Translate this page into:
A simple, rapid, expedient and sustainable green strategy for the synthesis of benz-/naphthimidazoles
⁎Corresponding author. sraju@ksu.edu.sa (Raju Suresh Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
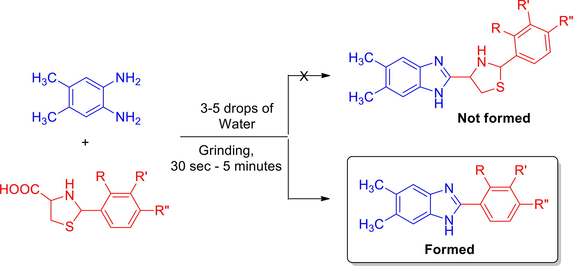
A versatile green chemical procedure for the highly selective construction of 2-aryl substituted benz-/naphthimidazoles starting from the reaction of aromatic 1,2-diamines with a series of substituted arylthioprolines with three to five drops of water under simple grinding at ambient temperature in good yields is described. The short reaction time, simplified experimental procedure, the absence of extraction and chromatographic purification steps in addition to the environment affability makes this green strategy highly attractive in view of green chemistry. The expected reaction to furnish the thiazole grafted benz-/naphthimidazole did not occur. Perhaps the arylthioprolines could be in zwitterionic form, which could react with 1,2-diamine giving dihyrobenzimidazole, which undergoes air oxidation to furnish the 2-aryl benzimidazole rather than the expected thiazole grafted imidazoles.
Keywords
Green chemistry
Arylthioprolines
Benzimidazole
Naphthimidazole
Selectivity
1 Introduction
Fused imidazole analogues have acquired a protuberant place in medicinal chemistry due to their noteworthy properties as therapeutics. The benzimidazole structural motif, a renowned biologically interesting N-containing heterocyclic hybrid (Emery et al., 2002; Sondhi et al., 2002) is ubiquitous in diverse naturally occurring and synthetic organic compounds is a vital pharmacophore and is a ‘privileged sub-structure’ for drug design in medicinal chemistry (Evans et al., 1988; Mason et al., 1999). As these heterocyclic molecular scaffolds involves benzene and imidazole sub-units, owing to their electron-rich scaffold and nitrogen atoms, these structural motifs can interact with diverse biological targets (Tahlan et al., 2019). The biological significance of benzimidazole tethered compounds have been highly recognized in the literature (Wright, 1951; Amari et al., 2002). Besides their biological significance, these molecular scaffolds are being employed as synthons for the construction of diverse building blocks and ligands (Samolova et al., 2019). Due to the huge importance as synthons and diverse bioactivities displayed by benzimidazole derivatives (Mederski et al., 2004; Kus et al., 2008) (Fig. 1), efforts have been made for the generation of libraries of these biologically important compounds (Breslin et al., 2003; Valdez et al., 2002). The augmented attention for these molecular scaffolds has also been due to their exceptional stability, bioavailability and noteworthy biological profiles (Yadav and Ganguly, 2015). A thorough retrospect over the reported routes for the synthesis of benzimidazoles reveals plenty of preparative methods (Katritzky and Boulton, 1980) amongst which the two momentous routes involves condensation of o-phenylenediamine with (i) aryl aldehydes (Moghaddam et al., 2006) and (ii) carboxylic acids (Dudd et al., 2003) or their analogues like nitriles, amidates and orthoesters (Chi and Sun, 2000; Huang and Scarborough, 1999) at high temperature in the presence of strong acids like polyphosphoric acid (Preston et al., 1981) or mineral acids (Kartritzky and Rees, 1984) and thermal or acid mediated cyclization of N-(N-arylbenzimidoyl)-1,4-benzoquinoneimines