Translate this page into:
A review on microbial degradation of drinks and infectious diseases: A perspective of human well-being and capabilities
⁎Corresponding author. mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Various microorganisms present in beverages as contaminants, yet few can develop in the presence of acidic and low oxygen conditions. Notably, yeast is the dominant microbe group present in the fruit juices and drinks in the fruit juices and drinks. Due to the microbe development and secondary metabolite production, such as polluting compounds, carbon dioxide, and spoilage, the beverages were identified. Yeast and molds are considered to be a vital microbe that causes deterioration. The primary reason for the deterioration in fruits and fruit juices is because of the contamination caused by fungi and yeast, and sometimes damage may cause by insects. Sugars and sugar concentrates are generally polluted with osmophilic yeasts, for instance, Z. rouxii. This review mainly focuses on the types of spoilages in soft drinks and beverages and their control measures.
Keywords
Beverages
Microbial spoilage
Infectious diseases
Prevention
Human well-being
1 Introduction
During the manufacturing of soft drinks and beverages, many microbes have been involved, and a few will be a reason for causing spoilage in it. The quality of sensory will be degraded with the spoilage and leads to changes in visual, odour, and flavour changes. Microbial growth has to reach a certain quantity, like 105 to 106 cells/ml, to spoil the beverages (Stratford, 2006). Secondary metabolites from the microbe will also lead to indirect spoilage other than the microbe's growth. If the raw materials are contaminated, that will lead to the product's spoilage, failure in the production process, higher production of foam, and loss of flavors (Davenport, 1996). Deteriorating microbe must survive in an acidic condition that is low in oxygen and supplements and generally rich in CO2. As different microorganisms prefer different environments for growth, a specific beverage will enhance a particular microbe (Tribst et al., 2009) even if the products are manufactured in better production practices. The non-specific adaptable species will be grown if the production process will be failed and these species are highly usual. The microbe, which grows in that specific environmental conditions, will cause the deterioration of beverages. The species which is already unknown to be present in the beverages will also start to develop if novel ingredients or new process development with known components.
2 Soft drinks
Soft drinks are usually considered as non-alcoholic beverages that consist of different types of beverages (Table 1). According to the carbonation level, functionality, non-water as a main ingredient juice, flavouring, juice content, and sugar basis, it can be classified. Among them, functional soft drinks are currently in trend. These functional drinks are of various types such as nutraceuticals, beverages for wellness, energy drinks, sports drinks, and fortified drinks like juices that will be enriched with minerals and vitamins. Various types of microbe will involve spoiling soft drinks (Table 2).
Category of soft drinks
Example
Ingredients used
Carbonation level
pH
Sugar Concentration
Lemonades and colas
7up, Coca- cola, Pepsi
Additives, Flavouring agent, Acids, Sugars, Sweetener
Medium to high carbonation level
2.4 to 3.2
0 to 10%
Drinks for wellness
Hyvaa Paivaa, Fenix
Additives, Minerals, vitamins, soluble fibres, herbal extracts
Low to medium carbonation level
3.5 to 4.5
2 to 7
Beverages based on malt
Naturade, Bionade
Sweetener agent, organic flavours, Fermented wort
Low to medium carbonation level
Data not available
Data not available
Drinks for Energy
Battery, Red Bull
Additives, Vitamin B, glucuronolactone, sugars, L-carnitine amino acids, extracts from herbs, taurine, caffeine
Low to medium carbonation level
2.5 to 3.2
1.4 to 14%
Drinks for sports
Gatorade
Additives, Sugars, amino acids, caffeine, salts
Negligible to low carbonation level
3.2 to 4.0
5.5 to 8%
Beverages which is friendly for tooth
Good for me
Additives, carbohydrates, Non-nutritive
Negligible to low carbonation level
More than or equal to 5.0
No sugars
Type of Microbe
Hazardous substance
Effect on humans
Specific genus and species
Mainly affecting
Yeasts
Products by fermentation
Emetic responses
Not known
Juices from fruits
Protozoa
Infecting humans
Gastrointestinal disorders
Cyclospora cayatenensis, Cryptosporidium hominis, Cryptosporidium parvum.
Concentrates from fruits, and juices
Viruses
Infecting humans
Gastroenteritis, Liver inflammation
Rotavirus, Noravirus, Hepatitis A
Water and juices from fruits
Bacteria
Reactions of allergic, intoxications, infection by food borne microbe
Inconstant
Listeria monocytogenes, Escherichia coli O157:H7, Salmonella
Water, Concentrates and juices from fruits
Moulds
Mycotoxins
Acute and chronic toxicity
Byssochlamys, Aspergillus, Penicillium
Cereal and juices from fruits
2.1 Spoilage of soft drinks by yeast
Yeasts are considered to be a common contaminant in soft drinks. It will generally be found in constitutes, raw materials, or the beverage processing environment. Yeasts have an excellent ability to tolerate the range of carbonation beyond 3.0 vol; thus, it is considered to be an essential beverage spoilage microbe. Yeast could also endure acidic conditions; it has a wide range of pH requirements for its growth from 1.5 to 8.5 (Sperber, 2009) with optimum growth conditions between 3.0 and 6.5 (Lawlor et al., 2009). The production of ascospores from yeasts is the primary cause of spoilage in soft drinks prepared thermally. According to the spoilage capability, the yeast is classified into four groups (Table 3). The first group is the additive resistant and the fermentative microbe, which are the primary trouble makers in all the production process steps. The presence of extremophiles are scarce in the production environment, and even if it is present, its concentration will be very low or negligible. Most of the spoilage was caused by these type of microbe. Spoilage by this group will be controlled by additive or preservatives (James and Stratford, 2003). The third group's presence is an indication that the sanitation in the production plant is abysmal, but still, this group of microbe will not spoil the end product. The microbe, which is not typically connected or associated with soft drinks, is the fourth group of yeasts. The intermediate caused by fermentation and turbidity leads to the formation of flavorless end product. Occasionally, swelling of the package or even bursting of the box may occur due to the presence of carbon dioxide. The presence of fermentation spoilage yeasts may lead to gas pressure up to 2 to 7 bars, after growing for 14 days in a soft drink that contains glucose (1 M). Yeasts develop themselves in the soft drinks and pave the way for other microbes to grow in the drinks by solubilizing the preservatives of a weak acid in nature. The authorized limit of alcohol will sometimes exceed in the soft drinks, which are spoiled during fermentation metabolism and lead to ethanol formation and cause the ceiling to exceed. Notably, in the fruit juices and lemonades, the primary contaminant will be Saccharomyces cerevisiae (Back 2005), as it produces a massive quantity of carbon dioxide and also extremely fermentative. Some of the Saccharomyces strains will also have the capability to endure sulphates, sorbates and benzoates (Mollapour and Piper, 2008), which can also produce pectinase, results in clearing the products with hazy natureaert.
