A study of insect succession of forensic importance: Dipteran flies (diptera) in two different habitats of small rodents in Riyadh City, Saudi Arabia
⁎Corresponding authors. famekhlafi@ksu.edu.sa (Fahd A. Al-Mekhlafi), ralajmi@ksu.edu.sa (Reem A. Alajmi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study focused on comparing between dipteran fauna recovered from small mice (Mus musculus Linnaeus, 1758) and albino rat (Rattus norvegicus Berkenhout, 1769) carcasses placed in different habitats (a botanical garden and on a over the roof of 3rd floor of the faculty of science‘s building in the university campus). It was conducted during winter 2018 in Al-Dir'iya, Riyadh, Saudi Arabia. Collected specimens were identified morphologically and by molecular techniques based on the mitochondrial cytochrome oxidase subunit I (COI) gene. The number of flies recovered from the different decomposition stages of rat and mice carcasses at the two study sites was 5244, representing 11 families and 14 species. A tota nol of 4665 flies, comprising 11 families and 14 species, invaded carcasses located in the botanical garden, compared to the carcasses placed on the rooftop, which attracted 579 flies from 6 families and 9 species. The most abundant species recovered from both sites was Chrysomya albiceps (Wiedemann, 1830), followed by Musca domestica (Linnaeus, 1758), suggesting that these species are the most valuable for estimating Post Mortem intervals (PMIs) in this region of the Kingdom of Saudi Arabia. The data collected from this research can serve as the baseline data for homicide investigation as these flies can help determine PMI.
Keywords
Forensic entomology
Carrion
PMI
Decomposition
Diptera
Riyadh
1 Introduction
Forensic entomology deals with the study of arthropods associated with dead bodies, and helps in determining the Post Mortem Interval (PMI) (Hall, 2001). This field is far-reaching; criminal investigations have been supported and crimes solved with the help of insect evidence. Insects use carrion as food and as a habitat for mating, larviposition, and oviposition (Rivers and Dahlem, 2014). The first step in helping to solve a criminal case or to know the exact time, manner, and cause of death through using insects is to identify such insects correctly (Byrd and Castner, 2001). Insects arrive on decomposing corpses in a relatively sequence that is named ecological succession. They arrive shortly after death and quickly begin their activities providing valuable scientific information (Martinez et al., 2007). This makes it easier to solve occurrences such as murder, sudden death, rape, suicide, and unexplained death (Cruise et al., 2018).
The order Diptera contains approximately 120,000 species of fly (Grimaldi and Engel, 2005). These flies belong to “blowflies (Calliphoridae), flesh flies (Sarcophagidae), and house flies (Muscidae)” they are most crucial carrion insects and commonly feed on human and animal remains, so that they are considered as forensically important. According to previous study (Al-Qahtnia et al., 2019), these three dipteran families are prevalent in early stages of decomposition. Carrion decomposition is a continuous process comprising different stages: fresh, bloated, advanced decay, and skeletal or dry remains (Reed, 1958). The decomposition process depends on environmental conditions such as Temperature (Wang et al., 2008) and habitat (Mashaly, 2017; Haddadi et al., 2019). Moreover, other factors related to the carrion including changes in the chemical constituents of the body (Coe, 1974), autolysis of tissue and decomposition due to bacterial activity within the body (Kashyap and Pillay, 1989).
Morphological keys are generally utilized in the identification of adult forensic insects (Marshall et al., 2011). Even with the availability of such keys, morphological identification requires a taxonomist with specialized taxonomic knowledge. To overcome morphological identification difficulties, molecular identification techniques can be used (Simon et al., 1994). These techniques can identify species quickly and accurately and can work with different insect stages (Aly and Wen 2013).
Many insect succession studies have been conducted in different provinces in Saudi Arabia. These investigated the families Calliphoridae, Sarcophagidae, and Muscidae found on animal carcasses such as rabbit carcasses (Abouzied, 2014; Al-Shareef and Al-Mazyad, Mashaly and Al-Mekhlafi, 2016; Haddadi et al., 2019; Shaalan et al., 2017), other different animal carcasses such as cows, camels and sheep (Al-Ghamdi et al., 2015; Mashaly, 2017) and human carcasses (Al-Qahtnia et al., 2019).
The aim of this research is to investigate if differences in rodent type, carcass size, location and habitat influence flies diversity, succession patterns and to assess the importance of this data in forensic entomology.