under harsh dehydrating conditions (Benincori and Sannicolo, 1988). Even though, some of these methods are acceptable for the construction of benzimidazoles, there are some disadvantages like the severe reaction conditions, luxurious reagents, usage of lethal organic solvents and prolong reaction times limit the usage of these methodology. Therefore, the research lasts for a better synthetic protocol, in terms of simple workup, economic feasibility, ecofriendly, and in specific with better selectivity as this would extend the scope in organic synthesis.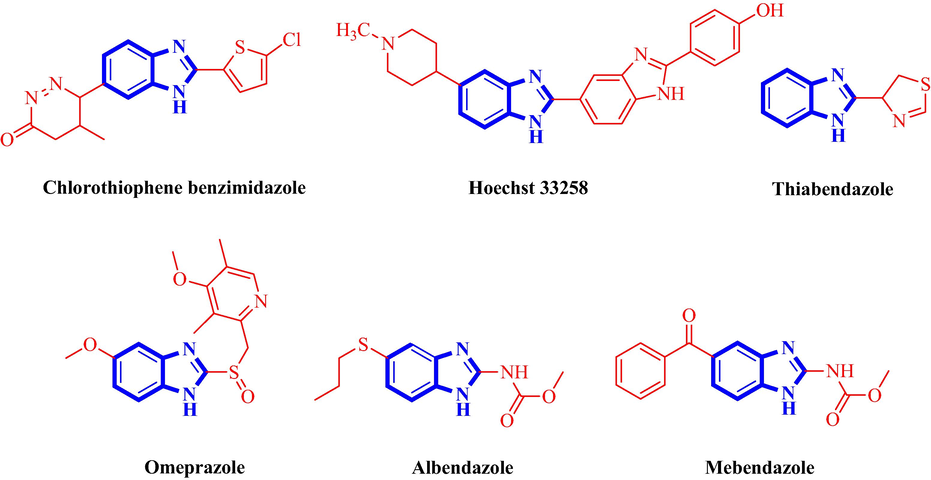
Selected benzimidazole derivatives of pharmacological and biological interest.
Advancement of eco-friendly green synthetic protocols for the assembly of new chemical units is acquiring inordinate significance and in current years, organic transformations mediated by water without employing conventional organic solvents has turn out to be one of the most significant features in synthetic organic chemistry as to meet the ecological demands. Performing organic reactions in water phase is very interesting both from the synthetic view point and also from the influence of the ecological pollution (Lindstrom, 2007). As the benzimidazole structural sub unit is the vital synthon for a variety of compounds of biological interest, there is a continuous and increasing attention over the years for an easy and convenient synthesis of compounds associated with imidazole derivatives. For these reasons and in extension of our earlier works towards the synthesis of novel biologically active hybrid heterocycles containing nitrogen and oxygen through tandem/multicomponent/green chemical transformations (Almansour et al., 2017, 2019; Kumar et al., 2011, 2013, 2018), in the present study, we report a facile, handy and green approach for the construction of benz-/naphthimidazoles under simple grinding with few drops of water. However, this would be the first report for the synthesis of benz-/naphthimidazole analogues employing 1,2-aryldiamine and arylthioprolines.
2 Experimental
2.1 General procedure for the synthesis of benz-/naphthimidazoles (3 and 8)
An equimolar mixture of mixture of 1,2-diamine 1/7 and substituted arylthioprolines 2 in 3-5 drops of water in a semi micro boiling tube were mixed thoroughly and ground well at ambient temperature. Progress of the reaction was indicated by the transformation of color of the reaction mixture, which became yellow/brown at the end when the reaction is complete. After completion of the reaction as evinced by the appearance of yellow/brown colour, the reaction mixture was dispensed into 50 mL of water. The solid settled down was filtered through a sintered glass crucible and washed repeatedly with water to furnish the benz-/naphthimidazoles as the only reaction product which was further purified by recrystallization from ethyl acetate.
2.1.1 2-(2,4-Dichlorophenyl)-5,6-dimethyl-1H-benzo[d]imidazole (3d)
Yellow solid (92%); m.p. 226–227 °C; IR (KBr): 3402, 3029, 1648, 1582 cm−1; 1H NMR (400 MHz, CDCl3): δH2.20 (s, 6H, 2xCH3), 4.09 (brs, 1H, NH), 6.60 (s, 1H, Ar-H), 6.91 (s, 1H, Ar-H), 7.30 (d, J = 8.0 Hz, 1H, Ar-H), 7.43 (s, 1H, Ar-H), 8.18 (d, J = 8.8 Hz, 1H, Ar-H). 13C NMR (100 MHz, CDCl3): δC 19.1, 19.8, 117.4, 118.1, 126.6, 127.5, 129.2, 129.8, 132.5, 134.2, 136.1, 136.9, 137.4, 140.8, 150.7. Anal. calcd for C15H12Cl2N2: C, 61.87; H, 4.15; N, 9.62. Found: C, 61.70; H, 4.27; N, 9.73%.
