Translate this page into:
A study on predominance of keratinophilic flora in soil of Jaipur, India
⁎Corresponding author. sharmaanima6@gmail.com (Anima Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fungi are heterotrophic organisms possessing cryptic lifestyles and have an individual hierarchy in the evolution of microorganisms on earth. Out of which, Keratinophilic fungi are allied molds that produce keratinase enzymes and diminish the keratin matters in or on the soil. Fifty soil samples were collected from various sites of Jaipur district, India to analyze incidence of keratinophilic fungi. Out of 162 isolates, a total of 24 keratinophilic fungal species of 13 genera were recovered, including Trichophyton sp. (17.90%), Aspergillus sp. (17.90%), Chrysosporium sp. (14.81%). Selective keratinophilic fungi were also discussed about phylogenetic studies. In multiple sequence alignment of sequences, species were reported to have closest relationships between teleomorphic and anamorphic form of keratinophilic fungi which exhibited a reason for phase changes between geophilic and dermatophytic fungi by accident or opportunistic where they may cause infections in both humans and animals on the opportunity. In the anti-fungal activity, Eucalyptus globulus (Inhibition Zone: 23.5 mm; Activity Index: 0.82) and Ocimum tenuiflorum (Inhibition Zone: 20.75 mm; Activity Index: 1.46) were inscribed to dispense a greater potential of antifungal activity against all selected keratinophilic fungi.
Keywords
Fungi
Heterotrophic
Keratinophilic
Teleomorphic
Anamorphic
1 Introduction
Keratin is an insoluble fibrous and recalcitrant protein that is arranged into soft and hard state depending on their amino acids and sulfur contents. Both of Keratin construct fundamental ingredients of mammals, birds, and fishes in form of wool, feathers, hairs, nails, horns, and hooves. Keratin is densely packed and stabilized with a mechanical strength by certain hydrogen bonds, disulfide bonds, and hydrophobic interactions. Moreover, the cross-linking of protein chains hinders keratin degradation by common proteases (Hassan et al., 2020a; Fraser and Parry, 2003) (Figs. 1–5).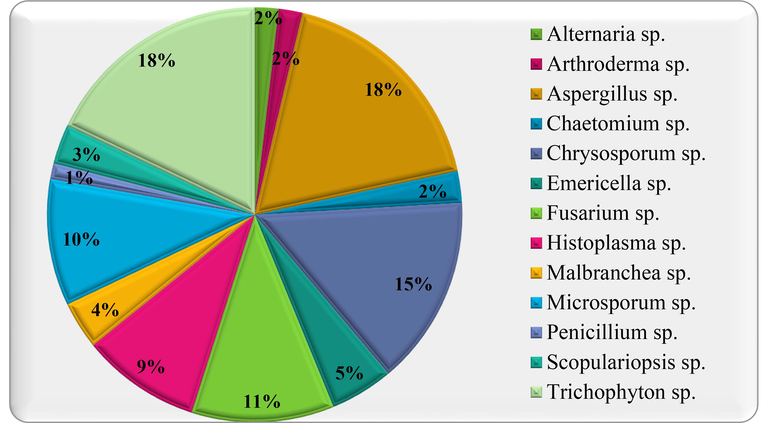
Prevalence of Keratinophilic Fungal Genera on Various Baits.
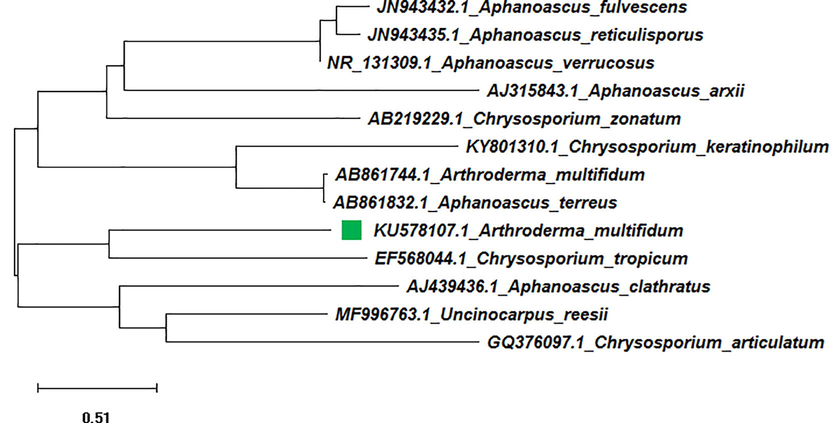
Neighbor joining tree of isolated Arthroderma multifidum (KU578107) with reference data.