Group I – Resistant to preservatives and fermentation type
Group II – Hygiene and spoilage type
Group III – Hygiene type
Group IV – Aliens type
Zygosaccharomyces rouxii
Saccharomyces cerevisiae
Rhodotorula mucilaginosa
Kluyveromyces lactis
Zygosaccharomyces lentus
Saccharomyces bayanus
Rhodotorula glutinis
Kluyveromyces marxianus
Zygosaccharomyces bisporus
Pichia membranifaciens
Debaryomyces etchellsii
Zygosaccharomyces bailii
Pichia anomala
Cryptococcus laurentii
Schizosaccharomyces pombe
Lodderomyces elongisporus
Cryptococcus albidus
Saccharomyces cerevisiae (atypical strains)
Hanseniaspora uvarum
Candida tropicalis
Saccharomyces exiguous
Issatchenkia orientalis
Clavispora Lusitania
Dekkara naardenensis
Debaryomyces hansenii
Candida solani
Dekkara bruxellensis
Candida parapsilopsis
Candida sake
Dekkara anomala
Candida davenportii
Aureobasidium pullulans
A new microbe that causes spoilage in soft drinks, beverages and artificially flavored drinks is identified as Candida davenportii, which rarely causes spoilage and falls in group two spoilage microbe. Zygosaccharomyces bailii is highly notorious because of its characteristic features like higher sugar fermentation, better tolerance for salt, high resistance to weak acids (Steels et al., 2002; Martorell et al., 2007) which is commonly found in sauces and concentrates made with fruits. The Z. bailii also oxidatively degrade and catalyze benzoate and sorbate, paving the way for other spoilage microbes to grow better (Mollapour and Piper, 2008). A few viable cells in the package will affect the quality of end products (Van Esch, 1987). Another Zygosaccharomyces species, Z. lentus was identified to be similar in the physiological properties of Z. bailii. Dekkera yeasts, which produce ascospores, are the usual soft drink spoilers, which grows slowly, and it will take many weeks to develop the symptoms of spoilage. This dekkera yeast has the potential to withstand and grow in a severe concentration of carbonation but significantly less tolerance to benzoates and sorbates. This microbe usually is responsible for the formation of condensed sediments and clouds, which also oxidizes sugars into acetic acid. Dekkara yeast also has the characterization of weak fermentation in less concentration of oxygen. Another common microbe present in soft drinks is D. anomala, which is low fastidious for the vitamin requirement compared to D. naardenensis or D. bruxellensis.
2.2 Bacterial spoilage
Among bacteria, acetic acid and lactic acid bacteria are the common microbes that cause spoilage in soft drinks. These are highly potential microbes to endure highly acidic conditions, which is a key factor in growing and developing soft drinks. Leuconostoc mesenteroides and Lactobacillus paracasei are the common beverage spoiling species. Other strains that are commonly present in polluted products are Weissella confuse, Lactobacillus perolens, Lactobacillus plantarum, Lactobacillus buchneri and Lactobacillus brevis. Various other strains also have the potential to spoil the beer products (Hammes and Hertel, 2009). In apple juice, formic acid indicates that the drink is spoiled. Less astringency and carbonation are due to lactic acid bacteria. Sometimes, ropiness has been seen in the end product; the reason behind this is due to the production of glucose or fructose molecule from sucrose.
Gluconobacter and Acetobacter are the important genera which consist of many spoilage acetic acid bacteria. Apart from these two, Asaia and Gluconacetobacter sp. are commonly associated with soft drinks. The genus Asaia consists of eight species discovered in 2000 (Yamada and Yukphan, 2008). The major microbe group present in ethanol and sugar enriched drinks are acetic acid bacteria (Suzuki et al., 2008). The higher concentration of these microbes indicates poor cleanliness maintained in the production processing environment (Raspor and Goranovich, 2008).
Various microbe forms a biofilm on the surfaces (Horsáková et al., 2009). An important feature of acetic acid bacteria is its acid-tolerant capacity, which tends to grow at a very low pH like 3.6 to 3.8 and sometimes even at 3.0 pH (Suzuki et al., 2008). The ideal temperature for the multiplication of this microbe is from 25 to 30 °C, and its growth and developments in soft drinks may lead to swelling in the package, changes in the flavours, sediment, haze and ropiness formation. Acetic acid bacteria are not as regular contaminants as lactic acid bacteria in soft drinks because of their strict oxygen requirement for its growth. The evolving strain Asaia spp, spoils packed water with fruit flavours and ice tea.
Propionibacterium cyclohexanicum was identified from sour orange juice, which had no flavour, yet this microbe has the potential to grow in psychrophilic conditions in other fruit juices. Optimum temperature and pH for this microbe's growth ranges between 20 and 40 °C and 3.6, respectively. Potassium sorbate and natrium benzoate at a higher concentration, such as 500 mg/l and 1000 mg/l will resist the microbe's growth in juices (Walker and Phillips, 2008). P.cyclohexanicum is thermophilic and could withstand up to 95 °C for 10 min; thus, it can’t be removed in normal pasteurization methods (Walker and Phillips, 2007). Bacillus and Clostridium genus are spore formers, usually not grown in low pH, but the spores will be active in the products. These species will found to spoil the juices from vegetables (pH > 4) when compared to juices made from fruits. The beverage spoiler’s concentration is identified to increase in mixed beverages made from fruit or vegetable juices with cereal fibers. Especially, Clostridium species such as Clostridium sporogenous and Clostridium butyricum causes sour flavour in the end product by spoiling the sugar syrup either during the manufacturing stage or during storage, Since this is highly acid-tolerant and can grow in pH between 3.6 and 3.8 (Hawthorne et al., 1991).
Another important genus in spoiling the fruit juices is Alicyclobacillus is found to spoil the ice tea, lemonade, isotonic water, and fruit juices, which are carbonated and has a wide range of temperature optimum for its growth from 20 to 70 °C which also has the potential to grow in the restricted supply of oxygen supply (Smit et al., 2011). The visual identification of spoilage will be discoloration, haze and sediment formation will be seen in the juices, but it will be difficult to identify. Streptomyces griseus is also a major contaminant in apple juices (pasteurized) which produces earthy and musty flavour, which is a gram-positive and spore producers, which is highly resistant to increased temperatures (Siegmund and Pöllinger-Zierler, 2007).
2.3 Fungal spoilage
The fungus can contaminant the end product, by-product, or raw materials either with mycelium or spores (Filtenborg et al., 2004). The major source of fungal contaminants will be from soil (Tribst et al., 2009). Some species like Rhizopus and Fusarium will grow at a low oxygen concentration, i.e., about 0.01% v/v. Mycotoxin production can be inhibited by increasing the carbon dioxide concentration in media if the oxygen couldn’t be removed. Some of the heat-tolerant microbes belong to the genus of Talaromyces, Neosartorya and Byssochlamys are the major contaminants in products made from heat processes such as purees and juices from fruits and canned fruits (Hocking and Pitt, 2001). Millions of dollars will be lost if the fungus contaminates the beverage industry, especially by Eupenicillium brefeldianum, Neosartorya fischeri, Talaromyces flavus and Byssochlamys nivea (Scholte et al., 2004). Another important microbes group responsible for the spoiling beverage industry is Cladosporium and Penicillium genus (Wareing and Davenport, 2005). Inducers of gushing will be seen if the raw material is highly contaminated by fungus (Table 4). An impulsive over foaming of the beer immediately after the opening of the packed beer is known as gushing. It is considered to be a problematic phenomenon. This activity might be due to the production of gushing factors by the fungus, which presents either in the cereal of malt-based raw materials chosen for brewing (Amaha and Kitabatake, 1981; Munar and Sebree, 1997; Sarlin et al., 2005). It has been shown that contagious hydrophobins from strains of the genera Trichoderma, Fusarium and Nigrospora are responsible for this beer gushing (Sarlin et al., 2007). Hydrophobins are active fungal proteins that are believed to stabilize the bubbles from carbon dioxide by covering the microbubbles with a layer (Draeger, 1996; Pellaud, 2002).