2.1.2 2-(2,4-Dichlorophenyl)-3H-naphtho[2,1-d]imidazole (8d)
Brown solid (85%); m.p. 231–232 °C; IR (KBr): 3408, 3035, 1652, 1596 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.25–8.10 (m, 7H, Ar-H), 8.25 (d, J = 8.8 Hz, 1H, Ar-H), 9.05 (s, 1H, Ar-H), 10.40 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δC 116.5, 118.5, 121.6, 125.8, 126.0, 128.8, 129.7, 130.6, 131.2, 133.4, 134.7, 136.0, 136.7, 137.2, 138.9, 141.5, 150.9. Anal. calcd for C17H10Cl2N2: C, 65.20; H, 3.22; N, 8.94. Found: C, 65.41; H, 3.09; N, 8.85%.
3 Results and discussion
With an aim of developing a sustainable reaction protocol for the construction of imidazoles, we reported in the current study, the reaction of a mixture of 4,5-dimethyl-1,2-phenylenediamine 1 (1 mmol) and substituted arylthioprolines 2(a-e) (1 mmol) with two to three drops of water under simple grinding at ambient temperature for 1–5 min (Scheme 1) furnishing a series of 2-arylbenzimidazoles 3(a-e) in good yields (84–92%). The expected reaction to furnish the thiazole grafted benzimidazole 4 has not been favored (Scheme 1). The aryl substituted thioprolines 2(a-e) required for the present study was synthesized by following the literature reported procedure (Liu et al., 2011), by the reaction of an equimolar mixture of L-cysteine hydrochloride hydrate, NaHCO3 in water (200 mL) with benzaldehyde in ethanol (200 mL). The reaction mixture was stirred for 6 h and the precipitate obtained was filtered using a sintered glass crucible, washed with ethanol, and dried to afford 2(a-e) as white solids.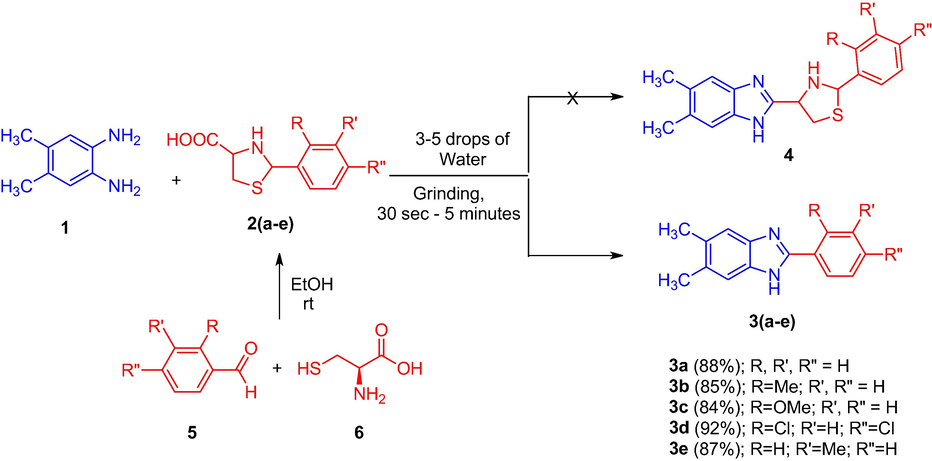
Synthesis of 2-aryl substituted benzimidazoles 3(a-e).