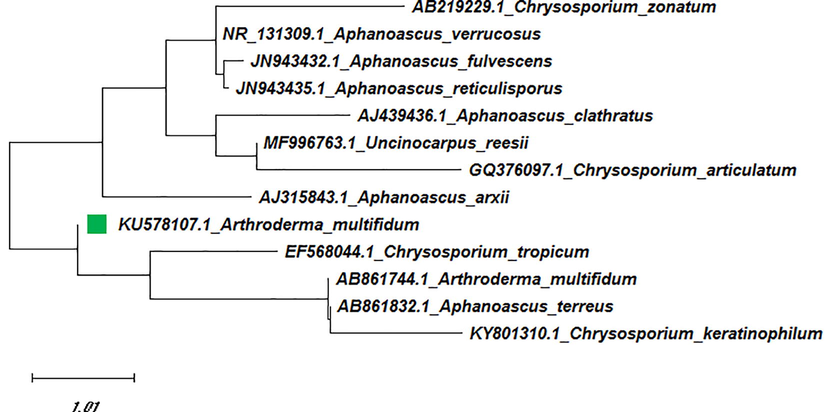
Maximum Likelihood tree of isolated Arthroderma multifidum (KU578107) with reference data.
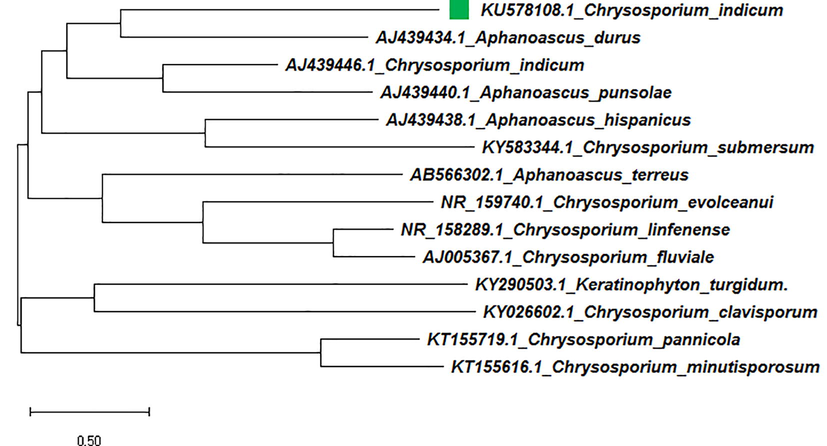
Neighbor joining tree of isolated Chrysosporium indicum (KU578108) with reference data.
Maximum Likelihood tree of isolated Chrysosporium indicum (KU578108) with reference data.
Keratinophilic fungi are imperfect molds which serve as saprophytic and consume their nourishment via decomposing the keratin or other organic matters (Kwon-Chung and Bennett, 1992; Rippon, 1988). Geographically, these are found in soils of forest, public places, marketplace, poultry sheds, herbivore or carnivore muck parks, as well as sediments of rivers and oceans containing humus and organic material, sewage and bird’s nest, barber’s hair dumping area (Kumawat et al., 2020; Gupta et al., 2012). In recent years, keratinophilic microbiota is attaining remarkable attention throughout the world (Sharma et al., 2020; Bohacz and Korniłłowicz-Kowalska, 2019; Gupta et al., 2012; Bentubo et al., 2006) (Tables 1 and 2). Note: IZ = Inhibition Zone (in mm) with the diameter of disc (6 mm), AI = Activity Index.