Gram-positive bacteria
Rod-shaped Lactobacillus spp. Lb. brevis Lb. brevisimilis Lb. buchneri Lb. casei Lb. coryneformis Lb. curvatus Lb. lindneri Lb. malefermentans Lb. parabuchneri Lb. plantarum
Cocci Pediococcus spp. P. damnosus P. dextrinicus P. inopinatus Micrococcus sp. M. kristinae
Gram-negative bacteria
Rod-shaped Pectinatus spp. P. cerevisiiphilus P. frisingensis Selenomonas sp. S. lacticifex Zymophilus sp. Z. raffinosivorans
Cocci Megasphaera sp. M. cerevisiae Zymomonas sp. Z. mobilis
3 Alcoholic beverages:
Drinks an alcohol concentration of about 2.8% or more by volume is considered to be an alcoholic beverage. Alcoholic beverage production isn't an aseptic procedure. However, very few microbes will be able to spoil alcoholic beverages because of the following characteristics: intensively carbonated like 25 to 3.0 vol, highly acidic with a pH range from 2.4 to 3.5, low oxygen levels, and higher carbon to nitrogen ratio. Thus, fermentative yeast, lactic acid bacteria with acid tolerant capability, are the major microbe present in alcoholic beverages and carbonated drinks (Fig. 1).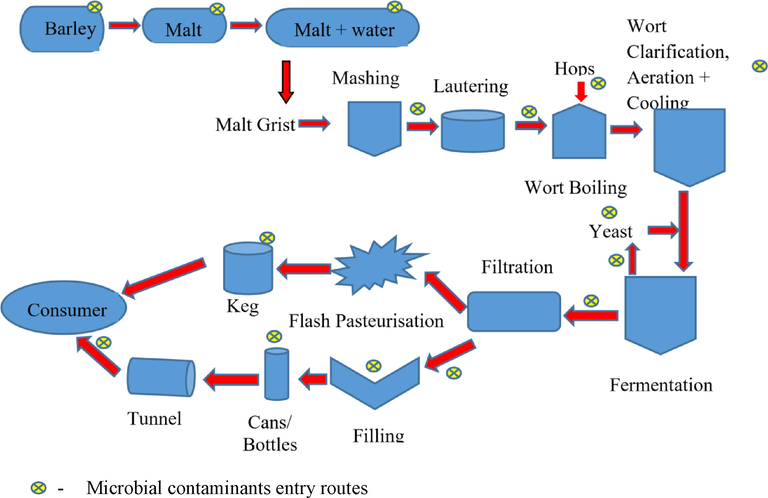
Brewing process flow chart and entry route of the microbe and its contaminant have been represented.
3.1 Yeast spoilage
Fermentative yeasts are the possible spoilers of alcoholic beverages from brewing due to its fermenting capability of sugars in an acidic and restricted oxygen environment. Most of the spoiling strains are resistant to additives. Ciders made from conventional methods was spoiled mostly by Saccharomycodes ludwigii, as it strongly resists the presence of sulphite at a concentration of 1000 to 1500 mg/l and grows in its presence. It can develop during all the manufacturing processes; however, spoilage usually is seen in packed products and might be contaminated during filling of the bottles. High amounts of volatile acids and esters will be produced by the overgrowth of Kloeckera apiculata. A mousy flavour by the presence of tetrahydropyridines compound was produced by Dekkara or Brettanomyces species and Pichia strains at the maturation stage. For the synthesis of tetrahydropyridines, lysine and ethanol are the precursors. Some of the volatile phenol producing microbe from phenolic acids are S. cerevisiae, Sz. Pombe, D. bruxellensis and Z. bailii, these volatile phenols are very dangerous at higher concentrations (Malfeito-Ferreira et al., 2009). By metabolizing the phenolic compounds, they detoxify and protect their cells from the effects of inhibition. Especially, brewers and wild yeast spoiled most beverage spoiling capability (Hutzler et al., 2008).
3.2 Bacterial spoilage
Alcoholic beverage spoiling bacteria mainly belong to Pediococcus and Lactobacillus strains (Fig. 2). During conventional cider manufacturing, Lactobacillus sp, which is alcohol resistant, will switch off the flavours and reduce alcohol yield (Hammes and Hertel, 2006). Lactobacillus sp. juices mainly spoiled cider of Scandinavian type. Due to the higher production of acetic acid by heterofermentative strains, the end product flavour was destroyed (Jarvis, 2003). Bitterness was seen due to the conversion of glycerol 3-hydroxypropionaldehyde, which can modify the acrolein and blend with polyphenols leads to the formation of bitterness (Sauvageot et al., 2000; Garai-Ibabe et al., 2008). Diacetyl will be produced by some Lactobacilli, which results in buttery flavour (Jarvis, 2003). The tetrahydropyridines from hetero fermenters result in off mousy flavour. The usual spoilage seen in natural cider is the ropiness characteristic (Ibarburu et al., 2010). This polysaccharide is usually made up of glucans, especially from glucose.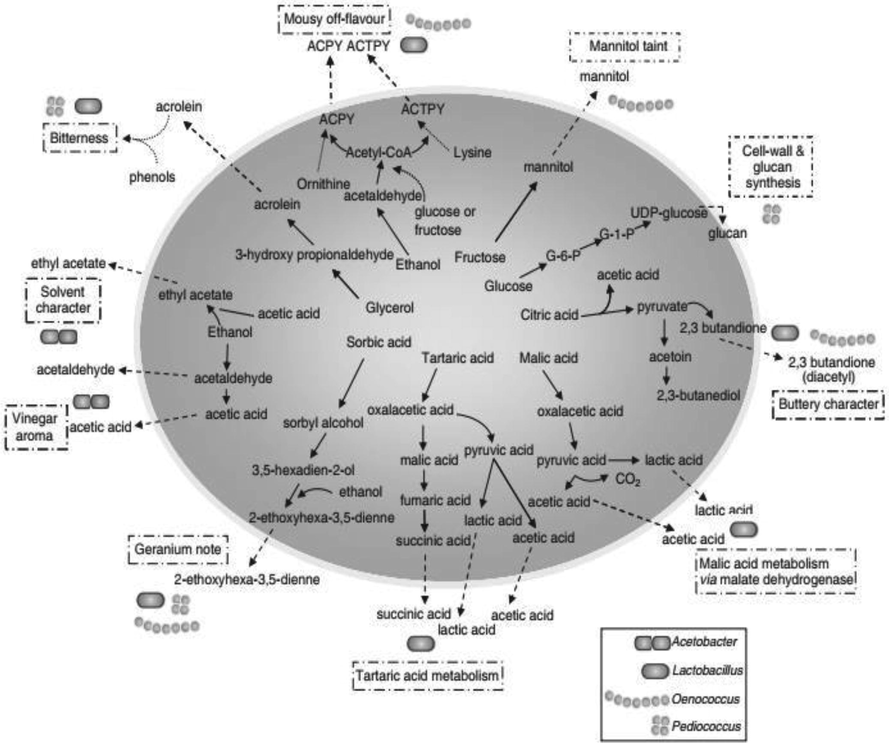
Summary of bacterial pathways leading to spoilage aroma and flavour compounds of wine (Sponholz, 1993; Bartowsky and Pretorius, 2008).
