Translate this page into:
A study of fermentation characteristics, nutrient content, and microbial population of triticale silage produced with different lactic acid bacteria: Long-term preserved triticale silage for livestock
*Corresponding author: Email address: choiwh@korea.kr (K. Choon Choi)
-
Received: ,
Accepted: ,
Abstract
There has been a lot of attention paid to lactic acid bacteria (LAB) in forage fermentation due to rapid acidification through the production of lactic acid (LA). Triticale is considered one of the best crops to develop high-quality silage due to its high crude protein (CP) content. The objectives of the present study is to develop high quality triticale silage with Pediococcus pentosaceus (2), Lactobacillus plantarum (1), and Lactobacillus rhamnosus (1) as single or cocktail forms and stored for 6 and 12 months. Triticale was wilted under field conditions and then manually cut into 1.5-2.5 cm lengths. Samples of 250g per bag were placed in 28 x 36 cm polythene bags. Different types of LAB were used to ferment the triticale at different moisture conditions by ensiling process. Vacuum sealed bags were stored in laboratory conditions for 6 and 12 months. After the storage period, microbial profiles, fermentative acids, and nutritional content were determined. A correlation triangle matrix was used to determine interactions among fermentative metabolites, nutritive values, and microbes using Python software. A significant reduction in pH was observed for both high moisture (HM) and low moisture (LM) silages produced with LAB in either individual or cocktail form compared to non-inoculum silage. On 6-month fermentation, the pH range of control and inoculum-treated HM silage was 6.11 ± 0.03 to 4.02 ± 0.08, and LM silage was 6.05 ± 0.09 to 3.98 ± 0.52. The pH was reduced in a similar manner on month 12. In addition, LAB significantly increased LA content from 0.48 ± 0.19 to 6.58 ± 0.28 DM% in HM and 0.00 ± 0.00 to 4.34 ± 0.19 DM% in LM on month 6. Silage fermented for 12 months also retained its LA content. Despite this, inoculated silage had higher levels of LA than control silage. Butyric acid (BA) content was significantly lower in inoculum-treated silage than in non-treated silage. In both experimental silages, marginal levels of AA were produced. High LAB and lower yeast and mold counts were found in inoculum-treated silage compared to non-inoculum silage. A cocktail of LAB treatments significantly increased LA content in silages over non-inoculum or single LAB treatments. The correlation study revealed that LA positively correlated with LAB and negatively interacted with yeast and mold. In this study, either single or cocktail LAB treatments significantly improved silage fermentation quality through increased LA content and reduced undesirable microbial populations. Cocktail LAB has a greater potential than single LAB. This evidence suggests that silage developed by combining multiple strains as a cocktail is more suitable for long-term storage of livestock.
Keywords
Cocktail LAB
Fermentation
Fermentative acids
LAB
Microbes
Nutrients
Triticale
1. Introduction
It has become increasingly important to develop high quality silage from grasses and legumes with enriched fermentative metabolites because animal farming has increased dramatically. Triticale is a hybrid variety of wheat and rye that produces more biomass and CP than wheat alone, making it useful in livestock animal feed production. The chemical composition of triticale is closer to wheat than rye; so, it could be used as a food source for humans and animals. In recent years, triticale has been considered good forage for making high-quality silage for livestock due to its high CP content (Harper et al., 2017; Soundharrajan et al., 2019; Lin et al., 2021; Jung et al., 2022; Negi et al., 2022). It is well known that microbial additives have gained considerable attention for the purpose of achieving a controlled fermentation of plant-based silages and increasing their digestibility, as evidenced by the fact that inoculants have been developed as silage additives for over four decades (Okoye et al., 2023). LA bacteria have been extensively studied for their potential to improve human and animal health (Amaral et al., 2020). Furthermore, LAB is widely regarded as an efficient and reliable method of storing forage in animal production (Guo et al., 2023). Researchers have found that understanding biological transitions in the ensiling process has helped them focus on unique strains that are becoming more efficient during silage ensiling (Xu et al., 2019). Due to their high safety and feasibility, non-corrosiveness, eco-friendliness, improved dry matter (DM) recovery, fermentation characteristics, and animal performance, LAB is considered to be a more suitable inoculum for silage production than the other additives (Okoye et al., 2023). LAB produces LA, AA, BA, succinic acid, and other organic acids during ensiling. Silage acidification inhibits microbial growth and prevents aerobic degradation (Muck 2010; Muck et al., 2018). Animal health and food quality can also be affected by spoilage microorganisms. LAB has been used for many years to improve the silage quality at LM and HM conditions and to increase milk production, body weight, and feed efficiency (Muck et al., 2018). Addition of LAB additives to crop biomass during ensiling to increase the positive fermentation, reduce DM loss, reduction of aerobic deterioration during feed out, improve the hygienic quality of the silage and aerobic stability restrict or limit the secondary fermentation via inhibition of undesirable microbes, increase the nutritive values of the silage, and mitigate the methane emission (Kaewpila et al., 2021; Ridwan et al., 2023).
Lactobacillus sp, pediococcus sp, and enterococcus sp are the major genera involved in fermenting silage anaerobically. Inoculants used intensively for silage production are Lactobacillus plantarum, Lactobacillus brevis, Pentosaceus pentosaceus, Lactobacillus rhamnosus, and Lentilactobacillus bucbneri, as well as Enterococcus faecium (Ogunade et al., 2019; Puntillo et al., 2020; Jung et al., 2022). Ensiled forage material deteriorates in the presence of oxygen when the silo is opened, particularly lactate-assimilating yeast. When silage temperature and pH rise, undesirable microbial growth occurs. In order to address this issue, LA bacteria have been inoculated into silage for many decades (Filya 2003; Kung et al., 2018). Direct ensiling is difficult due to the limited number of LAB in native plants. In recent years, new LABs have been isolated to produce silage, yet this activity remains important worldwide (Paradhipta et al., 2020; Dos Santos Leandro et al., 2021), since more strains are being sought not only as silage inoculants, but also for other plant-based foods for humans and animals (Wuyts et al., 2020). LAB strains promote silage fermentation as a single culture in most studies. It is interesting to note that silage produced with LAB in mixed cultures (more than one strain) accelerated its acidification, enhanced its LA production, and reduced BA by its synergistic effects, compared with silage produced with single LAB (Filya 2003; Zhang et al., 2023). Considering these factors, we examined different types of LA bacteria such as Pediococcus pentosaceus (2), Lactobacillus plantarum (1), and Lactobacillus rhamnosus (1) effects on acidification, fermentative metabolites, microbial population, and nutrient levels in triticale silage at the different moisture conditions after 6 and 12 months. In addition, a triangle map with a correlation matrix was used to determine the interactions among microorganisms, organic acids, and nutrient contents of the experimental silages.