2 Material and methods
2.1 Study area
The study area is located in Al-Dir'iya, Riyadh, Saudi Arabia. Al-Dir'iya is a town located west of Riyadh and is a semiarid, urban area. The climate of Al-Dir'iya is hot and dry in summer, with cool winters. The present experiment was conducted in winter for 40 days, from January 14 to February 23, 2019. Albino rat and mice carcasses were placed in two different study areas, which are approximately 1.5 km apart; the botanical gardens (surrounded by shrubs and trees) and 12 m above the ground on the roof of a building which is covered with cement and clear of any vegetation. Both sites are located in King Saud University campus in Riyadh, Saudi Arabia (24° 43′ 19.2″ N, 46° 37′ 37.2″ E), the temperature in the study sites were recorded daily (Table 1).
| Site | Stage | Daily temperature (C°) | ||
|---|---|---|---|---|
| Max | Min | Mean | ||
| Botanical garden | Fresh | 26 ± 3.25 | 12 ± 3.50 | 19 ± 7 |
| Bloated | 24.75 ± 2.75 | 11.5 ± 1.89 | 18.13 ± 2.94 | |
| Decay | 22.56 ± 0.86 | 11.48 ± 0.73 | 17.02 ± 0.97 | |
| Dry | 20.40 ± 0.85 | 10.10 ± 1.06 | 15.25 ± 1.36 | |
| All stages | 22.33 ± 0.65 | 11.15 ± 0.55 | 16.74 ± 0.76 | |
| Rooftop | Fresh | 28 ± 00 | 13 ± 00 | 20.5 ± 7.5 |
| Bloated | 26.25 ± 2.56 | 12.5 ± 1.94 | 19.38 ± 2.99 | |
| Decay | 24 ± 0.86 | 11.96 ± 0.71 | 17.98 ± 1.02 | |
| Dray | 21.8 ± 0.87 | 10.5 ± 1.02 | 16.15 ± 1.45 | |
| All stages | 23.78 ± 0.65 | 11.68 ± 0.54 | 17.73 ± 0.80 | |
2.2 Experimental design
Twelve carcasses were used in this study (six albino rats and six mice were killed by cervical dislocation), divided equally into two groups. The first group was placed in the botanical garden, while the second group was placed on the building roof. Each rat and mice weighed 300 g and 40 g respectively. At each location, carcasses were then placed in a plastic boxes covered with steel wires and some weight was provide above each cage to keep cages constant and protect carcasses from the attack of large animals 15 m away from each other. Insects attracted to the animal carcasses were collected daily for one hour from 13:00 to 14:00. During insect collection, the climatic conditions were monitored in the field using a hygro-thermometer (model MM 5202-Incoterm™.(The mean temperature in all stages of decomposition was 17.73° (Table 1).
Adult flies were collected as per the method described by Mashaly and Al-Mekhlafi (2016). The collected flies were preserved in 96% ethanol after washing them in tap water, and then transferred in labelled tubes to the laboratory. Preserved specimens were kept at 4 °C for further use.
2.3 Morphological identification
The collected samples were examined under stereomicroscope (SMZ18; Nikon-Japan), and identified following morphological identification key of Marshall et al. (2011). A part of the collected samples was preserved and deposited in the entomology laboratory in zoology department at King Saud University. After the morphological examination, some of the collected insects were stored in 96% ethanol in preparation for molecular identification.
2.4 DNA extraction and polymerase chain reaction (PCR)
From 49 randomly selected samples, Mitochondrial DNA (mt DNA) was extracted using a QIAamp DNA Mini Kit (50) (QIAGEN, Germany), following the manual instructions. The extracted DNA was then preserved at −20 °C.
A partial sequence of the mitochondrial gene COI was amplified using the Cyto1-primers 5′-GGTCAACAAATCATAAAGATATTGG-3ʹ(forward) and 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ (Folmer et al., 1994).
PCR was performed in a 20 μl reaction volume, containing 2.0 μl of DNA template, 0.6 μl of each primer, 4.0 μl of 5 × FIREPol® Master Mix (Solis Biodyne, Spain) (Reagents, FIREPol® DNA polymerase, 5 × Reaction Buffer B [0.4 M Tris-HCl, 0.1 M (NH4)2SO4, 0.1% w/v Tween-20], 12.5 mM). Targeted sequence were amplified using thermal cycler (Applied Biosystems By Thermo Fisher Scientific- USA) under the flowing conditions: an initial denaturation step at 95°c for 15 min followed by a 35 cycles each cycle consist of denaturation at 95°c for 45 s, annealing at 41°c for 45 s and extension at 72 °c for 45 s. A final extension step at 72 °c for 10 min was added. With the help of ethidium bromide staining (Vivantis Technologies, Malaysia), the PCR products were electrophoresed and visualized using 1% agarose gel (Haddadi et al., 2019).
2.5 Sequencing of DNA and data analysis
For molecular identification of collected species, DNA sequencing was performed at the DNA Sequencing Unit of Macrogen (Seoul, Korea), a precision medicine and biotechnology company. Obtaining sequences were edited using Sequencher v4.0.5 software (Gene Codes Corp., United States), identified using the Basic Local Alignment Search Tool (BLAST; National Centre for Biotechnology Information [NCBI]) (Samerjai et al., 2020).