S. No.
Name of fungal Genera
Human Hair
Human Nails
Chicken Feathers
Animal Hair
Total Plates
%
1.
Alternaria sp.
0
0
3
0
3
1.85
2.
Arthroderma sp.
1
1
0
1
3
1.85
3.
Aspergillus sp.
5
4
11
9
29
17.9
4.
Chaetomium sp.
0
1
2
1
4
2.47
5.
Chrysosporum sp.
8
2
8
6
24
14.81
6.
Emericella sp.
1
3
2
2
8
4.94
7.
Fusarium sp.
9
1
2
6
18
11.11
8.
Histoplasma sp.
4
1
4
6
15
9.26
9.
Malbranchea sp.
1
0
1
4
6
3.7
10.
Microsporum sp.
4
6
2
4
16
9.88
11.
Penicillium sp.
0
1
0
1
2
1.23
12.
Scopulariopsis sp.
1
1
2
1
5
3.09
13.
Trichophyton sp.
14
8
5
2
29
17.9
Total
48
29
42
43
162
Antifungal activity of essential Oils Extracted from Plants
Test Fungi
Ocimum tenuiflorum
Eucalyptus globulus
Citrus grandis
Inhibition Zone (IZ) (in mm)
Activity Index (AI)
Inhibition Zone (IZ) (in mm)
Activity Index (AI)
Inhibition Zone (IZ) (in mm)
Activity Index (AI)
Arthoderma multifidium
20.5 ± 1.75
0.72
23.5 ± 1.32
0.82
12.62 ± 0.26
0.44
Chrysosporium indicum
20.75 ± 1.66
1.46
17.5 ± 0.5
1.23
10 ± 0.57
0.70
Fusarium oxysporum
16.75 ± 0.52
1.08
16.5 ± 0.50
1.06
10.5 ± 0.5
0.68
Therefore, a precise distribution and appearance of keratinophilic fungi help to view advantageous and unhealthy impacts on nature. As a point of industrial and ecological interests, these could possess a role in degradation of keratinous waste as well as production of Keratinolytic enzymes. The primary outcomes are of great interest in their utilization in a natural and environmentally friendly way. These may appropriate in fabrication of bioplastics; animal feed and fertilizer productions; biogas production; detergent activity to tolerate various surfactants, detergents, and organic solvents; dehairing in leather and textile industry (Hassan et al., 2020b; Bohacz and Korniłłowicz-Kowalska, 2019).
Although keratinophilic fungal appearance on earth manifests an emphatic impression, besides also designates a reservoir for primary infection. It may be at least for some pathogenic fungi or also for those which are potentially pathogenic for small to big wild fauna (Garg et al., 1985). All keratinophiles endure as self-sufficient saprophytes in soil until the availability of favorable environmental conditions. Opportunistically or accidentally, being a contagion, these organisms adhere and penetrate to both humans and animal tissue through specific metabolic reactions and lead up to superficial and cutaneous fungal infections. Such keratinophiles are referred to as dermatophytes (Sharma et al., 2020; Bentubo et al., 2006). In the present study, the main objective was to evaluate prevalence of keratinophilic flora in soil of Jaipur (Rajasthan), India with their sustainable management.
2 Materials & methodologies
2.1 Soil collection
The selection of collecting soil sample areas for this study was based on the contamination of higher keratin levels. In the present study, a total of 50 soil samples was collected from various sites in Jaipur, India. After collection of soil samples, initial pH of soil was determined following the protocol of Rousk et al., 2009 using calibrated pH meter (Model 181, Electronics India).