2. Materials and methods
2.1 Preparation of inoculants for silage production
The LA bacteria such as L. plantarum- KCC - 34 (GeneBank No: KP091750.1); L. plantarum- KCC - 48 (GeneBank No: MT318652.1), P. pentosaceus - KCC-45 (GeneBank No: MN049504), P. pentosaceus - KCC-53 (GenBank No: MZ505239), and L. rhamnosus – KCC-54 (GenBank No: MZ505240) were isolated from various sources (Alfalfa, and Triticale), and published previously (Soundharrajan et al., 2023). The LAB isolates were cultured in MRS broth (CONDA, Madrid, Spain) and incubated at 37°C for 30 h with mild shaking at 150 rpm in an orbital shaker under a micro aerobic environment. After incubation, colonies were collected by centrifugation at 4000 g for 45 minutes at 4°C. The total bacterial colonies were calculated by a Quantom Tx Microbial cell counter (Logos Bio-system, Gyeonggi-do, South Korea). The collected pellets were washed twice with PBS, pH 7.4. After that, the bacterial pellets were diluted with sterile distilled water.
2.2 Production of triticale silage at heading stage with LAB
Triticale (Joseong cultivar) at heading stage from Jangsoo, Chunbuk (latitude: 35.6185318, longitude: 127.5107881), Korea, was collected and allowed wilted for 8–10 hours or 24–36 hours for HM and LM silage, respectively (Jung et al., 2022). After reaching the expected moisture level (HM: 62 ± 1.8%; LM: 43 ± 0.2%), the grass was manually cut into 1.5-2.5 cm size. Samples of 250g/bag were placed in 28 x 36 cm silage bags (Aostar Co., Ltd., Seoul). There were eight groups of five replicas each (n=5). Experimental groups included the non-inoculant group, L. plantarum- KCC - 34 group, P. pentosaceus- KCC - 45 group, L. plantarum - KCC - 48 group, P. pentosaceus- KCC - 53 group, L. rhamnosus- KCC - 54 group, cocktail-I group (KCC - 45+48+53), and cocktail-II group (KCC - 34+45+54). Density of LAB/ gram of forage was 105/CFU. Bags were vacuum sealed (MK Corporation, Seoul, Korea, Food Saver V48802). The bags were stored for 6 and 12 months in the laboratory.
2.3 Sampling and analysis of fermentative acids
For organic acids analysis, 10gms of each sample were mixed with 90 mL of water and kept in an orbital shaker for an hour. After passing through multiple layers of cheesecloth and filter membrane (0. 2 μm), the pH of the filtrate was measured with a pH meter (Thomas Scientific, NJ, USA). For organic acid analysis, samples were reduced to a pH of 2 with 50% sulfuric acid and frozen at -20°C for high-performance liquid chromatography system (HPLC) analysis. In order to determine the LA content, a HPLC (HP1100, Agilent Co., USA) was used. To elute the sample, 0.1 M H2SO4 was used on a Hi-Plex Ligand exchange column from Agilent (300 x 7.7 mm). The wavelength was fixed at 220 nm, and the flow rate was 0.6 mL/min. To determine the AA and BA content of silage, a CP7485 column fused with silica gel (length–25 cm, diameter–0.32, and film thickness–0.30) was used at temperatures ranging from 20°C to 270°C. Flow rates of 10 microliters per minute were used (Arasu et al., 2014; Jung et al., 2022).
2.4 Analysis of nutrient contents
The samples were weighed before being dried at 60°C in an oven. The DM of each sample was calculated immediately, and the samples were then pulverized and stored for further analysis. The Kjeldahl method is used to calculate the CP content in samples (AOAC 1990). The contents of acid detergent fiber (ADF) and neutral detergent fiber (NDF) were also determined (Van Soest et al., 1991).
2.5 Microbial population enumeration in experimental samples
The samples were filtered with sterilized cheesecloth, serially diluted 10 times in sterile distilled water, and 0.1 mL of each sample was poured onto a MRS agar plate (MRS agar, CONDA, Madrid, Spain) and incubated for 48 hours at 37°C under aerobic conditions. In addition, one mL of the diluted sample was spread on Petrifilm (3M microbiology products, St. Paul, USA) and incubated at 37°C for 70 to 120 hours. A specific incubation period was used to count yeast and mold (Jung et al., 2022).
2.6 Statistical analysis
A randomized strategy was followed with eight treatments and five replicates per treatment. The least significant difference test was used to investigate significant differences using SPSS16 software (one-way ANOVA, multivariate analysis, post-hoc, Duncan, and descriptive analysis parameters). P-values less than 0.05 were used to determine statistical significance. Python software (version 3.12.6, 2024) was used to perform a triangle heatmap correlation among organic acids, microbial population, and nutrient contents of silage.
3. Results
3.1 Acidification of silages in response to LAB treatments
Tables 1 and 2 show the acidification of experimental silages in response to LAB treatments, storage periods, and HM and LM levels. Silage produced without inoculants exhibited higher pH values after 6 months (HM: pH 6.11 ± 0.03 LM: 6.05 ± 0.09) and 12 months (HM: pH 5.15 ± 0.34 and LM: 5.65 ± 0.08). However, LABs produced as a single culture significantly reduced triticale silage pH values. The pH of HM triticale silage varied between 3.96 ± 0.12 and 4.25 ± 0.11 in month 12. For LM triticale silage, pH values ranged from 4.01 ± 0.08 to 4.35 ± 0.12 on month 6, 4.23 ± 0.12 and 4.49 ± 0.06 on month 12. Likewise, triticale silage produced with cocktail-I and cocktail-II significantly reduced pH values under HM conditions ranging between pH 4.02 ± 0.08 and 4.31 ± 0.13 on month 6; 3.96 ± 0.12 and 3.99 ± 0.02 on month 12. For LM, acidification levels varied between pH 3.98 ± 0.52 and 4.09 ± 0.12 on month 6; 3.94 ± 0.08 and 4.07 ± 0.08 on month 12 at heading stage compared to control.