Regarding to insect succession and number of collected specimens (form each identified species), the Obtained data were represented as mean ± SE and subjected to one-way (ANOVA) tests. All statistical analysis procedures were carried out with SPSS statistics 26 software (SPSS Inc., Chicago, IL); when p-values were ≤0.05, differences between means were considered significant.
3 Results
3.1 Carcass decomposition
The carcasses of both mice and rats undergo four decomposition stages: fresh, bloated, decay, and dry remains, determined by the physical changes to carcasses (Figs. 1 and 2; Table 2). The mean temperatures (both minimum and maximum) recorded during each stage are displayed in Table 1. The decomposition process for rat carcasses lasted for an average of 27.66 and 39.66 days on the building roof and in the botanical garden, respectively. However, for mice carcasses, decomposition lasted for an average of 14.75 and 26.33 days on the building roof and in the botanical garden, respectively.
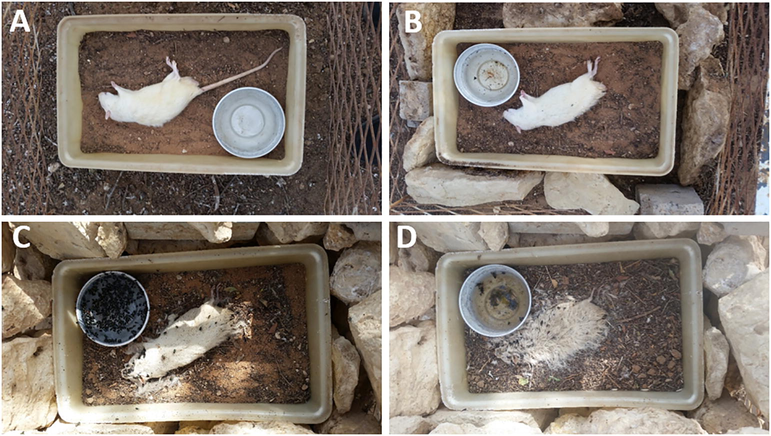
- Stages of rat, Rattus norvegicus, decomposition: (A) Fresh, (B) Bloat, (C) Decay, and (D) Dry.
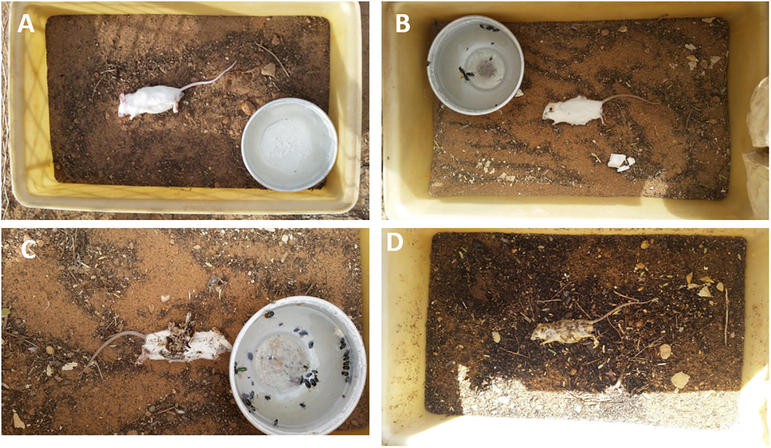
- Stages of mice, Mus musculus, decomposition: (A) Fresh, (B) Bloat, (C) Decay, and (D) Dry.
| Carcass type | Habitat | Duration of decomposition (days) | ||||
|---|---|---|---|---|---|---|
| Stage | ||||||
| Fresh | Bloat | Decay | Dry | Total | ||
| Rat | Botanical garden | 1 | 4.33 ± 0.33 | 24 ± 0.58 | 10.33 ± 0.33 | 39.66 |
| Rooftop | 1 | 3.33 ± 0.33 | 19 ± 0.58 | 4.33 ± 0.67 | 27.66 | |
| Mice | Botanical garden | 1 | 2 ± 0.0 | 14 ± 0.58 | 9.33 ± 0.88 | 26.33 |
| Rooftop | 0.42 | 1.66 ± 0.33 | 9 ± 0.58 | 3.67 ± 0.33 | 14.75 | |
3.2 Insect succession on carcasses in different stages of decomposition and habitats
The mean number of flies recovered from the different decomposition stages of rat and mice carcasses at the two study sites was 1,748, representing 11 families and 14 species. Insect number and species composition differed between decomposition stages and habitats. The results of the statistical analysis showed significant variations in the abundance of insects depending on the habitat and the stages of decomposition (p ≤ 0.05; Table 3).