The common rope forming strains are Lb. suebicus, Lb. diolivorans and Lb. collinoides. Volatile phenols and off medicinal flavors were formed by most Lactobacilli (Hammond et al., 1999). Another important spoilage microbe is Pediococci, which is uniform round cells commonly found in tetrads (Holzapfel et al., 2006). Pediococci may also induce ropiness by producing volatile phenols (Barthelmebs et al., 2000; Couto et al., 2006). P. parvulus is identified in french cider, which is ropy. Other important strains of Pediococci responsible for ropy are P. claussenii and P. damnosus and (Dobson et al., 2002). In the brewing industry, two new strains of Pediococci which are tolerant to ethanol were P. ethanolidurans and P. cellicola were identified (Zhang et al., 2005; Zhang and Dong, 2006) at the highly acidic condition with pH 3.5 (Fig. 3). Two important genera Gluconobacter and Acetobacter could affect the beverage at any time with oxygen availability (Bartowsky and Henschke, 2008). Normal contaminants present in the conventional cider brewing industry are G. oxydans and A. pasteurianus (Jarvis, 2003). Acetic acid bacteria did ethanol oxidization and this will further catabolize the acetic acid into water and carbon dioxide (Bartwosky and Henschke 2008). The product, which is spoiled by this microbe, results in vinegar flavour with ethanol with reduced pH (Raspor and Goranovich, 2008), which will also cause cider sickness. Another microbe responsible for casing cider sickness is Zymomonas mobilis (Coton et al., 2006), a gram-negative anaerobe tolerates a little bit of oxygen produced carbon dioxide and ethanol fermenting glucose and fructose.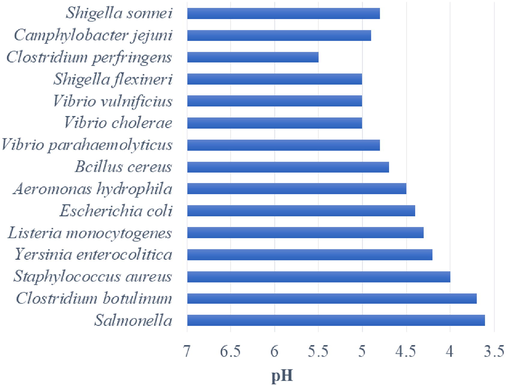
Minimum requirement of pH for the growth of pathogenic microbe in beverages.
3.3 Microbiological health risks linked with beverages
Soft drinks are generally considered safe, which will not create any food-borne illness, but that can’t be completely kept out. Around 32 outbreaks had been documented since 1922, with respect to food-borne diseases, mainly by consuming partially sterilized fruit juices. These outbreaks will be mostly by the low production process and cleanliness in the production plant (Vojdani et al., 2008). These outbreaks from beverages were mainly caused by an enteric group of pathogens, including protozoans, viruses and bacteria. But in most of the cases, the identification of the causative agent will be challenging and impossible (Parish, 2009). Another important risk for health with these beverages is mycotoxin from fungus (McCallum et al., 2002).
4 Presence of pathogenic bacteria
Some of the pathogenic bacteria will have the potential to grow in carbonated and acidic drinks, not in packed products. A research study showed that Salmonella and Escherichia coli were found to survive in soft drinks of cola-type for about 48 hrs (Sheth et al., 1988). Another study was done with Staphylococcus aureus, Listeria monocytogenes, Salmonella enterica and E. coli (Medina et al., 2007). A study with Yersinia enterocolitica, showed its survival potential in soft drinks made from orange with a pH of 3.5 for about 3 days at 30 °C (Akond et al., 2009). There were no reports for bacterial pathogens in soft drinks that are commercially available in developed countries. Mainly, juices from orange and apple are common disease-carrying mediators. Various studies have shown that these bacteria can survive and transmit the diseases in acidic juices. The enteric group's pathogens are not a common spoiling microbe and the contamination will be by the contamination by faeces either by indirect or direct contact (Tribst et al., 2009). Listeria monocytogenes has not yet been involved in outbreaks related to juice but found to be survived in the concentrates made from frozen juice (Oyarzabal et al. 2003). Recently conducted research had shown that the Y. enterocolitica found to survive for a longer period of time in juices made from orange with a pH of 6.3 (Estrada et al., 2010). Various pathogenic bacteria will also grow in sweet wort (García and Heredia, 2009). During the beer wort fermentation, the microbes like L. monocytogenes, Salmonella typhimurium and E. coli H157:07 were rapidly losing its activity. The pathogen's survival is found to be enhanced in higher pH, less concentration of ethanol with original gravity.
4.1 Presence of viruses and parasites
Viruses and Parasites were also associated with the outbreaks of disease after fruit juice consumption. Mainly, protozoa will survive only in the host at a resting stage like oocyst, which will be secreted by the hosts already infected by faeces (Dawson, 2005). Through the faecal oral route, the contamination will spread in beverages and food. Some of the oocysts may even cause gastroenteritis (Erickson and Ortega, 2006). From 1990s to 2000s, a protozoan Cryptosporidium partum was identified in juices and ciders. The unprocessed or less processed end products was the main reason for the outbreaks. C. partum oocysts have been appeared to lose >85% of its viable cells for about 24 hrs in beverages like beer of pH around 3.81 to 3.85 and a cola drink with a pH of 2.46 at temperatures of 4 and 22 °C (Friedman et al., 1997). Another important protozoan transmitted through fresh products like raspberries is Cyclospora cayatensis. In developed countries, protozoa's food-borne illness is found to be very less. Since viruses need living cells for its replication, it don't develop in food products. Nonetheless, just a couple of infection particles may bring about a high likelihood of disease. Some viruses like, Hepatitis A, rota, and nota viruses transmit food-borne illnesses by maintaining the worst cleanliness in the production and packaging process. In 1960s, Hepatitis A was found to be transmitted through juices made from orange (Parish, 2009). An outbreak from norovirus was associated with the raspberries cultivated by using contaminated water (Newell et al., 2010).