2.2 Isolation and identification of keratinophilic fungi
Isolation of keratinophilic fungi was done according to Vanbreuseghem’s hair bait technique (Vanbreuseghem, 1952). After the visible appearance of mycelium on applied baits, a fungal colony was transferred to Sabouraud’s Dextrose Agar (SDA) medium (Hi-Media). After the purity of isolated fungal growth, fungus was identified on the basis of macroscopic characteristics. Subsequently, microscopic characteristics were also observed by flag scotch tape method at 100X power under microscope.
The cultural and morphological characteristics of fungal colonies and their identification were done by referring laboratory methods in basic mycology (Forbes et al., 2002), Descriptions of Medical Fungi (Kidd et al., 2016) and Pictorial Atlas of Soil and Seed Fungi (Watanabe, 1937). Keratinolytic activity of fungal isolates was determined following the protocol of Kacinova et al., 1989. On the basis of occurrence and highest keratinolytic activity, most prevalent fungi were selected for further studies.
2.3 Molecular identification
Whole-cell DNA from mycelial growth of isolate was extracted by following the protocol of Lee and Taylor (1990). The quality of extracted DNA was monitored by agarose gel electrophoresis using 1.5%agarose gel with ethidium bromide fluorescence. ITS (Internal transcribed spacer) regions of fungal DNA were amplified using ITS primer such as ITS1(3′TCCGTAGGTGAACCTGCGG5′) and ITS4 (5′TCCTCCGCTTATTGATATGC3′) respectively (Sigma-Aldrich, Bengaluru, India) by Eppendorf’s DNA gradient thermal cycler (White et al., 1990; Sharma et al., 2017). Sequencing reactions were carried out using Big Dye terminator cycle sequencing kit, version3.1 (Applied Biosystems, CA, USA) and analyzed by the ABI3130 genetic analyzer (Applied Biosystems, CA, USA).
The resultant sequence was compared with the GenBank data base using NCBI Basic Local Alignment Search Tool (BLAST). The sequence showing ≥ 99% match with data base sequences of the reference taking as for species identification. The sequences were aligned with Clustal-ω computer program in MEGA-10 software. Phylogenetic trees were prepared by the Neighbor-joining and Maximum Likelihood methods using MEGA10 (Kumar et al., 2018).
2.4 Anti-fungal assay
The anti-fungal activity against selected test fungi was evaluated by disc diffusion (Gould and Bowie, 1952) method. Essential oils were extracted from Ocimum tenuiflorum (Tulsa), Citrus grandis (Pomelo), and Eucalyptus globulus (Bluegum) plants. In assay, sterilized discs impregnated with 10 µL of crude essential oil extracted from respective plants were aseptically transferred onto SDA plates inoculated with test fungi. The plates were then incubated at 30 °C for 48–72 h and were observed for the formation of zone of inhibition. The activity index was also calculated by following formula.
Minimum inhibitory concentration was determined by micro dilution method for active crude essential oils against selected test fungi. Various dilutions of essential oil were prepared with the help of DMSO. Each sterile disc soaked in 100 µL of various concentrations of essential oil was aseptically placed onto petri plates previously inoculated with fungal suspension of each test fungi. The plates were incubated at 30 °C for 48–72 h and observed for the formation of zone of inhibition.
2.5 Statistical analysis
All Experiments were performed in triplicate and data analyzed are mean ± SE subjected to one-way ANOVA with significant (P < 0.05).
3 Results and discussion
In pH analysis of soil samples, maximum pH 10.6 was recorded for soil samples collected from Sambhar Lake site followed by soil samples collected from dump sites of barbers shops which were exhibited more alkaline pH between pH 7.9–9.55. Likewise, other sites: road side, poultry farms, cattle yard, and public places of Jaipur city were found to have a pH range of 6.5–8.95.
In the present study, numerous mycelium structures were observed as hyphae and spores which were recorded to be keratinophilic fungi. Total twenty-four species distributed in thirteen genera were isolated as keratinophilic and related fungi. Chrysosporium sp. (14.81%) was second most dominant keratinophilic fungi afterward to Trichophyton sp. (17.90%) and Aspergillus sp. (17.90%) and the least account was recorded for Penicillium sp. (1.23%) (Figure-1 & Table-1). The major incidence ratio of keratin degradation in baiting technique was obtained on Human Hair > Animal Hair > Chicken Feathers > Human Nails (high to low growth) baits.