| Carcass type | Habitat | Duration of decomposition (days) | df | F | |||
|---|---|---|---|---|---|---|---|
| Stage | |||||||
| Fresh | Bloat | decay | dry | 3 | |||
| Rat | Botanical garden | 7 ± 1.53c A |
129 ± 9.96b A |
1123.33 ± 16.83a A |
101.33 ± 8.88b A |
3 | 2376.12 |
| Rooftop | – | 60 ± 6.43b B |
118 ± 5.77 a B |
31 ± 3.79c B |
3 | 113.33 | |
| Mice | Botanical garden | 1 ± 0.58c B |
21.33 ± 2.85b C |
92 ± 3.21 a B |
24.33 ± 2.60b BC |
3 | 245.48 |
| Rooftop | 1 ± 00d B |
12 ± 2.00b C |
36 ± 1.53a C |
6.33 ± 0.88Cb C |
3 | 134.19 | |
| df | 3 | 3 | 3 | 3 | |||
| F | 15.38 | 74.99 | 3309.09 | 69.13 | |||
The same lowercase letters in the horizontal column means there is no significance at p ≤ 0.05.
The same uppercase letters in the vertical column means there is no significance at p ≤ 0.05.
Fourteen dipteran species attracted to rat carcasses in the botanical garden were identified as the following: Chrysomya albiceps (Wiedemann, 1830), Chrysomy megacephala (Fabricius, 1794), Musca domestica (Linnaeus, 1758), Musca sorbens (Wiedemann, 1830), Atherigona sp., Sarcophaga dux (Thomson 1868), Sarcophaga ruficornis (Fabricius, 1794), Megaselia scalaris (Loew, 1866), Drosophila birchii (Dobzhansky & Mather, 1961), Drosophila jambuli (Parshad & Paika, 1965) (D. jambuli), Leucophenga sp., Physiphora alceae (Preyssler, 1791), Chiastocheta trollii (Zetterstedt, 1845) and Crossopalpus aeneus (Walker, 1871). There were also many flies that were identified only to the family level, belonging to the families Milichiidae, Ephydridae, and Psychodidae. On other hand, rat carcasses placed on the building rooftop attracted only nine dipteran species (C. albiceps C. megacephala, M. domestica, M. sorbens, S. dux, Me. scalaris, Leucophenga sp., P. alceae, and Atherigona sp. Table 4). Nine dipteran species (C. albiceps C. megacephala, M. domestica, M. sorbens, Atherigona sp., S. dux, M. scalaris, P. alceae, and Ch. trollii) were attracted to mice carcasses placed in the botanical garden, with only four such species attracted to those placed on the building roof (Table 4).
| Family | Species | Habitat | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat carcasses | Mice carcasses | ||||||||||||||||
| Botanical garden | Rooftop | Botanical garden | Rooftop | ||||||||||||||
| Fresh | Bloat | Decay | Dry | Fresh | Bloat | Decay | Dry | Fresh | Bloat | Decay | Dry | Fresh | Bloat | Decay | Dry | ||
| Calliphoridae | Chrysomya albiceps | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Chrysomya megacephala | √ | √ | √ | √ | √ | ||||||||||||
| Muscidae | Musca sorbens | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Musca domestica | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Atherigona sp. | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Sarcophagidae | Sarcophaga dux | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Sarcophaga ruficornis | √ | √ | |||||||||||||||
| Phoridae | Megaselia scalaris | √ | √ | √ | √ | √ | |||||||||||
| Ulidiidae | Physiphora alceae | √ | √ | √ | √ | √ | |||||||||||
| Drosophilidae | Drosophila birchii | √ | √ | ||||||||||||||
| Drosophila jambulina | √ | ||||||||||||||||
| Leucophenga sp. | √ | √ | √ | ||||||||||||||
| Anthomyiidae | Chiastocheta trollii | √ | √ | √ | √ | √ | |||||||||||
| Hybotidae | Crossopalpus aeneus | √ | |||||||||||||||
| Milichiidae | √ | √ | |||||||||||||||
| Ephydridae | √ | ||||||||||||||||
| Psychodidae | √ | ||||||||||||||||
From the previous results, it is clear that greater number of species were attracted to rat carcasses than mice, placed in the same location. Also more insect species were attracted to carcasses in the botanical garden than on the building roof. Also, results showed that a greater number of species are attracted to rat carcasses than mice carcasses placed in the same location. Additionally, regardless of the rodent type, more insect species were attracted to carcasses in the botanical garden than on the building roof (Fig. 3).