4.2 Mycotoxins in beverages
The development of filamentous fungi is typically not to be expected in the production of beverages. Nevertheless, during process and storage, various filamentous growths will have the potential to secrete secondary metabolites under stress conditions. When these mycotoxins are absorbed or inhaled or ingested, it will lead to illness or death in humans and animals. Almost in all taxonomy groups, these toxic producing groups and heterogeneous groups will be present (Drusch and Ragab, 2003). Though various mycotoxins are present, only a few possess high food products (Murphy et al., 2006). Usually, the genus of Alternaria, Fusarium, Penicillium, and Aspergillus produces mycotoxins that pose a severe threat to food-borne illness. Fusarium toxins are produced by zearalenone and trichothecenes, while aflatoxin was produced by A. patulin and ochratoxin. A huge amount of mycotoxin may have toxin effects ranging from chronic to acute like immune system suppression and high cancer risk to liver and kidney damages, respectively (Table 5). Mycotoxin will transmit to animals via animal feed and affects its health. This mycotoxin is highly stable and can survive in the entire production process and enter the end product. The major reason for mycotoxin entry in the production process is through the contaminated raw materials like cereal extracts and fruit juices (Boeira et al., 1999). According to the fermentation time, the type of yeast involved, the concentration of toxin the degree of growth will be inhibited (Kelsall and Lyons, 2003).
Mycotoxin
Source microbe
Beverage and food source
Aflatoxins
Aspergilus paracitus and Aspergilus flavus
Spices, dry fruits, plant seeds, nuts and cereals
Ochratoxin A
P. verrucosum and Aspergilus carbonarius,
Coffee, wine, grape juice, cereals and dry fruits
Fumonisin
F. nygamai, F. proliferatum, F. verticillioides,
Grits, corn meal and corn
Zearalenone
F. semitectum, F. graminearum, F. equiseti, F. culmorum, and F. crookwellense
Products from Cereals
Trichothecenes
F. sporotrichoides, F. langsethiae, F. culmorum F. gramine-arum, F. cerealis and F. acuminatum
Products from Cereals
Patulin
A. clavatus, A. terreus, P. expansum, B. nivea
Juices from fruits with low acid, cereals, olives, berries, apples, pears, grapes and apricots
Another important mycotoxin patulin has drawn considerable attention among researchers because of its spoiling capability of juices from berry and fruits (Delage et al., 2003). Penicillium spp produced this toxin found to contaminate apple ciders, juices from pears and apples. In post-harvest fruits, a blue mould rot disease will be caused by P. expansum. An indirect mutagenic effect like cross-links of DNA to DNA and cell mutation was induced by patulin, which also directly reacts with DNA, which also possess carcinogenic effects. Though the mechanism of the patulin toxicity mechanism was unknown in humans, the U.S. FDA had set 50 ppm is the maximum limit for tolerance in products derived from apple. Other mycotoxins can damage DNA such as penicilpenicillic acid, luteoskyrin, citrinin, zearalenone, sterigmatocystin are a group of aflatoxin (Paterson and Lima, 2010). Various research was done to determine the levels of patulin in cider and apple juice. A study by Harris et al. (2008) found that the patulin concentration in Michigan juices and apple cider to be below the maximum allowed. A report by Murillo-Arbizu et al. (2009) found the patulin to be exceeded in Spain's apple juices. In Northeast China, a survey had been conducted and identified that a higher concentration of patulin of about >50 Pl/l was seen in 16 percentage of apple products like concentrates from apple, baby food, the juice from apple and mixed juices, Youngsters are frequent consumers of apple beverages, and reduce the value from 50 Pg/l in order to protect them (Tangni et al., 2003).
5 Other microbial metabolites in beverages
Biogenic amines such as, polyamines like spermidine and spermine; diamines like, cadaverine and putresciene; aromatic amines like phenyletylamine and tyramine and heterocyclic amines like tryptamine and histamine, which was usually found in many types of beverages and foods. Biogenic amines will be produced in the food either by the presence of decarboxylase producing microbe such as lactic acid bacteria, Photobacterium, shigella, Pseudomonas, Proteus, Escherichia, Klebsiella, Clostridium, Citrobacter and Bacillus or by the activity of amino acid decarboxylase endogenously in the raw materials (Silla Santos, 1996). Biogenic amines concentration will be enhanced in the food by fermentation by microbe or during the spoilage steps (Karovicova and Kohajdova, 2005). Some amines have higher concentrations in the end products because of the worst processing of food, storage, contamination by microbe and low quality of raw materials. Biogenic amines will cause health hazards at higher concentrations being ingested (Donhauser et al., 1993). Hypertensive emergencies have been seen after the patients consumed monoamine oxidase inhibitors (MAOI) containing beer (Tailor et al. 1994). The presence of tyramine in tap and alcoholic beer has found to cause adverse effects, particularly if the concentration exceeds 6 mg within the period of 4 hr or beer contains >10 mg/l of tyramine, leads to potent danger to the patients who consumed it. Healthy persons doesn’t report any health risks (Shalaby, 1996). For the synthesis of carcinogenic nitrosamines, bigenic amines have acted as potential precursors. A research report also showed that the production of carcinogenic amines from some of the azo dyes would also be produced by lactic acid. These azo dyes are broadly used in soft drinks industries in various countries, but it is added in the list of restricted preservatives in Finland. These biogenic amines can be used as an indicator of the quality of packed products as a signal of activity of microbe (Rokka et al., 2004). The presence of a higher concentration of biogenic amines like histamine, cadaverine, and tryptamine in fermented beverages of in beers indicates the poor quality of the brewing production process. A report by Halasz et al. (1999) showed that the presence of histamine in beverages could be considered as an indicator, as histamine will not be present in malt or barley. Hence it is an excellent indicator of cleanliness in brewing, malting, or storage. Accumulation of amines in beverages and beer was due to lactic acid bacteria. The arginine in normal fruit juices will be utilized lactic acid bacteria to produce biogenic amines like agmatine and putrescine. Inadequate pasteurization will lead to the survival of lactic acid bacteria in beers, resulting in the formation of histamine and tyramine (Kalac et al., 2002). An important key factor for the production of drinks is biological acidification A test by Donhauser et al. in the year 1993, was done with eleven lactic acid bacteria on functional drinks and found that there was no increase in biogenic amine concentration when compared to the normal wort (Table 6). A spontaneous malolactic and alcoholic fermentation will occur with the addition of lactic acid bacteria and yeast (Garai et al., 2006). Thus, biogenic amine production is possible.
Technological improvements
Effects
Enzymology
Activation of important mash enzymes
Nutrients
Improved zinc bioavailability
Elimination of proteins
Improved break formation
Redox potential
Better hot trub precipitationLower sensitivity to oxygen, more buffering substances
Fermentation
Rapid decrease in pH Higher final attenuation
Filtration
Lower wort viscosity, faster lautering
Sensory improvements
Lower beer viscosity, faster filtration
Taste
Fuller and smoother flavour profile
Hop
bitterness Smoother bitterness
Mouthfeel
Fresh character
Foam
Finer bubbles Stable, longer lasting
Colloidal stability
Lower risk of protein haze
Microbial stability
Lower risk of microbial contamination
In natural ciders, the presence of putrescine, tyramine and histamine will be commonly seen. The level of amine levels will be varied, ranging from negligible to 23 mg/l in ciders. The production of biogenic amines like tyramine and histamine in cider synthesis by lactic acid bacteria. Lb. diolivorans were considered to a rigorous producer of histamine (Lachenmeier and Sohnius, 2008).