| Treatments | High-moisture (%) | |||
|---|---|---|---|---|
| pH | LA(DM%) | AA(DM%) | BA(DM%) | |
| Control | 6.11 ± 0.03 | 0.48 ± 0.19 | 0.05 ± 0.07 | 0.56 ± 0.26 |
| KCC34 | 4.27 ± 0.04** | 4.62 ± 0.68** | 0.26 ± 0.06 | 0.20 ± 0.14 |
| KCC45 | 4.23 ± 0.05** | 4.11 ± 0.20** | 0.93 ± 0.09* | 0.00 ± 0.00* |
| KCC-48 | 4.25 ± 0.05** | 6.08 ± 0.07** | 0.43 ± 0.10 | 0.10 ± 0.04 |
| KCC-53 | 4.47 ± 0.10** | 4.52 ± 0.25** | 0.16 ± 0.01 | 0.18 ± 0.10 |
| KCC-54 | 4.42 ± 0.05** | 4.44 ± 0.02** | 0.00 ± 0.00 | 0.01 ± 0.01* |
| Cocktail-I | 4.31 ± 0.13** | 6.58 ± 0.28**# | 0.41 ± 0.12# | 0.03 ± 0.01 |
| Cocktail -II | 4.02 ± 0.08**# | 5.69 ± 0.41**# | 0.38 ± 0.04# | 0.00 ± 0.00* |
| Low-moisture (%) | ||||
| Control | 6.05 ± 0.09** | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| KCC34 | 4.21 ± 0.03** | 3.17 ± 0.20** | 0.50 ± 0.02** | 0.03 ± 0.01 |
| KCC45 | 4.23 ± 0.07** | 2.04 ± 0.05** | 0.42 ± 0.06** | 0.00 ± 0.00 |
| KCC-48 | 4.01 ± 0.08** | 4.10 ± 0.20** | 0.55 ± 0.15** | 0.03 ± 0.01 |
| KCC-53 | 4.31 ± 0.09** | 3.06 ± 0.15** | 0.19 ± 0.16 | 0.03 ± 0.02 |
| KCC-54 | 4.35 ± 0.12** | 3.00 ± 0.51** | 0.44 ± 0.06** | 0.02 ± 0.01 |
| Cocktail-I | 3.98 ± 0.52** | 4.34 ± 0.19** | 0.38 ± 0.09** | 0.04 ± 0.01 |
| Cocktail -II | 4.09 ± 0.12** | 3.11 ± 0.26** | 0.54 ± 0.27** | 0.01 ± 0.02 |
KCC-34: L. plantarum; KCC-45: P. pentosaceus; KCC-48: L. plantarum; P. pentosaceus; KCC-53-L. KCC-54: L. rhamnosus; Co-Cultures-I: Cocktail-I: KCC-45+48+53; Cocktail -II: KCC-34+45+54; LA: Lactic acid; AA: Acetic acid; BA: Butyric acid; DM: Dry matter content; LAB: Lactic acid bacteria. High-Moisture: 62 ± 1.8%; Low-Moisture: 43 ± 0.2%. The data are expressed as the mean ± STD of three replicates. **P< 0.01 compared to non-inoculum treatment; *P< 0.05 compared to non-inoculum treatment; #P< 0.05 compared to some single LAB treatment.
| Groups | High-moisture (%) | |||
|---|---|---|---|---|
| pH | LA | AA | BA | |
| Control | 5.15 ± 0.34 | 1.28 ± 0.66 | 0.46 ± 0.04 | 0.48 ± 0.05 |
| KCC34 | 4.08 ± 0.12** | 4.48 ± 0.25** | 0.48 ± 0.10 | 0.06 ± 0.00** |
| KCC45 | 4.25 ± 0.11** | 3.74 ± 0.14** | 1.65 ± 0.17** | 0.00 ± 0.00** |
| KCC-48 | 4.11 ± 0.10** | 3.96 ± 0.24** | 0.67 ± 0.08* | 0.07 ± 0.01** |
| KCC-53 | 3.98 ± 0.09** | 4.84 ± 0.42** | 0.45 ± 0.06 | 0.04 ± 0.02** |
| KCC-54 | 4.02 ± 0.07** | 4.78 ± 0.53** | 0.36 ± 0.28 | 0.10 ± 0.02** |
| Cocktail-I | 3.99 ± 0.02** | 6.22 ± 0.63**# | 0.57 ± 0.06 | 0.05 ± 0.05**# |
| Cocktail -II | 3.96 ± 0.12** | 5.62 ± 0.63**# | 0.63 ± 0.04 | 0.25 ± 0.02* |
| Low-moisture (%) | ||||
| Control | 5.65 ± 0.08 | 0.00 ± 0.00 | 0.29 ± 0.10 | 0.07 ± 0.00 |
| KCC34 | 4.23 ± 0.12** | 2.43 ± 0.25** | 0.29 ± 0.07 | 0.01 ± 0.01** |
| KCC45 | 4.35 ± 0.05** | 2.07 ± 0.13** | 0.32 ± 0.07 | 0.00 ± 0.00** |
| KCC-48 | 4.28 ± 0.05** | 3.21 ± 0.44** | 0.28 ± 0.05 | 0.03 ± 0.01** |
| KCC-53 | 4.34 ± 0.13** | 2.64 ± 0.30** | 0.23 ± 0.03 | 0.03 ± 0.00** |
| KCC-54 | 4.49 ± 0.06** | 2.82 ± 0.22** | 0.41 ± 0.06* | 0.02 ± 0.02** |
| Cocktail-I | 4.07 ± 0.08** | 3.54 ± 0.24**# | 0.23 ± 0.03# | 0.01 ± 0.01**# |
| Cocktail -II | 3.94 ± 0.08** | 3.47 ± 0.23**# | 0.32 ± 0.03 | 0.01 ± 0.01**# |
KCC-34: L. plantarum; KCC-45: P. pentosaceus; KCC-48: L. plantarum; P. pentosaceus; KCC-53-L. KCC-54: L. rhamnosus; Cocktail-I: KCC-45+48+53; Cocktail -II: KCC-34+45+54; LA: Lactic acid; AA: Acetic acid; BA: Butyric acid; LAB: Lactic acid bacteria. High-Moisture: 62 ± 1.8%; Low-Moisture: 43 ± 0.2%. The data are expressed as the mean ± STD of three replicates. **P< 0.01 compared to non-inoculum treatment; *P< 0.05 compared to non-inoculum treatment; #P< 0.05 compared to some single LAB treatment.
3.2 Organic acids production in experimental silages after 6 and 12 months
Tables 1 and 2 show the determination of fermentative metabolites such as LA, AA, and BA in experimental silages at 6 and 12 months. The LA content of control silage were 0.48 ± 0.19 and 1.28 ±0.66 % DM in HM conditions after 6 months and 12 months, respectively. But in LM condition, LA content in non-inoculated silages was not detected at both experimental periods. LAB treatments in single form significantly increased the LA content of both HM and LM silages compared to non-inoculated silages (p<0.01). Among the strains, KCC-48 significantly increased LA content in both HM (6.08 ± 0.07 DM%) and LM silages (4.10 ± 0.20 DM%) compared to other strains as non-inoculum treated silage (p<0.05). At the same time, a higher content of LA was found in HM silage produced with cocktail-I or cocktail-II treatments than in the single LAB treatment (p<0.05). But, LM silage produced with cocktail-I and cocktail-II showed almost similar ranges of LA production compared to single strain treatments except KCC-45 after 6 months. Triticale forage treated with single LAB, cocktail-I or cocktail-II remained higher in LA content after 12 months of fermentation than non-inoculated silages (p<0.01). LA content in HM silage treated with single LAB revealed a wide range of concentrations from 3.74 ± 0.14 to 3.74 ± 0.14 DM% between the strains.