According to results, growth of Malbranchea saccardo (3.70%), Chaetomium kunze (2.47%) and Arthroderma multifidum (1.85%) were reported for the first time in Jaipur, Rajasthan. The results of present study are in line with several researchers who have documented the distribution of keratinophilic fungi from soil of India (Randhawa and Sandhu, 1965; Deshmukh, 1999; Vidal, 2000). Pakshir et al. (2013) also reported that most (66.42%) of the keratinophilic fungi grow in the soil with pH 7.0–8.0. Findings of Kumawat et al., 2020 are in support of the present study that has reported 154 isolates belonging to 16 genera and 31 species and recovered to Chrysosporium tropicum as most predominant fungal species in the soil of Rajasthan.
In molecular identification, DNA was isolated using the phenol–chloroform method and its purity was confirmed to be between 1.9 and 2.6 at 260 nm/280 nm ratios by using NanoDrop™ 2000/2000c Spectrophotometers. The PCR amplification of ITS region of the isolate KU7 and KU8 yielded PCR products of about 550 bp, respectively. Ramaraj et al. (2016) obtained the amplicon size of 650–800 bp for amplified regions (ITS-1 and ITS-2) of keratinophytes using primers ITS-1 and ITS-4 that is similar to our findings.
The PCR product with primer pair ITS1 was sequenced for species identification and found to be 90 to 100% similar in the BLAST program to sequences of the ITSI, 5.8S rRNA gene, and ITSII regions of respective fungi. On the basis of BLAST program results, ITS1 sequence of isolates was determined as Arthoderma multifidium (KU578107) and Chrysosporium indicum (KU578108) which was similar to morphological identification. After a preliminary identification, sequences of A. multifidum and C. indicum weredepositedintheGenBankdatabase(http://www.ncbi.nlm.nih.gov/Genbank/index.html) with the accession numbers KU578107 & KU578108 respectively.
The multiple sequence alignment analysis of isolated A. multifidum was found to similar with reference AB861744.1 and AB861799.1 at GenBank data. There were a total of 547 positions for A. multifidium in final dataset.
The data of thirteen fungal sequences including one test sequence and twelve reference sequences were used in phylogeny construction. In the phylogenetic tree, A. multifidum found to associate with A. multifidum, C. keratinophilum, C. articulatum, A. terreus, Aphanoascus fulvescens, A. verrucosus, A. reticulisporus, C. tropicum, C. zonatum, A. clathratus, A. arxii, and Uncinocarpus orissiare members in ITS1 homology group (Figure-1).
In the Neighbor-Joining tree of isolated Arthroderma sp. strain, the branch length was calculated by the method of Saitou and Nel, 1987and was obtained with the sum of 12.80 branch lengths. In this tree, A. multifidum (KU5781026) and A. clathratus (AJ439436.1) found to form cluster A, and all other Aphanoascus sp. and Chrysosporium sp. were recorded to form Cluster B with 100% bootstrap support. The members in cluster A were classified into two groups with ITS1 homology (groups a and b) according to their ITS1 DNA sequences. The group a was recorded to have very close relationships between sequence KU578107.1 and EF568044.1. The phylogenetic relationships mentioned above were also supported by the ML tree.
A. multifidum was revealed to be closely allied with the teleomorphs species C. tropicum and is on a well-supported branch that contains another major lineage with another telomorphs as C. articulatum lineage. But in case of subgroup b in cluster B, A. multifidum was shown to be closely allied with the A. terreus.
The phylogenetic relationships mentioned above were also supported by the ML tree where the reference sequence of A. multifidum (AB861744.1) was found to be closely allied with A. multifidum (AB861744.1) and a telomorphic sequence of C. tropicum. Hence, In ML tree, the tree was found with the highest log-likelihood of −8242.83.