6 Factors microbial growth in beverages
Various key factors like extrinsic and intrinsic play a major role in the microbe's stability in alcoholic beverages and soft drinks. Intrinsic factors such as the presence of antimicrobials, nutrient supplements, carbonation and acidity. The end product's microbiological quality will be determined by storage conditions, packaging, production process, cleanliness in the production plant, and the raw material (Sperber, 2009).
6.1 pH and acidity
pH and acidity are important factors that hinder the beverage quality (Mentz et al., 2010). Usually, the growth of the spoiling microbe will increase with an increase in pH. Naturally, the pathogens that cause food-borne illness will not grow at pH 4.6 (Lawlor et al., 2009). Hence the pH of the alcoholic beverage has to be set below this level. Low pH alone does not guarantee the stability and safety of the end product. The inactivation rate and the minimum pH requirement will be based on the acidulant nature inhibitor presence and the mechanism of resistance for the acids by microbe (Lücke, 2003). The microbe survival was highly affected by weak acids, as these weak acids will strongly inhibit the growth of the microbe in its undissociated forms at low pH conditions. Clostridium sps that produce butyric acids will have optimum growth conditions at the range of pH from 4.0 to 4.5 and at elevated temperatures (Lücke, 2003). Microbes that grow in sweet wort unhoped, Salmonella typhimurium and E. coli H157:07 at a pH of 4.5 and no growth and development were seen at pH 4.5 (Mentz et al., 2010). Some fruit juices with low acid values like watermelon, papaya, persimmon, melon, cantaloupe, and acai contain pathogen of beverage associated. Even though some foods highly acidic inhibit the multiplication of microbe but it couldn’t affect its survival. Hence the pathogenic microbe will survive for longer periods in these juices. The adaptation for acidic conditions will enhance microbe's adaptive capability to preservatives. Protozoans inactivation at lower pH has led to varied results. Most of the viruses associated with food-borne illness are highly acidic tolerant and can survive during fermentation of acidification as a method for preserving the food (Baert et al., 2009).
6.2 Carbonation and oxygen
Soft drinks that are carbonated are less susceptible to spoilage by microbe than soft drinks that are not carbonated. The spoiling microbe's inhibition will be done by lowering the cytoplasmic membrane's pH, sporulation induction, buffering of cytoplasmic perturbation, utilization of amino acid inhibition, cell division inhibition. The average level of carbonation will be around 3 volumes in soft drinks. However, some yeasts have potentially resisted the carbonation in the soft drinks at standard volumes, for example, Saccharomyces and Dekkera genus (Stratford, 2006). Some drinks will be less carbonated than conventional drinks, which will pave the way for spoilage microbe's growth. At a high concentration of CO2, some viruses will have the potential to survive in a modified atmosphere.
6.3 Nutritional status
The type of microbial spoiling and its rate depended highly on the composition of nutrients used in the brewing industry. Generally, the beverage production ingredients will provide nutrient supplements for the microbial growth that will lead to spoilage (Table. 7). Different beverages possess different nutritional qualities. The high juice containing fortified drinks will represent the rich environment while soft drinks manufactured synthetically represent a poor microbial growth environment. The nutrient less soft drinks will be spoiled by Dekkara species, which is highly tolerant of citric acid. Yeast's spoilage will be enhanced if additional sugar was added to the low sugar formulations as it will improve the susceptible of the juices to the yeast spoilage. Simultaneously, reducing the sugar concentration in rich sugar containing formulations will not reduce the spoilage by the yeast, which is fermentative. Soft drinks with 5 to 10% sugar concentration will also equally get spoiled. A test with an herbal drink without sugar was conducted and found that this drink supported around 150 species of yeast growth, but none can grow in the drink (Stratford, 2006).
Antimicrobial hurdles
Mode of inhibition
Intrinsic hurdles
Ethanol
Inhibits cell membrane functionality
Low pH
Affects enzyme activity Enhances inhibitory effects of hops
Hops
Inhibits cell membrane functions Affects Gram-positive bacteria only
Carbon dioxide
Creates anaerobic conditions Lowers pH Affects enzyme activity Affects cell membrane
Low oxygen levels
Creates anaerobic conditions
Lack of nutrients
Starves cells
Sulphur dioxide
Affects various metabolic systems
Extrinsic hurdles
Mashing
Causes thermal destruction of cells
Kettle boil
Causes thermal destruction of cells
Pasteurization
Causes thermal destruction of cells
Filtration
Removes cells by physical size exclusion Bottle
Conditioning Not applicable to all beers.
Creates anaerobic conditions
7 Control measures
The measures to control the spoilage microbe will be by maintaining irradiation by UV, surface waxes, surfactants, chemical sanitizers, immersion of the products in hot water, storage temperature control, filtration and equipment cleanliness (Fig. 4). The microbial load has minimized by using dimethyl dicarbonate, a preservative, to cold, pasteurize the fruit juices. Other methods like using UV light, pused electric field and processing the products by high pressure. Growth of the bacteria and yeast will be modeled, an effective technique to control the spoilage (Battey et al., 2001; Shearer, et al., 2002). Residues of fruits and sticky sugar-containing products are highly susceptible to contamination by moulds and yeasts. Excellent sterile practices and adherence to GMPs are the best control measures for microbial pollution in the soda pops industry, especially for yeasts. Sustenance processors around the globe have embraced the Hazard Analysis and Critical Control Point (HACCP) approach. In the United States, HACCP is mandatory for natural product juice processors, with great rural practices (GAP) to establish a fruitful HACCP framework.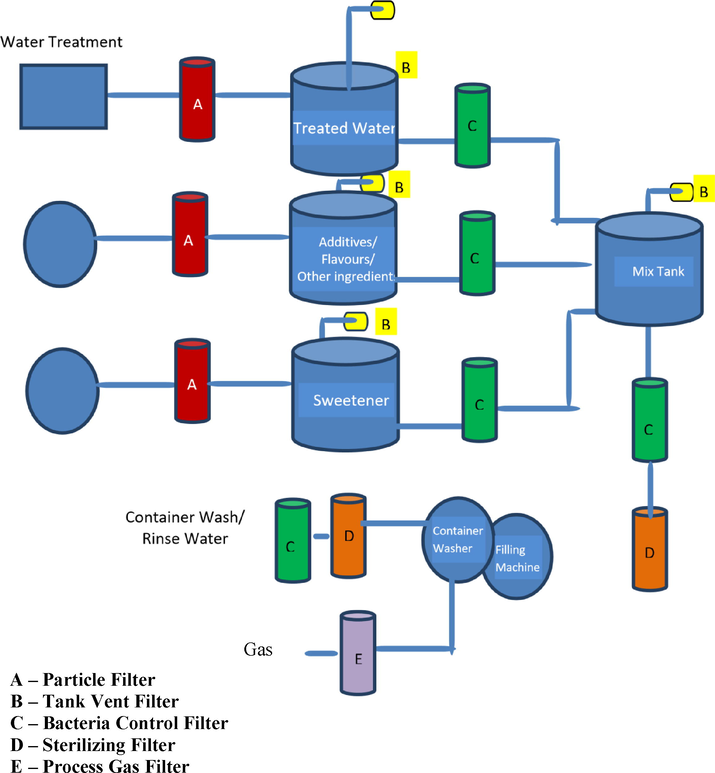
Filtration process to remove microbe in soft drink production.