The content of LA in cocktail-I (6.22 ± 0.63 DM%) or cocktail-II (5.62 ± 0.63 DM%) treated silages exhibited higher concentrations than single strain treatments (p<0.05). Same trend was noted for LM silage produced with single LAB treatments (ranging from 2.07 ± 0.13 to 3.21 ± 0.44 DM%) or cocktail-I (3.54 ± 0.24 DM%) or cocktail-II (3.47 ± 0.23 DM%) treatments compared to silage produced with non-inoculants (p<0.01). AA content of experimental silages at different moisture levels at 6 and 12 months is given in Table 1. AA level was significantly increased in silage treated with KCC-34, KCC-45, and KCC-48 as single form treatments compared to non-inoculated silages similarly cocktail-I or cocktail-II treatments also moderately increased AA levels compared to KCC-53, KCC-54, and KCC-34 as well as non-inoculated HM silages (p<0.05). AA level was significantly higher in silage treated with all LAB treatments either single or cocktail form compared to non-inoculated silages (p<0.01) after 6 months (Table 1). After 12 months’ fermentation, the AA content of silage was almost similar between non-inoculum and LAB treatments except KCC-45 (p<0.01) and KCC-48 (p<0.05) in HM silage and KCC-54 and cocktail-I in LM silage (Table 2). LAB treatments reduced the BA content of triticale silage at HM condition where as there are no significant changes in BA level in LM-treated silage between treatments (Table 1). In addition, after 12 months, the BA content of triticale silage produced with single strain or cocktail treatment was significantly decreased compared to non-inoculum treated silage at both moistures condition (p<0.01). Cocktail-I or cocktail-II treatments more strongly reduced the production of BA level of silage than single strain treatments (p<0.05) after 12 months (Table 2).
3.3 Microbial fluctuations in silage fermentation on different storage periods and LAB treatments
LAB, yeast, and mold populations were counted in triticale silages after 6 months with different LAB treatments (Table 3). The number of LAB count was higher in silage treated with different inoculum as single or cocktail form compare to non-inoculum treated silage (p<0.01). The LAB numbers in LM silage varied with inoculant treatments, ranging from 0.11 ± 0.08 to 8.72 ± 1.11 x 107 CFU/g. KCC-48 treatment significantly increased LAB count in triticale silage at both HM (2.4 ± 0.3 CFU/g) and LM (8.72 ± 1.11 CFU/g) conditions after 6 months compared to other strains’ treatment (Table 3). Cocktail-II treatment had higher LAB counts in HM triticale silage (7.6 ± 0.6 CFU/g) than other strains (p<0.05). The LAB counts in HM and LM silage varied with inoculant treatments after 12 months of storage. HM silage had a higher LAB population after 12 months of storage than HM silage after 6 months (Table 4). Both yeast and mold counts were sharply reduced but increased LAB counts after 6 and 12 months when silage treated with different LAB was compared with non-inoculum treated silage (Table 3 and Table 4).
| Groups | High-moisture (%) | Low-moisture (%) | ||||
|---|---|---|---|---|---|---|
| LAB (×107) | Yeast (×106) | Mold (×106) | LAB (×107) | Yeast (×106) | Mold (×106) | |
| Control | 0.3 ± 0.1 | 7.3 ± 1.4 | 0.3 ±0.1** | 0.11 ± 0.08 | 11.5 ± 2.83** | 0.00 ± 0.0 |
| KCC-34 | 1.7 ± 0.2** | 0.5 ± 0.0** | 0.1 ± 0.1** | 0.40 ± 0.40 | 0.22 ± 0.09** | 0.00 ± 0.0 |
| KCC-45 | 1.2 ± 0.3** | 0.6 ± 0.1** | 0.0 ± 0.0** | 1.04 ± 0.08** | 0.31 ± 0.08** | 0.00 ± 0.0 |
| KCC-48 | 2.4 ± 0.3** | 1.2 ± 0.2** | 0.0 ± 0.0** | 8.72 ± 1.11** | 2.09 ± 0.52** | 0.00 ± 0.0 |
| KCC-53 | 1.6 ± 0. 3** | 1.5 ± 0.5** | 0.1 ± 0.0** | 2.12 ± 0.29** | 5.97 ± 1.06** | 0.00 ± 0.0 |
| KCC-54 | 1.5 ± 0.1** | 1.2 ± 0.0** | 0.1 ± 0.0** | 3.23 ± 0.52** | 1.74 ± 0.23** | 0.00 ± 0.0 |
| Cocktail-I | 2.7 ± 0.2**# | 1.2 ± 0.0**# | 0.0 ± 0.0**# | 1.71 ± 0.17** | 2.63 ± 0.54**# | 0.00 ± 0.0 |
| Cocktail-II | 7.6 ± 0.6**# | 1.3 ± 0.0**# | 0.0 ± 0.0**# | 4.05 ± 0.28**# | 7.06 ± 0.28**# | 0.00 ± 0.0 |
KCC-34: L. plantarum; KCC-45: P. pentosaceus; KCC-48: L. plantarum; P. pentosaceus; KCC-53-L. KCC-54: L. rhamnosus; Cocktail-I: KCC-45+48+53; Cocktail -II: KCC-34+45+54, Microbial population (CFU: colony forming unit/ gram), LAB: Lactic acid bacteria. High-moisture: 62 ± 1.8%; Low-moisture: 43 ± 0.2%. The data are expressed as the mean ± STD. **P< 0.01 compared to non-inoculum treatment; *P< 0.05 compared to non-inoculum treatment ; #P< 0.05 compared to some single LAB treatment.