In support, multiple sequence alignment of C. indicum (KU578108) was found to similar with reference AJ439446.1 and AJ005369.1 at GenBank data. There were a total of 556 positions for C.indicum in final dataset and data of fourteen fungal sequences including one test sequence and thirteen reference sequences were used in phylogeny construction.
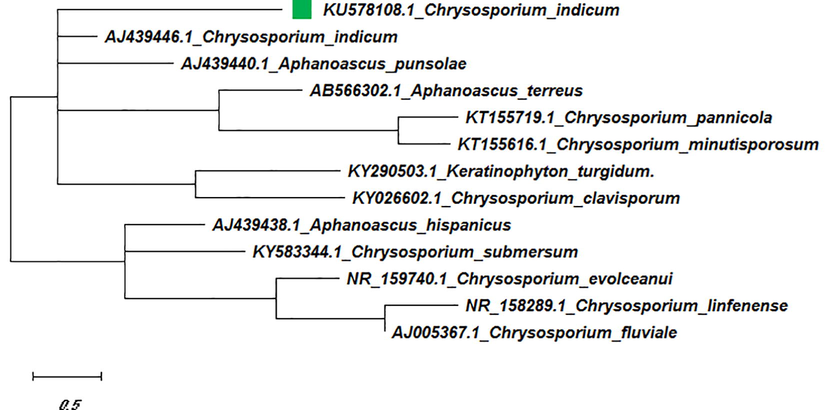
The aligned sequences of their ITS1 regions are presented in Figure-4. In the phylogenic trees, C. indicum found to associate with C. indicum, A. punsolae, A. terreus, A. durus, A. hispanicus, C. evolceanui, C. linfenense, C. fluviale, C. clavisporum, C. pannicola, Keratinophyton turgidum, C. submersum, C. minutisporosum and members in ITS1 homology group (Figure-4).
In the Neighbor-Joining tree of isolated Chrysosporium sp. strain, the branch length obtained with the sum of 16.95 branch lengths. In NJ tree, there are two major clusters A & B. The members in cluster A were classified into two groups with ITS1 homology (groups a and b) according to their ITS1 DNA sequences. In Cluster A, test (KU578108.1) and reference (AJ439446.1) sequence of C. indicum were found to have very close relationships with Aphanoascus sp. (AJ439440.1&AJ439434.1). Subsequently, in Cluster B, and group b of cluster A, maximum members were recorded for Chrysosporium sp. with 100% bootstrap support. C. indicumwas shown to be closely allied with the anamorphs species A. durus and is on a well-supported branch that contains another major lineage with another as A. punsolae lineage. The phylogenetic relationships mentioned above were also supported by the ML tree where the sequence of C. indicum (KU578108.1) was found to be closely allied with reference sequence AJ439446.1 with the highest log-likelihood of −9547.26. This species was also grouped together with Trichophyton anamorphs of Arthroderma sp. by Vidal, 2000.
Results of present study have indicated about phylogenetic relationships between the teleomorphic and anamorphic form of keratinophilic fungi and exhibited a reason for phase changes between geophilic and dermatophytic fungi by accident or opportunistic where they may cause infections in both humans and animals on the opportunity and may exist as parasite on the body. In this dimorphism character of fungi, a number of fungal (superficial and deep mycotic) infections has enhanced as a significant clinical problem and still is continued (NCCLS, 1997; Atraide et al., 2011). The limited therapeutic strategies, ranging from routes administration, efficacy, cost, solubility and stability of antifungal agents needed asearch of novel antifungal agents from biosynthetic laboratories i.e. plants (Sharma et al., 2014).
