of Keratinophilic fungi in selected soils of Ladakh (India) Nat. Sci.. 2010;2(11):1247-1252.
- [Google Scholar]
- Descriptions of Medical Fungi (2nd ed.). Australia: Mycology Unit, Women’s and Children’s Hospital, North Adelaide; 2007.
- Laboratory methods in basic mycology. In: Bailey and Scott’s Diagnostic Microbiology (11th ed.). St. Louis: Mosby; 2002. p. :711-798.
- [Google Scholar]
- Isolation of dermatophytes and other keratinophilic fungi from soils in India. Med. Mycol.. 1966;4(4):259-264.
- [Google Scholar]
- Diversity of keratinophilic fungi on human hairs and nails at four governorates in Upper Egypt. Mycobiology. 2006;34(4):180-184.
- [Google Scholar]
- Isolation and characterization of keratinophilic fungi and related dermatophytes from various public parks of Jaipur, India. Int. J. Pharma Bio Sci.. 2017;8(2):100-106.
- [Google Scholar]
- Prevalence of keratinophilic fungi in soils of St. Kitts and Nevis. J. Infect. Dev. Ctries.. 2012;6(4):347-351.
- [Google Scholar]
- Isolation and identification of keratinophilic fungi from soil of Gwalior region and their control by methanolic plant extracts. J. Biomed. Pharm. Res.. 2012;1(3):01-21.
- [Google Scholar]
- Effect of soil physicochemical parameters and seasonal variations on the occurrence of Keratinophilic Fungi of Murtala Amusement Park in Minna, Niger State, Nigeria. South Asian J. Res. Microbiol.. 2018;1(1):1-15.
- [Google Scholar]
- Safe, low-distortion tape touch method for fungal slide mounts. J. Clin. Microbiol.. 2000;38(12):4683-4684.
- [Google Scholar]
- Keratinophilic fungi from the soil of district, Jamshoro, Sindh, Pakistan. Pak. J. Bot.. 2007;39(4):1377-1382.
- [Google Scholar]
- A descriptive study of keratinophilic fungal flora of animal and bird habitat, Jaipur, Rajasthan. Afr. J. Microbiol. Res.. 2012;6(42):6973-6977.
- [Google Scholar]
- Isolation of keratinophilic fungi from soil in Isfahan province, Iran. J. Mycol. Med.. 2012;22(1):8-13.
- [Google Scholar]
- Native feather degradation by a keratinophilic fungus. Int. J. Chemtech. Res.. 2013;5(6):2947-2954.
- [Google Scholar]
- Keratinase production from feathers wastes using some local streptomyces isolates. Aust. J. Basic Appl. Sci.. 2009;3(2):561-571.
- [Google Scholar]
- Keratinophilic fungi from poultry farm soils of Jammu, India. Mycologist. 2000;14(2):89-91.
- [Google Scholar]
- Biodegradation of keratinous waste substrates by Arthroderma multifidum. Asian J. Appl. Sci.. 2016;9(3):106-112.
- [Google Scholar]
- Chrysosporium queenslandicum: a potent keratinophilic fungus for keratinous waste degradation. Int. J. Recycl. Org. Waste Agric.. 2017;6(2):143-148.
- [Google Scholar]
- Diversity of keratin degrading fungal flora in industrial area of Jaipur and keratinolytic potential of Trichophyton mentagrophytes and Microsporum canis. Int. J. Biotechnol. Bioeng. Res.. 2013;4(4):359-364.
- [Google Scholar]
- Physiology of keratinophilic fungi. In: Kushwaha R.K.S., Guarro J., eds. Biology of Dermatophytes and Other Keratinophilic Fungi. Bilbao: Revista Iberoamericana de Micologia; 2000. p. :77-85.
- [Google Scholar]
- Distribution of soil keratinophilic fungi isolated in summer beaches of the east sea in Korea. Kor. J. Med. Mycol.. 2011;16(2):44-50.
- [Google Scholar]
- Keratinophilic fungi: their occurrence in the environment. J. Mycopathol. Res.. 2010;50(2):193-198.
- [Google Scholar]
- Scopulariopsis brevicaulis: a keratinophilic or a keratinolytic fungus? Mycoses. 2000;43(7–8):281-292.
- [Google Scholar]
- Investigation of keratinophilic fungi from soils in Western Australia a preliminary survey. Mycopathologia. 1980;72(3):155-165.
- [Google Scholar]
- Isolation of dermatophytes and correlated species from the soil of public gardens and parks in Rome. Sabouraudia. J. Med. Vet. Mycol.. 1980;18(2):123-128.