| Groups | High-moisture (%) | Low-moisture (%) | ||||
|---|---|---|---|---|---|---|
| LAB (×107CFU/g) | Yeast (×104 CFU/g) | Mold (×104 CFU/g) | LAB (×107 CFU/g) | Yeast (×104 CFU/g) | Mold (×104 CFU/g) | |
| Control | 0.52 ± 8.5 | 759 ± 60 | 17 ± 0.74 | 0.38 ± 0.1 | 13.5 ± 0.44 | 17.3 ± 3.8 |
| KCC-34 | 23.9 ± 2.0** | 371 ± 33** | 3.3 ± 0.62** | 17.4 ± 4.2** | 3.30 ± 0.33** | 2.33 ± 1.25** |
| KCC-45 | 26.3 ± 1.7** | 121 ± 18** | 5.3 ± 0.09** | 13.8 ± 0.7** | 1.23 ± 0.08** | 1.67 ± 0.94** |
| KCC-48 | 16.7 ± 1.4** | 1.51 ± 0.2** | 0.3 ± 0.47** | 16.7 ± 1.4** | 1.51 ± 0.24** | 0.33 ± 0.47** |
| KCC-53 | 19.6 ± 1.4** | 35.7 ± 6.6** | 0.3 ± 0.05.** | 18.3 ± 4.6** | 4.93 ± 1.28** | 1.00 ± 0.08** |
| KCC-54 | 14.9 ± 1.8** | 23.0 ± 6.4** | - | 18.1 ± 4.4** | 1.60 ± 0.22** | - |
| Cocktail-I | 25.4 ± 3.2**# | 14.6 ± 2.0**# | 0.33 ± 0.47**# | 15.6 ± 2.6** | 4.43 ± 0.11**# | - |
| Cocktail -II | 27.8 ± 1.8**# | 10.3 ± 1.2**# | 0.72 ± 0.90**# | 15.6 ± 2.2** | 0.70 ± 0.25**# | 0.67 ± 0.17** |
KCC-34-L. plantarum; KCC-45-P. pentosaceus; KCC-48-L. plantarum; KCC-53-P. pentosaceus; KCC-54-L.rhamnosus; Co-Cultures-I: KCC-45+48+53; Cocktail-I: KCC-45+48+53; Cocktail-II: KCC-34+45+54; Microbial population (CFU: colony forming unit/gram), LAB: Lactic acid bacteria. High-moisture: 62 ± 1.8%; Low-moisture : 43 ± 0.2%. The data are expressed as the mean ± STD. **P < 0.01 compared to non-inoculum treatment; *P < 0.05 compared to non-inoculum treatment; #P < 0.05 compared to some single LAB treatment.
3.4 Nutrient content of experimental silages
The nutritional contents such as ADF, NDF, and CP of experimental silages were determined after 6 and 12 month storage periods. The content of ADF, NDF, and CP was not changed at significant levels in both non-inoculum and LAB treated silage after 6 and 12 month storage periods (Supplementary Table 1 and Supplementary Table 2).
3.5 Triangle heatmap correlation between fermentative parameters, nutrient contents, and microbial population
An interaction among pH, organic acids, nutrient content, and microbes was investigated using Python software. The pH of the silages was negatively correlated with LA, AA, and LAB populations. In contrast, yeast and mold were positively correlated with pH and negatively interacting with AA in the silages after 6 months in both HM (Fig. 1a) and LM (Fig. 1b) silages. Lactate content of the silages was positively correlated with LAB, and negatively correlated with BA. After 12 months’ storage period, similar patterns of interactions were noted among pH, LAB, LA, AA, yeast, and mold in both HM (Fig. 1c) and LM (Fig. 1d) silages.
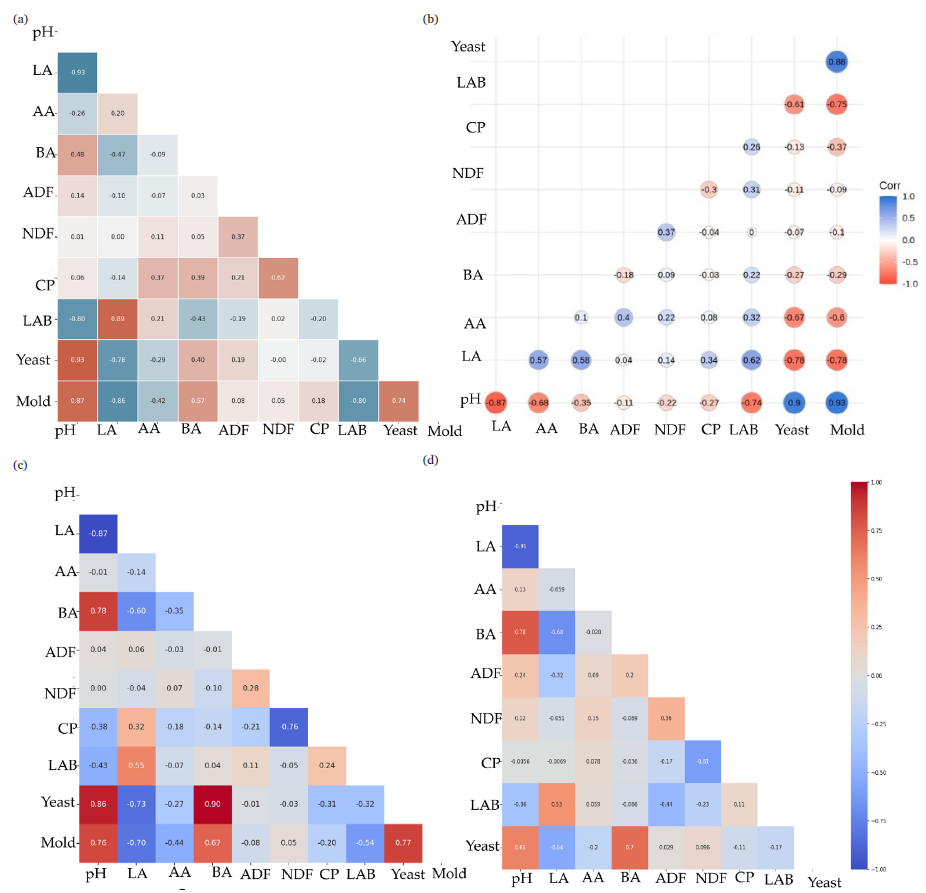
- Triangle heatmap-interactions among fermentative acids, nutrient contents, and microbial population by Python software. (a) Six month fermented silage at HM condition, (b) six month fermented silage at LM condition, (c) twelve-month fermented silage at HM condition, and (d) twelve-month fermented silage at LM condition. LA: Lactic acid, AA: Acetic acid, BA: Butyric acid, ADF: Acid detergent fiber, NDF: Neutral detergent fiber, CP: Crude protein, LAB: Lactic acid bacteria.