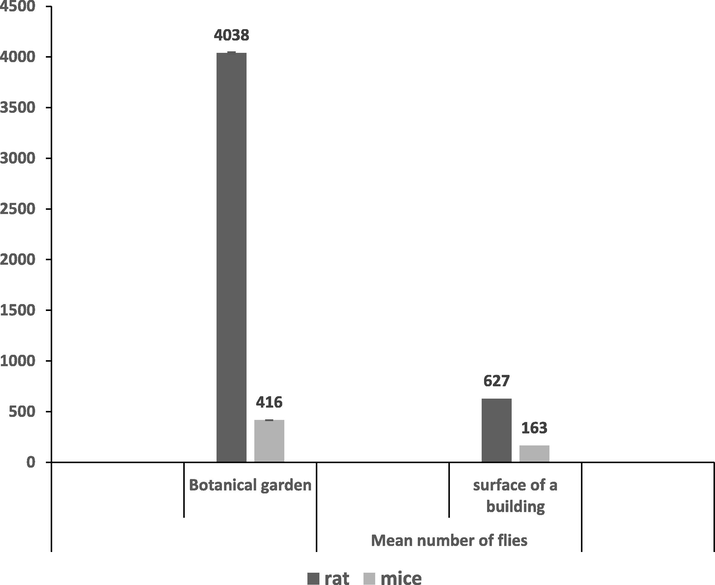
- Abundance of flies in the two different study habitats, botanical garden and rooftop.
In addition to species number, the total number of flies was also affected by carcass type and location. Tables 3–6 showed the mean fly abundance and the number of flies attracted to different decomposition stages of rat and mice carcasses in different habitats.
| Family | Species | Habitat | |||||
|---|---|---|---|---|---|---|---|
| Rat | Mice | ||||||
| Botanical garden | Rooftop | Botanical garden | Rooftop | df | f | ||
| Calliphoridae | Chrysomya albiceps | 633 ± 9.29a A |
51 ± 2.08b B |
57 ± 8.62b A |
18.67 ± 3.18c A |
3 | 2787.88 |
| Chrysomya megacephala | 35.33 ± 2.19a F |
7.33 ± 1.45b EFG |
8.33 ± 1.20b CD |
– | 3 | 115.43 | |
| Muscidae | Musca sorbens | 118.67 ± 8.11a C |
35.67 ± 2.33b C |
8.33 ± 0.88c CD |
0 ± 00c B |
3 | 163.12 |
| Musca domestica | 194 ± 7.00a B |
67 ± 3.61b A |
12 ± 2.52c C |
16 ± 1.00c A |
3 | 416.25 | |
| Atherigona sp. | 86 ± 3.79a D |
8 ± 1.15b EF |
10 ± 2.65b C |
– | 3 | 285.65 | |
| Sarcophagidae | Sarcophaga dux | 74 ± 3.21a ED |
20 ± 3.46b D |
23 ± 2.52b B |
16.67 ± 2.91b A |
3 | 101.50 |
| Sarcophaga ruficornis | 26.33 ± 4.10a FG |
– | – | – | 3 | 41.33 | |
| Phoridae | Megaselia scalaris | 5 ± 0bc H |
11 ± 2.08a E |
8.67 ± 1.45ab CD |
– | 3 | 14.28 |
| Ulidiidae | Physiphora alceae | 58.33 ± 2.67a E |
6 ± 1.53b EFG |
5.67 ± 1.20b CD |
4 ± 0.58b B |
3 | 251.63 |
| Drosophilidae | Drosophila birchii | 6.67 ± 1.67a HG |
– | – | – | 3 | 16.00 |
| Drosophila jambulina | 6 ± 1.00a HG |
– | – | – | 3 | 36.00 | |
| Leucophenga sp. | 16 ± 2.31a HFG |
3 ± 1.73b FG |
– | – | 3 | 27.96 | |
| Anthomyiidae | Chiastocheta trollii | 17 ± 2.89a HFG |
– | 5.33 ± 0.33b CD |
– | 3 | 30.43 |
| Hybotidae | Crossopalpus aeneus | 3.67 ± 1.20a H |
– | – | – | 3 | 9.31 |
| Milichiidae | 3.33 ± 2.03a H |
– |
– | – | 3 | 2.70 | |
| Ephydridae | 3.33 ± 0.88a H |
– | – | – | 3 | 14.29 | |
| Psychodidae | 10 ± 0.58a HG |
– | – | – | 3 | 300.00 | |
| df | 17 | 17 | 17 | 17 | |||
| F | 1431.46 | 175.70 | 68.71 | 38.76 | |||
The same lowercase letters in horizontal columns mean there is no significance at p ≤ 0.05.
The same uppercase letters in vertical columns mean there is no significance at p ≤ 0.05.