8 Conclusion
Current additives and common antimicrobials have, to some degree, constrained antimicrobial action. It is improbable that new nourishment additives will be acknowledged sooner rather than later. In this manner, abuse of the synergistic impacts of the current characteristic antimicrobials and deep physical safeguarding strategies is the most powerful approach for controlling unsafe microorganisms in refreshments later on. Organic concentrates and smell mix officially utilized as a part of the refreshment details have potential as normal antimicrobials and could be abused with the end goal of protection. Natural fermentation of the beverage bases is another approach with tremendous potential for upgrading soundness, well-being, and the refreshments' healthful and tangible nature. The fate of refreshment conservation methods will be a talented mix of antimicrobial obstacles to keep up microbiological strength and security while keeping up the most significant tangible and nutritious quality.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the Project no. (IFKSURP-1435-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bacterial contaminants in carbonated soft drinks sold in Bangladesh markets. Int. J. Food Microbiol.. 2009;130:156-158.
- [Google Scholar]
- Gushing in beer. In: Pollock J.R.A., ed. Brewing Science. London, UK: Academic Press; 1981. p. :457-489.
- [Google Scholar]
- Back, W., 2005. Colour Atlas and Handbook of Beverage Biology. In: Back, W. (ed.), Verlag Hans Carl: Nürnberg, Germany, p. 317.
- The efficiency of preservation methods to inactivate food-borne viruses. Int. J. Food Microbiol.. 2009;31:83-94.
- [Google Scholar]
- Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveal the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Env. Microbiol.. 2000;66:3368-3375.
- [Google Scholar]
- Bartowsky, E.J., Pretorius, I.S., 2008. Microbial Formation and Modification of Flavor and Off-Flavor Compounds in Wine, In book: Biology of Microorganisms on Grapes, in Must and in Wine, pp. 209–231.
- Acetic acid bacteria spoilage of bottled red wine – a review. Int. J. Food Microbiol.. 2008;125:60-70.
- [Google Scholar]
- Modelling mould spoilage in cold-filled ready-to-drink beverages by Aspergillus niger and Penicillium spinulosum. Food Microbiol.. 2001;18:521-529.
- [Google Scholar]
- Inhibitory effect of Fusarium mycotoxins on growth of brewing yeasts Zearalenone and Fumonisin B1. J. Inst. Brew.. 1999;105:366-374.
- [Google Scholar]
- Yeast ecology in French cider and black olive natural fermentations. Int. J. Food Microbiol.. 2006;108:130-135.
- [Google Scholar]
- Ability of lactic acid bacteria to produce volatile phenols. Am. J. Enol. Viticul.. 2006;57:166-171.
- [Google Scholar]
- Forensic microbiology for soft drinks business. Soft. Drink. Manag. Int. 1996:34-35.
- [Google Scholar]
- Phylogenetic analysis of the genus Pediococcus: Pediococcus claussenii sp. nov., a novel lactic acid bacterium isolated from beer. Int. J. Sys. Evol. Microbiol.. 2002;52:2003-2010.
- [Google Scholar]
- Donhauser, S., Wagner, D., Geiger, E., 1993. Biogenic amines. Significance, occurrence and assessment. Brauwelt. Int. Part II, pp. 100–107.
- Draeger, M., 1996. Physical observations on the subject of gushing. Brauwelt. Int. IV, pp. 363–367.
- Mycotoxins in fruits, fruit juices and dried fruits. J. Food. Prote.. 2003;66:1514-1527.
- [Google Scholar]
- Inactivation of protozoan parasites in food, water, and environmental systems. J. Food. Prote.. 2006;69:2786-2808.
- [Google Scholar]
- Effects of organic acids, nisin, lyzozyme and edta on the survival of Yersinia enterocolitica population in inoculated orange beverages. J. Food Saf.. 2010;30:24-39.
- [Google Scholar]
- Filtenborg, O., Frisvad, J., Samson, R., 2004. Specific association of fungi to foods and influence of physical environmental factors. In: Introduction to food- and airborne fungi. 7th ed. Samson, R., Hoekstra, E. and Frisvad, J. (eds). Centraalbureau voor schimmelcultures, Utrecht, The Netherlands, pp. 306–320.
- The potential for Cryptosporidium parvum oocyst survival in beverages associated with contaminated tap water. J. Food Saf.. 1997;17:125-132.
- [Google Scholar]
- Glycerol metabolism and bitterness producing lactic acid bacteria in cidermaking. Int. J. Food Microbiol.. 2008;121:253-261.
- [Google Scholar]
- Food-borne pathogens and toxins: an overview. In: Heredia N., Wesley I., García S., eds. Microbiologically Safe Foods. John Wiley & Sons, Inc.; 2009. p. :15-52.
- [Google Scholar]
- The biogenic amine content of beer; the effect of barley, malting and brewing on amine concentration. Zeitschrift für Lebensmittel-Untersuchung und -Forschung.. 1999;208:418-423.
- [Google Scholar]
- The Genera Lactobacillus and Carnobacterium. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., eds. The Prokaryotes: A Handbook on the Biology of Bacteria (third ed). New York: Springer; 2006.
- [Google Scholar]
- Genus I. Lactobacillus beijerinck 1901, 212AL. In: De Vos P., Garrity G., Jones D., Krieg N.R., Ludwig W., Rainey F.A., Schleifer K.-H., Whitman W.B., eds. Bergey’s Manual of Systematic BacteriologyBergey’s Manual of Systematic Bacteriology. New York, USA: Springer; 2009. p. :465-513.
- [Google Scholar]
- Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Appl. Microbiol. Biotechnol.. 2008;78:997-1006.
- [Google Scholar]
- Moulds. In: Moir C.J., Anderw-Kabilafkas C., Arnold G., Cox B., Hocking A.D., Jensen I., eds. Spoilage of Processed Foods: Causes and Diagnosis. Australia: AIFST Inc. (NSW Branch) Food Microbiology Group; 2001. 361–281
- [Google Scholar]
- Asaia sp. as a bacterium decaying the packaged still fruit beverages. Czech J. Food Sci.. 2009;27:S362-S365.
- [Google Scholar]
- Beer mixed beverages: dangerous spoilage yeasts, susceptible beverages. Brauwelt. Int.. 2008;26:206-211.