For optimizing the reaction conditions and yield of 3, we started our investigation by considering the model reaction of an equimolar ratio of 4,5-dimethyl-1,2-phenylenediamine (1) and 2-(2,4-dichlorophenyl)thiazolidine-4-carboxylic acid (2d) under different conditions (Table 1). Initially an equimolar ratio of the aforesaid reactants, 1 and 2d in 5 mL of water were stirred at room temperature. The reaction progress being evidenced through change of colour of the reaction mixture and also monitored with thin layer chromatographic (TLC) analysis. The reaction progress was monitored at one-minute time intervals. In about two minutes the reaction mixture became yellow, the TLC analysis of the reaction mixture revealed the formation of a sole product without any side products. After completion, the reaction mixture was kept aside for 15 min. The precipitate settled down was then filtered carefully, washed with 50 mL of water and dried in vacuum, the yield of 3d obtained by this method was found to be 83% (Entry 1). In order to enhance the yield and to further reduce the reaction time, in a separate experiment the same reactants in 5 mL of water was heated under reflux, as expected the reaction time has considerably been reduced. The reaction was completed in 1 min, affording a gummy solid, TLC analysis revealed the formation of the product along with minor impurities. The reaction mixture was extracted and recrystallized from ethyl acetate to furnish the benzimidazole 3d in 78% yield (Entry 2). In a different attempt, the above model reaction was performed under neat conditions in the absence of solvent. The two reactants were simply mixed and ground thoroughly in a semi micro boiling tube, the reaction progression was monitored after each one-minute time interval. The reaction was completed in about 5 min, affording the product 3d in 81% yield (Entry 3). Furthermore, to ensure thorough mixing of aryldiamine 1 and substituted arylthioproline 2d, the reaction mixture was ground well in a semi micro boiling tube at ambient temperature with three drops of water. Progress of the reaction is being revealed by the weakening color of the reaction mixture, which became yellow at completion of the reaction. To our surprise, the reaction was completed in just 1 min and afforded solely the benzimidazole without the formation of any side products and therefore neither crystallization nor column chromatography was required for purification. Then 50 mL of water was added to the reaction mixture and the precipitate obtained was filtered to afford pure 2-(2,4-dichlorophenyl)-5,6-dimethyl-1H-benzo[d]imidazole in an excellent yield (92%). The results obtained were consolidated in Table 1, it is clearly evident from the above experiments that the best results were obtained by entry 4, in terms of yield and reaction time. Continuous monitoring of the reaction progress was not required in the optimized method as the reaction is completed in just 1 min and the reaction scale up does not envisage any decrease in either the yield or purity of the product, since the reaction necessitates only an in-depth mixing of the reactants at ambient temperature. The reactions with other substituted thioprolines 2(a-c) and 2e were performed under the optimized conditions, all these reactions afforded good yields of the product as expected in short reaction time. Among the five benzimidazoles synthesized, compounds 3a, 3c and 3e with phenyl, 2-OMe phenyl and 3-Me phenyl rings respectively has already been reported in the literature. There are plenty of literature reports available for the synthesis of 3a employing different reagents and conditions both by conventional and green chemistry approaches. Nguyen et al. Synthesized compound 3a by a cobalt- or iron-catalyzed redox condensation of 2-nitroanilines and benzylamines under a solvent-free conditions. The reaction was carried out at 120 °C for 24 h affording a reasonable yield (80%) of the product (Nguyen et al., 2013). Rostamizadeh et al. performed the reaction of o-phenylenediamine and benzaldehyde in 1 M solution of glucose at 60 °C. Although the reaction for the synthesis of 3a has been carried under green chemical approach, a quite long time of 7 h has been consumed for its completion (Shahnaz et al., 2011). Mukhopadhyay and Tapaswi (Mukhopadhyay and Tapasw, 2008) reported PEG-mediated synthesis of 3c under solvent free conditions. Even though a good yield of the product has been achieved, the reaction was performed at a quite high temperature, 110 °C. Singh and his co-workers (Singh et al., 2013) reported the two-step synthesis of 3e starting from benzoylisothiocyanates and ortho-phenylenediamines. The bisthioureas obtained undergoes cyclization on refluxing in pyridine furnishing 3e in 87% yield. The synthetic methodology described by us in the present study is advantageous than the above reported procedures for the synthesis of benzimidazoles of this kind with respect to short reaction time, operational simplicity and eco-friendliness. So far, no method has been reported for the synthesis of benzimidazoles (3) employing arylthioprolines and aromatic diamines.