4. Discussion
Forage conservation by ensiling has gained considerable attention for providing reliable and predictable feed for ruminants. A number of factors, such as plant oxidation, pathogens in plants, proteolytic activity, Clostridia fermentation, and microbial deamination and decarboxylation of amino acids, can be detrimental forage productivity, which increases the accumulation of anti-nutritional complexes in forage samples (Oliveira et al., 2017). Several decades, LA bacteria have been used to promote the fermentation process in plant samples for livestock due to its efficiency to improve the quality of the silages via acidification by production of LA at higher levels and reduced BA content and inhibition of undesirable microbial growth which favor the long-term storage of fermented silages (Feng et al., 2023; Guo et al., 2023). In spite of LAB’s benefits, maintaining quality of silage during long-term storage has several challenges which includes aerobic stability, temperature fluctuations, and moisture content, which may lead to spoilage. Furthermore, improper sealing and handling can intensify these issues, resulting in lower feed quality and higher economic losses.
In addition, epiphytic LAB in the plants has been varied from 101 to107 CFU/g based on several environmental factors (Pahlow et al., 2003) such as DM content of samples, temperature, and storage period. Epiphytic LAB in plants is not sufficient to induce rapid fermentation in ensiled silages. To produce excellent quality silage with high digestibility, different flavors, and additional inoculum must be incorporated into the ensiling method. LAB is used as an inoculant to increase the proportion of LA to AA in silage making to inhibit proteolysis and increase DM recovery (Muck 2013; Kim et al., 2017).
Numerous studies reported that adding LAB during the ensiling of different forages can accelerate positive fermentation (Kim et al., 2017; Guo et al., 2023). In addition, the production of high-quality silage with mixed forms of LAB (more than one strain) could accelerate the fermentation process due to its synergistic functions to enhance LA production and reduce BA levels compared to silage produced with single LAB treatments. Furthermore, mixed LAB could extend storage periods with preserved nutrients (Filya 2003; Kleinschmit and Kung 2006; Muthusamy et al., 2021; Zhang et al., 2023). In the present study, high-quality silage was made from heading stages triticale forage at different moistures with various LA bacteria such as L. plantarum, P.pediococcus, and L. rhamnosus in either single or cocktail form and stored for 6 / 12 months. After storage periods, the fermentative parameters such as nutrient and microbial profiles were evaluated. High-quality silage has always been enriched with nutrients that preserve fermentation potential. ADF and NDF are key indicators of silage quality (He et al., 2018; Li et al., 2018; Xian et al., 2022). Fermented samples with increased ADF and NDF content indicate poor quality silage and reduced animal digestion. Silage with high fiber contains lower protein and energy than lower fiber silage. Hence, reducing the fiber content of silages is a better strategy to improve feed value (Li et al., 2008). Our study found that silage produced with different types of LAB did not change nutrient contents such as ADF, NDF, and CP. But, in our previous study, silage produced with L. plantarum KCC - 48 and mixed LAB such as L. plantarum, P. pentosaceus, and L. rhamnosus combination significantly decreased ADF of HM early heading triticale silages after 180 and 360 days’ fermentation (Soundharrajan et al., 2023). The same strains were used in the present study to ferment heading stage of triticale. However, there is no response to degradation of fiber material in fermented silage after 6 and 12 months. It may be richer and stronger fibers in the heading stage of triticale than in the early heading stage, which is the reason for being unable to degrade by the currently used LAB.
Acidification is a key indicator for production of high quality silages which reduces proteolytic activities in silages, decomposition of nutrients, and inhibits undesirable microbial growth (Muck 2010). An ideal pH for the good fermentation of silages ranges between 3.8 and 4.2 pH (Ahmadi et al., 2019). In general, pH 4.2 is considered a benchmark for well-fermented silages (He et al., 2018). In the present study pH of the non-inoculated silages had higher pH values greater than five in both HM and LM condition after 6- and 12-month storage periods. It is due to lower LAB population and higher yeast and mold counts in non-inoculated silages, as evidenced by yeast and mold were positively correlated with pH of the silages determined by heatmap correlation. Silage produced with different LAB either single or cocktail form strongly reduced the pH of silage at both moisture and storage conditions due to higher LAB and lower yeast and mold populations. The previous supportive evidence for LAB strains actively suppressed fungal growth by pH 4.0 and the presence of LA (Broberg et al., 2007). The pH of the LAB mediated silages ranged from 3.98 to 4.47 and 3.94 to 4.49 in both HM and LM silage after 6 and 12 months’ fermentation, respectively. At both moisture levels and storage periods, the pH almost reached desirable levels in response to different LAB treatments. In particular, cocktail-I and cocktail-II treatments more strongly reduced the pH of the silage than single strain treatments. Even after 12 months, the pH of the silage made with cocktail maintained the pH below 4.2 benchmark level, it was consistent with heatmap correlation study, suggesting that the higher LAB in treatment was negatively correlated with pH and negatively correlated with yeast and mold after 6 and 12 months at both moistures.
A high-LAB population and a low yeast and mold count were achieved by adding LA bacteria in different forms to the silages. In order to confirm the fermentation quality of forage samples, it is important to measure the LA content, which is an essential acid and an indicator of a successful fermentation process. LA is a predominant acids present in the fermented silages than the other acids (Wang et al., 2021). It is more than 10-12 times higher than other organic acids that contribute to reducing the pH of silage (Kung et al., 2018). In both HM and LM conditions, non-inoculum treated silage had lower levels of LA after 6 and 12 months. This may be due to a low concentration of LAB was in non-inoculum treated silages, confirming that the native LAB present in the plants cannot induce LA fermentation. When silage is made with different LAB strains, either in single or cocktail forms, the amount of LA produced is more than 7 fold higher than non-inoculum treated silage. The production of LA after 6 months in HM silage made with cocktail-I or cocktail-II was more than 10 times compared to non-inoculum treated silage. In LM silage at 6 months, LA was not detected in non-inoculum treated silages. But, silage treated with LAB at the different forms produced LA ranging from 2.04 ± 0.05 to 4.34 ± 0.19 DM%, it confirmed that addition of LAB produced several folds more LA than non-inoculum treated silage. Similar trends were observed in silage treated with LAB after 12 months of storage periods. Higher production of LA in silages due to higher population of LAB and lower counts of yeast and mold. The correlation study confirmed that LA was positively correlated with LAB and negatively correlated with pH, yeast and mold counts, suggesting that LAB plays a main role in pH reduction and higher LA production. Subsequently, AA is another most leading acid in fermented silage (Hafner et al., 2013; Kung et al., 2018). Marginal level of AA has been accepted for good quality silage which inhibits yeast and mold growth and increases aerobic stability (Wilkinson and Davies 2013). The acceptable range of AA is from 10g/kgDM to 30g/KgDM (Muck et al., 2018; Gerlach et al., 2021). Triticale silage produced at heading stage with LAB in single or cocktail form significantly increased the content of AA compared to non-inoculum silages. The production of AA in LAB-treated silages varies among the strains used. KCC-45, KCC-48, cocktail-I, and cocktail-II produced higher AA acid than other strains (p<0.05) at HM conditions whereas all of the used LAB in any forms increased AA content after 6 months in LM. After 12 month of storage, KCC-45 and KCC-48 at HM and KCC-54 and cocktail-I in LM conditions significantly increased AA levels compared to non-inoculum silages. The production of AA at marginal levels could be beneficial for silage due to inhibition of yeast and mold, as evidence that the triangle heatmap correlation revealed that AA was negative correlation with yeast and mold growth at all moisture and storage periods.