| Family | Species | Decomposition stage | |||||
|---|---|---|---|---|---|---|---|
| Fresh | Bloat | Decay | Dry | df | f | ||
| Calliphoridae | Chrysomya albiceps | – | 12.92 ± 19.03b A |
156.25 ± 68.03a A |
3.83 ± 1.22b ABC |
3 | 4.93 |
| Chrysomya megacephala | – | 1.83 ± 1.01a AB |
2 ± 0.63a B |
2.17 ± 0.71a BCD |
3 | 2.14 | |
| Muscidae | Musca sorbens | – | 3 ± 1.80ab BC |
7.33 ± 1.56a B |
2.75 ± 1.37sb ABCD |
3 | 4.85 |
| Musca domestica | – | 4.75 ± 2.02b B |
15.67 ± 4.25 s B |
6.33 ± 1.02b A |
3 | 7.44 | |
| Atherigona sp. | 0.50 ± 0.23b AB |
1.17 ± 0.42b BC |
4.83 ± 1.28a B |
0.50 ± 0.29b CD |
3 | 8.80 | |
| Sarcophagidae | Sarcophaga dux | 0.25 ± 0.13c AB |
4 ± 0.67b BC |
11.33 ± 0.82a B |
5.08 ± 0.79b AB |
3 | 47.85 |
| Sarcophaga ruficornis | – | – | 4.58 ± 2.42a B |
2 ± 1.22a BCD |
3 | 2.57 | |
| Phoridae | Megaselia scalaris | – | – | 18.75 ± 6.54a B |
1.25 ± 0.65b CD |
3 | 7.80 |
| Ulidiidae | Physiphora alceae | – | 2.33 ± 1.36b BC |
14.83 ± 5.09a B |
1.33 ± 0.72b BCD |
3 | 6.68 |
| Drosophilidae | Drosophila birchii | 0.83 ± 0.47a A |
0.83 ± 0.47a BC |
– | – | 3 | 2.06 |
| Drosophila jambulina | – | – | – | 1.50 ± 0.81a BCD |
3 | 3.41 | |
| Leucophenga sp. | – | – | 4.08 ± 1.84a B |
0.67 ± 0.51ab CD |
3 | 4.21 | |
| Anthomyiidae | Chiastocheta trollii | 0.25 ± 0.18a AB |
0.83 ± 0.30a BC |
2.75 ± 1.02a B |
1.75 ± 0.97a BCD |
3 | 2.29 |
| Hybotidae | Crossopalpus aeneus | – | – | 0.91 ± 0.54a B |
– | 3 | 2.85 |
| Milichiidae | – | 0.25 ± 0.18a C |
0.58 ± 0.43a B |
– | 3 | 1.38 | |
| Ephydridae | – | – | 0.83 ± 0.47a B |
– | 3 | 3.09 | |
| Psychodidae | – | – | 2.50 ± 1.31a B |
– | 3 | 3.63 | |
| df | 17 | 17 | 17 | 17 | |||
| f | 2.86 | 12.52 | 4.94 | 5.80 | |||
The same lowercase letters in the horizontal column means there is no significance at p ≤ 0.05.
The same uppercase letters in the vertical column means there is no significance at p ≤ 0.05.
For rat carcasses placed in the botanical garden, C. albiceps and M. domestica were the two most abundant species, followed by M, sorbens, Atherigona sp, S. dux, and P. alceae. For rat carcasses placed on the roof of the building, M. domestica was the most abundant species by far, followed by C. albiceps, M. sorbens, and S. dux. In comparison, for mice carcasses, C. albiceps was the most abundant species in the botanical garden, followed by S. dux and Atherigona sp., while C. albiceps was also the dominant species on the building roof.
This study indicates that there are clear relationships between specific fly species and stages of decomposition (Table 6). The fresh stage attracted only four species of fly and a low number of specimens in general. Drosophila birchii was the most abundant species at this stage. Contrastingly, at the bloat stage, nine species were attracted; C. albiceps was the most abundant species, followed by M. domestica and M. sorbens. All fly species recorded in this study were observed at the decay stage with the exception of D. birchii and D. jambulina. Chrysomya albiceps flies were the most frequently recorded overall during this stage (p ≤ 0.05). At the dry stage, 12 fly species were frequently recorded at low numbers; M. domestica was the most abundant species at this stage.
4 Discussion
Depending on the type of carcass and the duration of the stages, the categorization of the decomposition differs in different environments. In rat and mice carcasses, approximately five decomposition stages were observed by Moretti et al. (2008). However, Kočárek (2003) reported four different decomposition stages in rat remains. The decomposition stages used in the current study followed the classifications of Reed (1958), which are also widely followed by many other authors (Al-Shareef and Al-Mazyad, 2016; Mashaly, 2017). Morris (1988) reported that the most satisfactory description of different decomposition stages is Reed’s classification, and recommended its approval by all researchers in the field of medicolegal entomology.
The time taken for rat carcasses to fully decompose was greater than that of mice. Additionally, within both types of remains, it took longer for bodies to decompose in the botanical garden compared to on the building roof. This variation might be due to the types and sizes of carcasses, study sites, variations in climatic conditions, and insect activities and abundance (Charabidze et al., 2017). Variation in microclimate depending on the location of carcasses relative to trees and shrubs obviously also influenced the decay rate (Mashaly and Al-Mekhlafi, 2016).