Entry
Reaction condition
Reaction time
Yield (%) of 3da
1
Stirring at ambient temperature with 5 mL of water
2 min
83
2
Heating under reflux with 5 mL of water
1 min
78
3
Simple grinding at ambient temperature under solvent free condition
5 min
81
4
Simple grinding at ambient temperature with 3 drops of water
30 s
92
A careful structural elucidation of benzimidazoles 3 was accomplished with the help of NMR spectroscopic and elemental analysis data. As a distinguishing case, structural explanation of compound 3d is discussed here. In the 1H NMR, the singlet at 2.20 ppm can be ascribable to two –CH3 groups while the other singlets in the downfield region at 6.60, 6.91 and 7.43 ppm are due to the aromatic ring protons. A doublet at 7.30 ppm (J = 8.0 Hz) is attributed to one of the aromatic ring protons of 2,4-dichloro phenyl ring. The other doublet at 8.18 ppm with J = 8.8 Hz related by a H,H-COSY correlation is due to the remaining aromatic ring protons. In 13C NMR spectrum (vide supplementary data), the two methyl carbons resonated at 19.07 and 19.77 ppm whereas the aromatic carbons appeared between 117.36 and 150.71 ppm. The structure of other compounds was also arrived through similar considerations and all new compounds were in agreement with their analytical and NMR spectroscopic data.
The versatility of this green protocol was further investigated by performing the reaction with a different diamine viz. 1,2-diaminonaphthelene 7. The reaction with this aromatic bicyclic diamine was performed adopting the optimized synthetic procedure. As in the previous cases, the naphthimidazoles 8(a-d) were also obtained in good yields (76–85%) (Scheme 2). Among the naphthimidazoles 8(a-d) synthesized, compound 8a has already been reported in literature by different research groups employing different synthetic methodologies. In one of the reports by Reddy et al. (Reddy et al., 1994), the synthesis of compound 8a involved a three-step reaction sequence starting from acetylnaphthelene in the presence of polyphosphoric acid at high temperatures. The yield obtained in this three-step reaction is low when compared to the yield obtained in the present study. Naphthoimidazole 8a has also been synthesized using different reagents and conditions. Our methodology for the synthesis of naphthoimidazole 8a is more advantageous that the reported methods in terms of reaction time, yield and safety. The structure of other compounds is in agreement with their NMR spectroscopic and analytical data, whilst the spectroscopic and physical data of the known compound correlate well with the reported results. The position and electronic or steric properties of the substituent at the aromatic ring of arylthioprolines 2(a-e) does not have any major influence on the yield or rate of the reaction. All the reactions (Scheme 1 and 2) proceeded well and afforded the benz-/naphthimidazoles in good yields. To the best of our knowledge the method developed in the present study is more advantageous than the literature reported methods.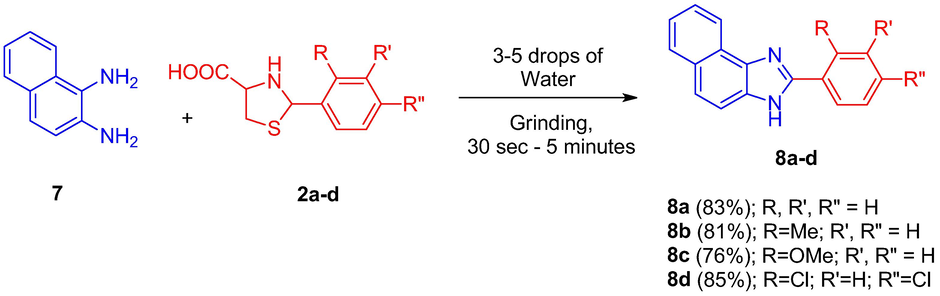
Synthesis of 2-aryl naphthimidazoles (8).