BA indicates poor quality silage due to clostridia-mediated fermentation (Pahlow et al., 2003; Zhang et al., 2023). Reduction or inhibition in total is a great strategy for producing high-quality silage with preserved nutrients for long-term storage. Since soluble nutrients have been degraded by clostridia fermentation, higher levels of BA reduce of silage DM and nutritional content (Kung et al., 2018). The study found that HM silage at 6 months had higher concentrations of BA in control silage which were reduced by LAB treatments in different forms, such as KCC-45, KCC-48, KCC-54, and both cocktail forms (p <0.05). Despite this, no significant amount of BA is produced in all experimental silages at LM levels. After 12 months of storage, LAB treatments significantly reduced BA levels in both high and low silages. Variations in BA reduction were observed among strains. Cocktail-I and cocktail-II treatments significantly reduced BA production compared to LAB alone. It is believed that the reduction in BA levels in LAB-treated silage is due to the inhibition of yeast and mold, and to LAB dominance. In addition, BA showed a positive correlation with yeast and mold, while LAB showed a negative correlation with BA. There are specific challenges in producing triticale silage at heading stages due to a lack of water soluble carbohydrates (WSCs). The heading stage triticale contains less WSC and more fiber (ADF and NDF) than the early heading stage (milky stage). WSC is essential to LAB fermentation since it increases LA levels which reduce pH. Generally, fiber content is not favorable for LAB-mediated fermentation. Thus, the use of LAB that produces cellulase during the ensiling of fiber-rich forages could enhance fermentation and increase essential organic acids content.
5. Conclusions
The production of high-quality silage from plant sources is essential for livestock farms. However, storing silage for a long period of time can lead to mold and yeast growth, as well as LA degradation. Thus, biological additives, such as LA bacteria, improve fermentation quality, and preserve nutrients for long-term storage. Therefore, high-quality silage was made from heading triticale with different types of LA, such as Pediococcus pentosaceus, Lactobacillus plantarum, and Lactobacillus rhamnosus, by the ensiling method to speed up fermentation by reducing pH of silages and periods after six and twelve months. LAB treatments also retained more LA and reduced BA levels by increasing LAB and reducing yeast and mold counts even after 12 months of storage. Cocktail-I (two P. pentosaceus and one L. plantarum) and cocktail-II (L. plantarum, P. pentosaceus and L. rhamnosus) treatments were significantly more effective at preserving nutrient values of fermented silages for livestock for long periods of time than single strain treatments. This suggests that silage developed using multiple strains as a cocktail is more appropriate for livestock for long-time storage. Despite this, further studies are required to confirm the efficiency of the different LAB used in this study in either single or cocktail form on fermentation of different forages, including grasses and leguminous plants.
Acknowledgments
The research was supported by a grant from National Institute of Animal Science, Rural Development Administration, Republic of Korea, titled “Efficient succession production of Domestic Maize and Triticale silage and feeding to Hanwoo cow and heifer for growth, reproduction and beef production (PJ017203).” This study was supported by 2024 the RDA Fellowship Program of National Institute of Animal Science, Rural Development Administration, Republic of Korea.
CRediT authorship contribution statement
Ilavenil Soundharrajan, Jeong Sung Jung, and Ki Choon Choi: Conceptualization, Investigation, Methodology, Resources, Formal analysis, Data curation, Validation, and Manuscript review and editing. Ilavenil Soundharrajan and Sathya Rengasamy: Writing–original draft, Writing–review and editing, and software analysis. Palaniselvam Kuppusamy, Yang Seung Hak, and Ouk Kyu Han: Formal analysis, Data curation, Validation, and Manuscript review and editing. Kaleeswaran Balasubramanian and Sathya Rengasamy: Data curation, Validation, Manuscript review and editing, and software analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
All experimental data were available in the original manuscript as well as in the supplementary file. Raw data will be provided on request.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://dx.doi.org/10.25259/JKSUS_47_2024
References
- Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manag. 2019;87:258-267. doi: https://doi.org/10.1016/j.wasman.2019.02.010
- [CrossRef] [PubMed] [Google Scholar]
- Novel lactic acid bacteria strains enhance the conservation of elephant grass silage cv. BRS Capiaçu. Animal Feed Science and Technology. 2020;264 114472-10.13039/501100004901. doi: https://doi.org/10.13039/501100004901
- [CrossRef] [PubMed] [Google Scholar]
- Official methods of analysis of the AOAC (15th ed). Arlington, VA, USA: Methods 932.06, 925.09, 985.29, 923.03; 1990.