The high average temperature (Table 1) of the rooftop is likely to have contributed to the faster decomposition rate in this location. We also observed that the bodies of mice placed on the roof surface were embalmed. This mummification may be due to dehydration of the small carcasses through the combination of high temperature and low humidity (Probst et al, 2020) and this may explain the lower number of insect species that attracted to carcasses placed on the building roof. No previous study has investigated insect succession on rat and mice carcasses in Saudi Arabia. However, Kočárek (2003) observed that rat carcasses took an average of 48.5 days to decompose in forest and 44 days in meadow habitat during spring in the Czech Republic.
Collected insects from the present study were identified morphologically and molecularly based on mitochondrial COI gene. The mt COI gene proved to be a good genetic marker to identify insects of different species of forensic importance. Chen et al. (2004) identified forensically important blow fly using COI gene in Taiwan. Also, Alessandrini et al. (2008) used the mt COI and mtCOII genes to identify insect samples collected from different human corpses in Italy. Moreover, Mashaly et al. (2018) used the same gene to identify carrion beetles in Saudi Arabia.
The number of species attracted to the different carcasses in the two locations ranged from fourteen to four. Ch. albiceps, M. sorbens, Atherigona sp., and M. scalaris were the most abundant species found across all carcasses in both locations. These species have been documented as important insects in many Saudi Arabian studies, including in Al-Baha (Abouzied, 2014), Jeddah (Al-Shareef and Al-Mazyad, 2016), Riyadh (Haddadi et al., 2019; Al-Qahtnia et al., 2019), and Al-Ahsaa (Shaalan et al., 2017). A number of the dipteran families that we identified are here reported as forensic insects for the first time in Saudi Arabia (Drosophilidae, Anthomyiidae, Hybotidae, Milichiidae, and Ephydridae). Drosophilidae and Anthomyiidae have previously been reported from rat carrion in Central Africa (Feugang et al., 2012), the presence of Crossopalpus humilis (Frey, 1913 Hybotidae) was recorded in the Iberian Peninsula on pig carcasses (Ventura et al., 2012), and Desmometopa sp. (Milichiidae) has been recovered from a human corpse in Malaysia (Kumara et al. 2012). Furthermore, Ephydridae species have been found on rat carrion in South Carolina (Tomberlin and Adler, 1998), and on rabbit carrion in Central Africa (Feugang et al., 2012).
Our results identified a number of Calliphoridae, Muscidae and Sarcophagidae flies, confirming that there is a range of species present throughout the winter study period. A higher diversity of arthropods has been found on rat and mice (Moretti et al., 2008) during winter. This is possibly because carcasses take a longer time to decay at lower temperatures and relative humidity, thereby permitting the arrival of a wider array of arthropods. Nonetheless, continued investigation of different strategies for dealing with cadavers of different sizes should be considered, as this may explain the different indices of fly diversity. In our study, the species and therefore the dimensions of the carcasses affected the frequency of adult insect and species richness and this is supported by the study of Moleón et al., 2015; Moretti et al., 2008). However, Norris (1965), reported that this parameter is only different when carcasses differ a great deal in size.
In conclusion, this study successfully identified many forensic important insect species. Also, it was clear that type, size and place of carcasses are factors affect insect succession and species richness on specific carcass in a specific environment. Such data are valuable for future studies into forensic entomological analysis in this region.
Acknowledgement
Research Supporting Project number (RSP-2019/112), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Forensic insects attracted to human cadavers in a vehicular environment in Riyadh, Saudi Arabia. Saudi J. Biol. Sci.. 2019;26:1499-1502.
- [Google Scholar]
- Insect colonization and succession on rabbit carcasses in southwestern mountains of the kingdom of Saudi Arabia. J. Med. Entomol.. 2014;51:1168-1174.
- [Google Scholar]
- DNA analysis for genetic identification of forensically important insects. Forensic Sci. Int.: Genet. Suppl. Series. 2008;1(1):584-585.
- [Google Scholar]
- Characterization of forensically important necrophagus flies (Diptera) of Jeddah, Saudi Arabia. Adv. Environ. Biol.. 2015;9(8):58-72.
- [Google Scholar]
- Insect faunal succession on decaying rabbit carcasses in urban area at Jeddah city, Kingdom of Saudi Arabia. Am. J. Sci.. 2016;12:78-88.
- [Google Scholar]
- Applicability of partial characterization of cytochrome oxidase I in identification of forensically important flies (Diptera) from China and Egypt. Parasitol. Res.. 2013;112:2667-2674.
- [Google Scholar]
- Insects of forensic importance. Forensic entomology: the utility of arthropods in legal investigations (2nd ed.). Boca Raton, FL: CRC Press; 2001. p. :43-79.
- Use of necrophagous insects as evidence of cadaver relocation: myth or reality? PeerJ. 2017;5:e3506
- [Google Scholar]
- Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J. Med. Entomol.. 2004;41(1):47-57.