A probable mechanism for the formation of benz-/naphthimidazoles (3/8) is shown in scheme 3. There are two plausible mechanistic routes, in the first pathway (Path A), the arylthioprolines 2(a-e) in the presence of few drops of water may dissociate into benzaldehyde (9) and 2-amino-3-mercaptopropanoic acid (10). The sp3 carbon of amino acid 2 was attacked by a hydroxyl group of water molecule furnishing the benzaldehyde 9 and 2-amino-3-mercaptopropanoic acid. The benzaldehyde obtained may react with 1,2-diamine (1) to give the imine intermediate 11 which upon intramolecular cyclization, i.e., the amine group attacks the electrophilic iminocarbon affording the dihydrobenzimidazole (12). The dihydrobenzimidazole (12) upon air oxidation may furnish the final 2-aryl benzimidazoles (3). In a separate experiment, the arylthioproline in presence of few drops of water was stirred/ground well, we didn’t observe the dissociation of arylthioproline in to benzaldehyde as postulated. Therefore, the possibility of this pathway was ruled out. In the second pathway (Path B), perhaps the arylthioprolines could be in zwitterionic form, which could react with 1,2-diamine giving dihyrobenzimidazole, which undergoes air oxidation to furnish the 2-aryl benzimidazole (3).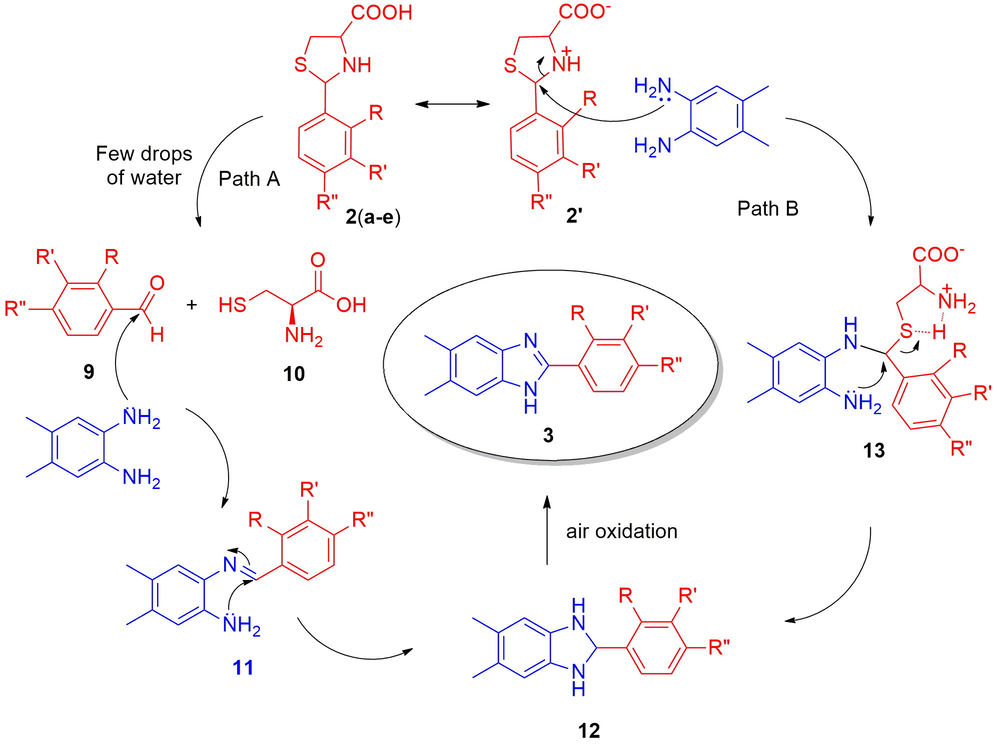
Plausible mechanism for the formation of 2-aryl benzimidazoles 3.
4 Conclusions
In conclusion, the present work describes a versatile, handy and environment friendly protocol for the synthesis of 2-substitued benz-/naphthimidazoles by the reaction of substituted 1,2-phenylene-/naphthalenediamines with arylthioprolines under simple grinding at ambient temperature. The synthetic methodology described in the present study is advantageous than the synthetic procedures reported in the literature for the synthesis of benz-/naphthimidazoles of this kind with respect to selectivity, short reaction time, operational simplicity and eco-friendliness. Further, studies on the synthesis of a wide range of heterocyclic hybrids employing green chemical transformations are under progress in our research group.
Acknowledgement
The authors acknowledge the Deanship of Scientific Research at King Saud University for funding this work through research group No. RGP-026.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and antiproliferative activity of decarbonyl luotonin analogues. Eur. J. Med. Chem.. 2017;138:932-941.
- [Google Scholar]
- Almansour, A.I., Kumar, R.S., Arumugam, N., Kotresha, D., Menendez, J.C., Anti-Cancer compound, US Patent US 10,357,485 B1 Jul. 23, 2019.
- Reactivity studies on 4-aminopyrones: Access to benzimidazole and benzimidazolone derivatives. J. Het. Chem.. 2002;39:811-816.
- [Google Scholar]
- Tripeptidyl-peptidase II (TPP II) inhibitory activity of (S)-2,3-dihydro-2-(1H-imidazol-2-yl)-1H-indoles, a systematic SAR evaluation. Part 2. Bioorg. Med. Chem. Lett.. 2003;13:4467-4471.
- [Google Scholar]
- Soluble Polymer-Supported Synthesis of a Benzimidazole Library. Synlett 2000:591-594.