- Enhancing Nutritional Quality of Silage by Fermentation with Lactobacillus plantarum. Indian J Microbiol. 2014;54:396-402. doi: https://doi.org/10.1007/s12088-014-0473-9
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Metabolite profiles of lactic acid bacteria in grass silage. Appl Environ Microbiol. 2007;73:5547-52. doi: https://doi.org/10.1128/AEM.02939-06
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Isolation, Identification, and Screening of Lactic Acid Bacteria with Probiotic Potential in Silage of Different Species of Forage Plants, Cocoa Beans, and Artisanal Salami. Probiotics Antimicrob Proteins. 2021;13:173-186. doi: https://doi.org/10.1007/s12602-020-09679-y
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ensiling hybrid Pennisetum with lactic acid bacteria or organic acids improved the fermentation quality and bacterial community. Front Microbiol. 2023;14:1216722. doi: https://doi.org/10.3389/fmicb.2023.1216722
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J Dairy Sci. 2003;86:3575-81. doi: https://doi.org/10.3168/jds.S0022-0302(03)73963-0
- [CrossRef] [PubMed] [Google Scholar]
- A data analysis on the effect of acetic acid on dry matter intake in dairy cattle. Animal Feed Science and Technology. 2021;272 114782-10.1016/j.anifeedsci.2020.114782. doi: https://doi.org/10.1016/j.anifeedsci.2020.114782
- [CrossRef] [Google Scholar]
- Current approaches on the roles of lactic acid bacteria in crop silage. Microb Biotechnol. 2023;16:67-87. doi: https://doi.org/10.1111/1751-7915.14184
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Emission of volatile organic compounds from silage: Compounds, sources, and implications. Atmospheric Environment. 2013;77 827-839-10.1016/j.atmosenv.2013.04.076. doi: https://doi.org/10.1016/j.atmosenv.2013.04.076
- [CrossRef] [Google Scholar]
- Inclusion of wheat and triticale silage in the diet of lactating dairy cows. J Dairy Sci. 2017;100:6151-6163. doi: https://doi.org/10.3168/jds.2017-12553
- [CrossRef] [PubMed] [Google Scholar]
- Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J Anim Physiol Anim Nutr (Berl). 2018;102:1429-1436. doi: https://doi.org/10.1111/jpn.12965
- [CrossRef] [PubMed] [Google Scholar]
- Improved performance and microbial community dynamics in anaerobic fermentation of triticale silages at different stages. Bioresour Technol. 2022;345:126485. doi: https://doi.org/10.1016/j.biortech.2021.126485
- [CrossRef] [PubMed] [Google Scholar]
- Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci Rep. 2021;11:1968. doi: https://doi.org/10.1038/s41598-021-81505-z
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of a new lactic acid bacterial inoculant for fresh rice straw silage. Asian-Australas J Anim Sci. 2017;30:950-956. doi: https://doi.org/10.5713/ajas.17.0287
- [CrossRef] [PubMed] [Google Scholar]
- The effects of Lactobacillus buchneri 40788 and Pediococcus pentosaceus R1094 on the fermentation of corn silage. J Dairy Sci. 2006;89:3999-4004. doi: https://doi.org/10.3168/jds.S0022-0302(06)72443-2
- [CrossRef] [PubMed] [Google Scholar]
- Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 2018;101:4020-4033. doi: https://doi.org/10.3168/jds.2017-13909
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour Technol. 2018;257:76-83. doi: https://doi.org/10.1016/j.biortech.2018.02.070
- [CrossRef] [PubMed] [Google Scholar]
- Effect of concentrate-forage ratio in diet on liveweight gain of stall-fed goats. Acta Prataculturae Sinica. 2008;17:85.
- [Google Scholar]
- Exploring the bacterial community and fermentation characteristics during silage fermentation of abandoned fresh tea leaves. Chemosphere. 2021;283:131234. doi: https://doi.org/10.1016/j.chemosphere.2021.131234
- [CrossRef] [PubMed] [Google Scholar]
- Silage microbiology and its control through additives. R. Bras. Zootec.. 2010;39 183-191-10.1590/s1516-35982010001300021. doi: https://doi.org/10.1590/s1516-35982010001300021
- [CrossRef] [Google Scholar]
- Recent advances in silage microbiology. AFSci. 2013;22 3-15-10.23986/afsci.6718. doi: https://doi.org/10.23986/afsci.6718
- [CrossRef] [Google Scholar]
- Silage review: Recent advances and future uses of silage additives. J Dairy Sci. 2018;101:3980-4000. doi: https://doi.org/10.3168/jds.2017-13839
- [CrossRef] [PubMed] [Google Scholar]
- Dual Culture Inoculation Enhanced Quality of Silage Produced from Leguminous Plants. J. Kor. Grassl. Forage. Sci.. 2021;41 176-182-10.5333/kgfs.2021.41.3.176. doi: https://doi.org/10.5333/kgfs.2021.41.3.176
- [CrossRef] [Google Scholar]
- Advances in bioconversion of spent tea leaves to value-added products. Bioresour Technol. 2022;346:126409. doi: https://doi.org/10.1016/j.biortech.2021.126409
- [CrossRef] [PubMed] [Google Scholar]
- Lactic acid bacteria and silage fermentation. In: Lactic Acid Bacteria (5th Edition). CRC Press; 2019. p. :275-286. doi: https://doi.org/10.1201/9780429057465-17.
- [Google Scholar]
- The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol Res. 2023;266:127212. doi: https://doi.org/10.1016/j.micres.2022.127212
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci. 2017;100:4587-4603. doi: https://doi.org/10.3168/jds.2016-11815
- [CrossRef] [PubMed] [Google Scholar]
- Microbiology of Ensiling. In: Buxton D.R., Muck R.E., Harrison J.H., eds. Silage Science and Technology. Vol Volume 42. 2003. p. :31-93.
- [Google Scholar]
- Dual-purpose inoculants and their effects on corn silage. Microorganisms. 2020;8:765. doi: https://doi.org/10.3390/microorganisms8050765
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Potential of lactic acid bacteria isolated from different forages as silage inoculants for improving fermentation quality and aerobic stability. Front Microbiol. 2020;11:586716. doi: https://doi.org/10.3389/fmicb.2020.586716
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A meta-analysis to observe silage microbiome differentiated by the use of inoculant and type of raw material. Front Microbiol. 2023;14:1063333. doi: https://doi.org/10.3389/fmicb.2023.1063333
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of different lactic acid bacteria in single or mixed form on the fermentative parameters and nutrient contents of early heading triticale silage for livestock. Foods. 2023;12:4296. doi: https://doi.org/10.3390/foods12234296
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Probiotic and triticale silage fermentation potential of pediococcus pentosaceus and lactobacillus brevis and their impacts on pathogenic bacteria. Microorganisms. 2019;7:318. doi: https://doi.org/10.3390/microorganisms7090318
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583-97. doi: https://doi.org/10.3168/jds.S0022-0302(91)78551-2
- [CrossRef] [PubMed] [Google Scholar]
- Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol. 2021;9:612285. doi: https://doi.org/10.3389/fbioe.2021.612285
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The aerobic stability of silage: key findings and recent developments. Grass and Forage Science. 2013;68:1-19. doi: https://doi.org/10.1111/j.1365-2494.2012.00891.x
- [CrossRef] [Google Scholar]
- Applications of plant-based fermented foods and their microbes. Curr Opin Biotechnol. 2020;61:45-52. doi: https://doi.org/10.1016/j.copbio.2019.09.023
- [CrossRef] [PubMed] [Google Scholar]
- Effects of cellulase and lactiplantibacillus plantarum on the fermentation parameters, nutrients, and bacterial community in cassia alata silage. Front Microbiol. 2022;13:926065. doi: https://doi.org/10.3389/fmicb.2022.926065
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative lactobacillus plantarum and heterofermentative lactobacillus buchneri. Front Microbiol. 2019;9:3299. doi: https://doi.org/10.3389/fmicb.2018.03299
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of different types of LAB on dynamic fermentation quality and microbial community of native grass silage during anaerobic fermentation and aerobic exposure. Microorganisms. 2023;11:513. doi: https://doi.org/10.3390/microorganisms11020513
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