In this approach, anti-fungal activity was used as research parameter to control incidence of fungal infections and recorded that essential oil extracted from all selected plants were found to have great antifungal potential against all keratinophilic fungal species tested. However, a comparison showed that essential oil of Eucalyptus globulus has a greater potential of anti-fungal activity than those of other essential oil. The Eucalyptus globulus exhibited highest zone of inhibition against A. multifidium with an activity index of (IZ: 23.5 mm; AI: 0.82). In support, essential oil of Ocimum tenuiflorum was found toexhibit highest zone of inhibition against Chrysosporium indicum with an activity index of (IZ: 20.75 mm; AI: 1.46). Citrus grandis was also found to exhibit its highestzone of inhibition against A. multifidium with an activity index of (IZ: 12.62 mm; AI: 0.44). In respect of Fusarium oxysporum, fungal growth was found to inhibit by essential oil of Ocimum tenuiflorum with an activity index of (IZ: 16.75 mm; AI: 1.08).
In Minimum inhibitory concentration (MIC), range 1/7(v/v) for all oil Ocimum tenuiflorumand Eucalyptus globulus was found to be effective for all tested fungi excluding to Citrus grandis, which was found to be effective in more decreased concentration.
The present results are in line with Basilico and Basilico, 1999 who reported the successful antifungal activity of O. basilicum oil against Aspergillus ochraceus (Basilico and Basilico, 1999). Vasudeva and Sharma (2012) reported maximum antifungal activity for essential oil extracted from Citrus species against Fusarium oxysporum with zone of inhibition 10.23 mm.
4 Conclusion
In the present study, the more prominent occurrence of Keratinophilic fungi in soils of Jaipur District confirms semi-arid environment of city. Certain reservoirs can part in approaching to superficial infections such as tinea or ringworm via frequent human contact under favorable conditions. The leading incidence of keratinophilic fungi generates an urgent need to manage the appearances of fungi. Results obtained regarding antifungal activity prove to be useful and may replace expensive therapies for controlling fungal infections and provide affordable remedial resources for millions of individuals or poor people around the world.
Although, the isolated keratinolytic fungi are also facilitated the breakdown of protein and di-sulphide bonds in keratin which could be exploited for many industrial applications. The crude keratinase enzyme could also be employed as an effective and eco-friendly alternative in leather processing industries. The results of this research work can guide and lead to the anticipated path for biotechnological interventions.
Acknowledgement
The authors are indebted to Head, Department of Biotechnology, JECRC University, Jaipur. The authors would also gratitude their heartiest expression to Dr. M R Shivaprakash, Professor at NCCPF, PGIMER, Chandigarh for their corporal help in analysis of isolated fungal cultures.
References
- The pattern of skin disorders in a Nigerian tertiary hospital. J. Public Health Epidemiol.. 2011;3:177-181.
- [Google Scholar]
- Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol.. 1999;29(4):238-241.
- [Google Scholar]
- Isolation of Microsporum gypseum from the hair coat of health wild felids kept in captivity in Brazil. Braz. J. Microbiol.. 2006;37:148-152.
- [Google Scholar]
- Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process Biochem.. 2019;80:119-128.
- [Google Scholar]
- Keratinophilic fungi isolated from soils of Mumbai, India. Mycopathologia. 1999;146(3):115-116.
- [Google Scholar]
- Laboratory methods in basic mycology. In: Bailey and Scott’s Diagnostic Microbiology (11th ed.). St.Louis: Mosby; 2002. p. :711-798.
- [Google Scholar]
- Macrofibril assembly in trichocyte (hard alpha -) keratins. J. Struct. Biol.. 2003;142(2):319-325.
- [Google Scholar]
- Ecology of keratinophilic fungi. Proc. Indian Acad. Sci. (Plant Sci.). 1985;94(2 & 3):149-163.
- [Google Scholar]
- The determination of bacterial sensitivity to antibiotics. Edinburgh Med. J.. 1952;59:178-199.
- [Google Scholar]
- Isolation and identification of keratinophilic fungi from soil of Gwalior region and their control by methanolic plant extracts. J. Biomed. Pharmaceut. Res.. 2012;1:1-21.