- [Google Scholar]
- A real time PCR assay for detection and quatification of 2-branched (1,3)- ȕ-D-glucan producing lactic acid bacteria in cider. Int. J. Food Microbiol.. 2010;143:26-31.
- [Google Scholar]
- Spoilage yeasts with emphasis on the genus Zygosaccharomyces. In: Boekhout T., Robert V., eds. Yeasts in Food: Beneficial and Detrimental Aspects. B. Behrs Verlag GmbH & Co; 2003. p. :171-186.
- [Google Scholar]
- Practical management of yeast: conversion of sugars to ethanol. In: Jacques K.A., Lyons T.P., Kelsall D.R., eds. The Alcohol Textbook. A Reference for the Beverage, Fuel and Industrial Alcohol Industries (fourth ed.). Nottingham, UK: Nottingham Univeristy Press; 2003. p. :121-133.
- [Google Scholar]
- The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: evidence from a large chemical survey. Food Chem. Toxicol.. 2008;46:2903-2911.
- [Google Scholar]
- Lawlor, K., Schuman, J., Simpson, P., Taormina, J., 2009. In: Sperber, W.H. and Doyle, M.P. (eds.) Compendium of the Microbiological Spoilage of Foods and Beverages, Food Microbiology and Safety, pp. 245–283, Springer, New York.
- The control of pH. In: Zeuthen P., Bogh-Sorensen L., eds. Food Preservation Techniques. Cambridge, UK: Woodhead Publishing Limited; 2003. p. :109-125. Part II
- [Google Scholar]
- Wine spoilage by fungal metabolites. In: Moreno-Arribas M.V., Polo M.C., eds. Wine Chemistry and Biochemistry. New York: Springer; 2009. p. :615-645. Chapter 11
- [Google Scholar]
- Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii isolated from high sugar environments. Int. J. Food. Microbial.. 2007;114:234-242.
- [Google Scholar]
- Factors affecting patulin production by Penicillium expansum. J. Food. Prote.. 2002;65:1937-1942.
- [Google Scholar]
- Weak organic acid resistance of spoilage yeasts. In: Anonymous British Mycological Society Symposia Series. Stress in Yeast and Filamentous Fungi. Academic Press; 2008. p. :143-155.
- [Google Scholar]
- Occurence of patulin and its dietary intake through apple juice comsumption by the Spanish population. Food Chem.. 2009;113:420-423.
- [Google Scholar]
- Foodborne diseases – The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol.. 2010;139:S3-S15.
- [Google Scholar]
- Food safety issues and the microbiology of fruit beverages and bottled water. In: Heredia N., Wesley I., García S., eds. Microbiologically Safe Foods. John Wiley & Sons, Inc; 2009. p. :291-304.
- [Google Scholar]
- Toxicology of mycotoxins. In: Luch A., ed. Molecular, Clinical and Environmental Toxicology. Clinical Toxicology. Basel: Springer; 2010. p. :31-63.
- [Google Scholar]
- Pellaud, J., 2002. Gushing: state of the art. The Xth Jean de Clerck Chair, Leuven Belgium, 2002.
- Biotechnological applications of acetic acid bacteria. Cri. Rev. Biotechnol.. 2008;28:101-124.
- [Google Scholar]
- Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions B. Biogenic amines as quality indicating metabolites. Food. Cont.. 2004;15:601-607.
- [Google Scholar]
- Fungal hydrophobins as predictors of the gushing activity of malt. J. Inst. Brew. 2005:111.
- [Google Scholar]
- Glycerol metabolism in Lactobacillus collinoides: production of 3-hydroxypropionaldehyde, a precursor of acrolein. Int. J. Food Microbiol.. 2000;55:167-170.
- [Google Scholar]
- Spoilage fungi in the industrial processing of foods. In: Samson R., Hoekstra E., Frisvad J., eds. Introduction to food- and airborne fungi (seventh ed.). Utrecht, The Netherlands: Centraalbureau voor schimmelcultures; 2004. p. :339-359.
- [Google Scholar]
- Significance of biogenic amines to food safety and human health. Food Res. Int.. 1996;29:675-690.
- [Google Scholar]
- Heat resistance of juice spoilage microorganisms. J. Food. Prote.. 2002;65:1271-1275.
- [Google Scholar]
- Survival of enteric pathogens in common beverages: An in vitro study. Am. J. Gastroenterol.. 1988;83:658-660.
- [Google Scholar]
- Growth behavior of off-flavor-forming microorganisms in apple juice. J. Agri. Food. Chem.. 2007;55:6692-6699.
- [Google Scholar]
- Biogenic amines: their importance in foods. Int. J. Food Microbiol.. 1996;29:213-231.
- [Google Scholar]
- Alicyclobacillus spoilage and isolation – a review. Food Microbiol.. 2011;28:331-349.
- [Google Scholar]
- Introduction to the. In: Sperber W.H., Doyle M.P., eds. Compendium of the Microbiological Spoilage of Foods and Beverages, Food Microbiology and Safety. New York: Springer; 2009. p. :1-39.
- [Google Scholar]
- Wine spoilage by microorganisms. In: Fleet G.H., ed. Wine Microbiology and Biotechnology. Sydney: Harwood Academic Publishers; 1993. p. :395-420.
- [Google Scholar]
- Zygosaccharomyces kombuchaensis: The physiology of a new species related to the spoilage yeasts Zygosaccharomyces lentus and Zygosaccharomyces bailii. FEMS Yeast Res.. 2002;2:113-121.
- [Google Scholar]
- Food and beverage spoilage yeasts. In: Querol H., Fleet G., eds. Yeasts in Food and Beverages. Berlin, Germany: Springer-Verlag; 2006. p. :335-379. Chapter 11
- [Google Scholar]
- Sake and beer spoilage lactic acid bacteria – review. J. Inst. Brew.. 2008;114:209-223.
- [Google Scholar]
- Patulin in domestic and imported apple-based drinks in Belgium: occurrence and exposure assessment. Food. Addi. Contam.. 2003;20:482-489.
- [Google Scholar]
- Review: Microbiological quality and safety of fruit juices–past, present and future perspectives. Cri. Rev. Microbiol.. 2009;35:310-339.
- [Google Scholar]
- Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food. Prote.. 2008;71:356-364.
- [Google Scholar]
- Microbiology of soft drinks and fruit juices. In: Ashurst P., ed. Chemistry and Technology of Soft Drinks and Fruit Juices (second ed.). Blackwell Publishing Ltd.; 2005. p. :279-299.
- [Google Scholar]
- Genera and species in acetic acid bacteria. Int. J. Food Microbiol.. 2008;125(1):15-24.
- [Google Scholar]
- Pediococcus ethanolidurans sp. isolated from the walls of a distilled-spirit-fermenting cellar. Int. J. Sys. Evol. Microbiol.. 2006;56:2405-2408.
- [Google Scholar]
- Pediococcus cellicola sp. a novel lactic acid coccus isolated from a distilled-spirit-fermenting cellar. Int. J. Sys. Evol. Microbiol.. 2005;55:2167-2170.
- [Google Scholar]