- [Google Scholar]
- Synthesis of benzimidazoles in high-temperature water. Green Chem.. 2003;5:187-192.
- [Google Scholar]
- Focus on New Drugs in Development Against Human Cytomegalovirus. Drugs. 2002;62:1853-1858.
- [Google Scholar]
- Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem.. 1988;31:2235-2246.
- [Google Scholar]
- A new “traceless” solid-phase synthesis strategy: Synthesis of a benzimidazole library. Tetrahedron Lett.. 1999;40:2665-2668.
- [Google Scholar]
- Comprehensive Heterocyclic Chemistry. 1984;5:457-474.
- Katritzky, A.R., Boulton, A.J., 1980. Advances in Heterocyclic Chemistry; Vol. 27; Academic: New York, 241.
- A facile three-component [3+2]-cycloaddition/annulation domino protocol for the regio- and diastereoselective synthesis of novel penta- and hexacyclic cage systems, involving the generation of two heterocyclic rings and five contiguous stereocenters. Tetrahedron. 2011;67:3132-3139.
- [Google Scholar]
- Three-component synthesis and 1,3-dipolar cycloaddition of highly functionalized pyrans with nitrile oxides: An easy access to 1,2,4-oxadiazoles. Synth. Commn.. 2013;43:2763-2772.
- [Google Scholar]
- Highly functionalized pyrrolidine analogues: stereoselective synthesis and caspase-dependent apoptotic activity. RSC Adv.. 2018;8:41226-41236.
- [Google Scholar]
- Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem.. 2008;16:4294-4303.
- [Google Scholar]
- Organic Reactions in Water. Oxford: Blackwell Publishing; 2007.
- Design, synthesis and biological activity of thiazolidine-4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg. Med. Chem.. 2011;19:2342-2348.
- [Google Scholar]
- New 4-Point Pharmacophore Method for Molecular Similarity and Diversity Applications: Overview of the Method and Applications, Including a Novel Approach to the Design of Combinatorial Libraries Containing Privileged Substructures. J. Med. Chem.. 1999;42:3251-3264.
- [Google Scholar]
- Halothiophene benzimidazoles as P1 surrogates of inhibitors of blood coagulation factor Xa. Bioorg. Med. Chem. Lett.. 2004;14:3763-3769.
- [Google Scholar]
- Facile and efficient one-pot protocol for the synthesis of benzoxazole and benzothiazole derivatives using molecular iodine as catalyst. Synth. Commun.. 2006;36:2543-2548.
- [Google Scholar]
- PEG-mediated catalyst-free expeditious synthesis of 2-substituted benzimidazoles and bis-benzimidazoles under solvent-less conditions. Tetrahedron Lett.. 2008;49:6237-6240.
- [Google Scholar]
- A. Cobalt- and iron-catalyzed redox condensation of o-substituted nitrobenzenes with alkylamines: a step- and redox-economical synthesis of diazaheterocycles. Org. Lett.. 2013;15:6218-6221.
- [Google Scholar]
- Novel pyrolytic reactions: simultaneous formation of 2-arylnaphth[1,2-d]oxazoles and 2-arylnaphth[1,2-d]imidazoles. Indian J. Heterocycl. Chem.. 1994;3:219-222.
- [Google Scholar]
- Bis(benzimidazole) as supramolecular building block in manganese(IV) chemistry. J. Mol. Struct.. 2019;1176:366-375.
- [Google Scholar]
- Aqueous 1 M Glucose Solution as a Novel and Fully Green Reaction Medium and Catalyst for the Oxidant-Free Synthesis of 2-Arylbenzimidazoles. Synth Commn.. 2011;41:1794-1804.
- [Google Scholar]
- A new and convenient synthesis of 2-arylbenzimidazoles through reaction of benzoylisothiocyanates with ortho-phenylenediamines. ARKIVOC (ii) 2013:213-219.
- [Google Scholar]
- Heterocyclic Compounds as Inflammation Inhibitors. Curr. Med. Chem.. 2002;9:1045-1074.
- [Google Scholar]
- Benzimidazole scaffolds as promising antiproliferative agents: a review. BMC Chem.. 2019;13:66.
- [Google Scholar]
- Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett.. 2002;12:2221-2224.
- [Google Scholar]
- Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem.. 2015;97:419-443.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.09.001.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