- [Google Scholar]
- Isolation of keratinophilic fungi from soil samples of forests and farm yards. Iranian J. Publ. Health.. 2006;35(4):62-69.
- [Google Scholar]
- Occurrence of keratinophilic fungi with special reference to Chrysosporium species in soil of India. Sydowea.. 1990;42:200-208.
- [Google Scholar]
- Keratinophilic fungi from the nest of birds in Czechoslovakia. Sabouraudia. J. Med. Vet. Mycol.. 1967;5(4):350-354.
- [Google Scholar]
- Isolation and Molecular Identification of Keratinophilic Fungi from Public Parks Soil in Shiraz, Iran. Biomed Res. Int.. 2013;2013:1-5.
- [CrossRef] [Google Scholar]
- A survey of Soil inhabiting dermatophytes and related Keratinophilic fungi of India. Sabouraudia. J. Med. Vet. Mycol.. 1965;4(2):71-79.
- [Google Scholar]
- Prevalence of dermatophytes and other keratinophilic fungi from soils of public parks and playgrounds of Riyadh, Saudi Arabia. J. Animal Plant Sci.. 2012;22(4):948-953.
- [Google Scholar]
- Disseminated infection due to Chrysosporium zonatum in a patient with chronic granulomatous disease and review of non-Aspergillus fungal infections in patients with this disease. J. Clin. Microbiol.. 1999;37(1):18-25.
- [Google Scholar]
- Keratinophilic fungi in soils of parks of Corrientes city, Argentina. Rev. Iberoam. Micol.. 2016;33(1):7-12.
- [Google Scholar]
- Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses. 2012;55(5):410-415.
- [Google Scholar]
- Influence of temperature and relative humidity on growth and sporulation of some common dermatophytes. Ind. J. Fund. App. Life Sci.. 2012;2(4):1-6.
- [Google Scholar]
- A review on antidermatophytic efficiency of plant essential oils. Int. J. Pure Appl. Biosci.. 2014;2(6):265-278.
- [Google Scholar]
- Incidence of dermatophytes and other keratinophilic fungi in the schools and college playground soils of Jaipur, India. Afr. J. Microbiol. Res.. 2010;4(24):2647-2654.
- [Google Scholar]
- Keratinophilic Fungi: Nature’s keratin degrading machines their isolation, identification and ecological role. Resonance. 2003;8(9):28-40.
- [Google Scholar]
- Prevalence of keratinophilic fungi at various pH in different areas of Jaipur, Rajasthan. J. Microbiol Biotech Res.. 2014;4(2):17-21.
- [Google Scholar]
- Dermatophytes: diagnosis of dermatophytosis and its treatment. Afr. J. Microbiol. Res.. 2015;9(19):1286-1293.
- [Google Scholar]
- Distribution and prevalence of dermatophytes in semi-arid region of India. Adv. Microbiol.. 2015;5(2):93-106.
- [Google Scholar]
- Occurrence of keratinophilic fungi from the soils of Chhattisgarh. Int. J. Sci. Res.. 2014;3(9):2041-2044.
- [Google Scholar]
- Keratinophilic fungi of Ghana Birds Sanctuary, Bharatpur. Adv. Plant Sci.. 1994;7(2):280-291.
- [Google Scholar]
- Dermatophytes and related keratinophilic fungi in soil of parks and agricultural fields of Uttar Pradesh. Indian J. Dermatol.. 2010;55(3):306-308.
- [Google Scholar]
- Arthroderma multifidum reported from soil of Ujjain, India. Life Sci. Int. Res. J.. 2015;2(1):376-379.
- [Google Scholar]
- Isolation of keratinophilic fungi from soil in Khairpur city, Sindh, Pakistan. Bangladesh J. Microbiol.. 2007;24(1):79-80.
- [Google Scholar]
- Incidence of dermatophytes and other keratinolytic fungi in the soil of Amravati (India) Trends Appl. Sci. Res.. 2007;2(6):545-548.
- [Google Scholar]
- Technique biologique pour 1’isolement des dermatophytes du sol. Ann. Soc. Belge. Med. Trop.. 1952;32:173-178.
- [Google Scholar]
- Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species’ (2nd ed.). Boca Raton, Florida: CRC Press LLC; 1937.
- Isolation and investigation of keratinophilic fungi in the parks of municipality districts of Tehran. Thrita. 2013;2(3):2-5.
- [Google Scholar]