- [Google Scholar]
- Postmortem chemistry: practical considerations and a review of the literature. J. Forensic Sci.. 1974;19(1):13-32.
- [Google Scholar]
- Ecological succession of adult necrophilous insects on neonate Sus scrofa domesticus in central North Carolina. PLoS One. 2018;13:e0195785
- [Google Scholar]
- Biodiversity study of arthropods collected on rat carrion in Yaounde, Cameroon: first study on forensic entomology in Central Africa. Int. J. Biosci.. 2012;2(1):1-8.
- [Google Scholar]
- A model of the complex between single-stranded DNA and the single-stranded DNA binding protein encoded by gene V of filamentous bacteriophage M13. J. Mol. Biol.. 1994;240:341-357.
- [Google Scholar]
- Evolution of the Insects. Cambridge: Cambridge University Press; 2005. xv+755 pp.
- A Comparative Study of Insect Succession on Rabbit Carrion in Three Different Microhabitats. J. Med. Entomol.. 2019;56(3):671-680.
- [Google Scholar]
- Introduction: perceptions and status of forensic entomology. In: Byrd J.H., Castner J.L., eds. Forensic entomology: the utility of arthropods in legal investigations (2nd ed.). Boca Raton, FL: CRC Press; 2001. p. :1-15.
- [Google Scholar]
- Efficacy of entomological method in stimation of postmortem interval: A comparative analysis. Forensic Sci. Int.. 1989;40(3):245-250.
- [Google Scholar]
- Decomposition and Coleoptera succession on exposed carrion of small mammal in Opava, the Czech Republic. Eur. J. Soil Biol.. 2003;39(1):31-45.
- [Google Scholar]
- Occurrence of oriental flies associated with indoor and outdoor human remains in the tropical climate of north Malaysia. J. Vector Ecol.. 2012;37(1):62-68.
- [Google Scholar]
- Blow flies (Diptera: Calliphoridae) of eastern Canada with a key to Calliphoridae subfamilies and genera of eastern North America, and a key to the eastern Canadian species of Calliphorinae, Luciliinae and Chrysomyiinae. Can. J. Arthropod Identif.. 2011;11:1-93.
- [Google Scholar]
- Succession pattern of carrion feeding insects in Paramo, Colombia. Forensic Sci.. 2007;166:128-189.
- [Google Scholar]
- Carrion beetles succession in three different habitats in Riyadh, Saudi Arabia. Saudi J. Biol. Sci.. 2017;24:430-435.
- [Google Scholar]
- Differential Diptera succession patterns on decomposed rabbit carcasses in three different habitats. J. Med. Entomol.. 2016;53(5):1192-1197.
- [Google Scholar]
- Molecular identification of the carrion beetles (Coleoptera) in selected regions of Saudi Arabia. J. Med. Entomol.. 2018;55(6):1423-1430.
- [Google Scholar]
- Carcass size shapes the structure and functioning of an African scavenging assemblage. Oikos. 2015;124:1391-1403.
- [Google Scholar]
- Insects on decomposing carcasses of small rodents in a secondary forest in Southeastern Brazil. Eur. J. Entomol.. 2008;105(4):691-696.
- [Google Scholar]
- Morris, B., 1988. Carcass decomposition and early arthropod succession, pp. 267. In Proceedings of the 18th International Congress of Entomology, 3-9 July 1988, Vancouver, Canada.
- A study of dog carcass communities in Tennessee, with special references to the insects. Am. Midl. Nat.. 1958;59:213-245.
- [Google Scholar]
- Rivers, D. B., Dahlem G. A., 2014. The science of forensic entomology, p. 400. Wiley-Blackwell, UK. ISBN: 978-1-119-94037-1.
- Mitochondrial DNA-Based Identification of Forensically Important Flesh Flies (Diptera: Sarcophagidae) in Thailand. Insects. 2020;11(1):2.
- [Google Scholar]
- A Preliminary Study of Insect Succession in Al-Ahsaa Oasis, in the Eastern Region of the Kingdom of Saudi Arabia. J. Forensic Sci.. 2017;62(1):239-243.
- [Google Scholar]
- Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am.. 1994;87:651-701.
- [Google Scholar]
- Seasonal colonization and decomposition of rat carrion in water and on land in an open field in South Carolina. J. Med. Entomol.. 1998;35(5):704-709.
- [Google Scholar]
- Ventura, D., Díaz, B., Saloña, M., 2012. Crossopalpus Humilis (Frey, 1913) En La Península Ibérica Y La Relación De La Familia Hybotidae Con Cadáveres De Vertebrados (Diptera: Empidoidea: Hybotidae). Boletín De La Sociedad Entomológica Aragonesa (S.E.A.). 50, 527-532.
- The succession and evelopment of insects on pig carcasses and their significances in estimating PMI in south China. Forensic Sci. Int.. 2008;179(1):11-18.
- [Google Scholar]