- [Google Scholar]
- Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: a review. Int. J. Biol. Macromol.. 2020;154:567-583.
- [Google Scholar]
- Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int. J. Biol. Macromol.. 2020;153:561-572.
- [Google Scholar]
- Production of Extracellular Keratinase byChrysosporium tropicum and Trichophyton ajelloi. J. Microbiol. Biotech. Food Sci.. 1989;3(1):103-106.
- [Google Scholar]
- Descriptions of medical fungi. South Australia: Newstyle Printing; 2016.
- Kumar S., Stecher G., Li M., Knyaz C., 2018. MEGA X- molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6), 1547-1549.
- Kumawat, T.K., Sharma, A., Sharma, V., Chandra, S., Bhadauria, S.., 2020. A study on the prevalence of keratinophilic fungal biota of semi-arid region of Rajasthan, India Journal of King Saud University - Science 32(1), 1014–1020. https://linkinghub.elsevier.com/retrieve/pii/S1018364719317793.
- Medical Mycology. Philadelphia, London: Lea & Febiger; 1992.
- Lee S.B., Taylor J.W., 1990 Isolation of DNA from fungal mycelia and single spores. In Innis MA, Gelfand D.H., Sninsky J.J., White T.J., In PCR protocols: A guide to methods and applications. San Diego, Academic Press Inc, p. 282–287.
- Performance standards for antimicrobial disk susceptibility tests: approved standard M2–A7. Wayne, PA, USA: National Committee for Clinical Laboratory Standards; 1997.
- Pakshir K., Ghiasi M.R., Zomorodian K., Gharavi A.R., 2013 Isolation and molecular identification of keratinophilic fungi from public parks soil in Shiraz, Iran. Biomed Res Int. 2013, 1-5, Article ID 619576.
- Ramaraj V., Vijayaraman R.S.,Rangarajan S., 2016 Incidence and prevalence of dermatophytosis in and around Chennai, Tamilnadu, India. Int J Res Med Sci. 4(3), 695-700.
- A survey of soil inhabiting dermatophytes and related keratinophilic fungi of India. Med. Mycol.. 1965;4(2):71-79.
- [Google Scholar]
- Rippon J.W., 1988 The pathogenic fungi and the pathogenic Actinomycetes. In Medical Mycology, W.B. Saunders, Philadelphia, p. 1-797.
- Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. AEM. 2009;75(6):1589-1596.
- [Google Scholar]
- The Neighbor-Joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- Phylogenetic analysis of Trichophyton mentagrophytes Isolated from Tinea Patients. Res. J. Biotechnol.. 2020;15(1):46-52.
- [Google Scholar]
- A review on anti-dermatophytic efficiency of plant essential oils. Int. J. Pure App. Biosci.. 2014;2(6):265-278.
- [Google Scholar]
- Evaluation of keratinolytic activity succeeds by keratinophilic fungi in Jaipur, India. Am. J. Appl. Sci.. 2017;14(7):678-681.
- [Google Scholar]
- Technique biologique pour L’isolement des dermatophytes du sol. Ann. Soc. Belg. Trop.. 1952;32:173-178.
- [Google Scholar]
- Chemical composition and antimicrobial activity of essential oil of Citrus limettioides. Tanaka J Pharmaceutical Tech. Drug Res.. 2012;1(2):1-7.
- [Google Scholar]
- Vidal P., de los Vinuesa A.M., Sanchez-Puelles J.M., Guarro J., 2000 Phylogeny of the anamorphic genus Chrysosporiumand related taxa based on rDNA internal transcribed spacer sequences. In Kushwaha R.K.S. (Ed.), Biology of dermatophytes and other keratinophilic fungi. Guarro J. Revista Iberoamericana de Micología, Bilbao.
- Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. Boca Raton, Florida: CRC Press LLC; 1937.
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., eds. PCR Protocols: A Guide to Methods and Applications. New York Academic Press Inc; 1990. p. :315-322.
- [Google Scholar]







