Translate this page into:
A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks
⁎Corresponding authors at: Unit of Vector Control, Phytochemistry and Nanotechnology, Department of Zoology, Annamalai University, Annamalainagar 608 002, Tamil Nadu, India. CO2 Research and Green Technologies Centre, VIT University, Vellore 14, India. mushahid@ksu.edu.sa (Shahid Mahboob), drgovind1979@gmail.com (Marimuthu Govindarajan), vijimicro21@gmail.com (Shankar Vijayalakshmi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carbon dioxide gas is the key element of the carbon cycle and a major source for photosynthesis, but for the past 150 years, the atmospheric CO2 has been increased drastically from 250 to 418 ppm due to the extreme utilization of fossil fuels. This accelerated release of CO2 acts as a major source for climatic change due to the greenhouse gas effect resulting in global warming and melting of polar ice caps, alteration in biogeochemical cycles, altered rainfall, ocean acidification, eutrophication of lakes, imbalance in the ecological communities and extinction of some species, effects on soil fertility, changes in the metabolism and at the molecular level. Reduce, reuse and recycle strategy can be applied to control elevated CO2 levels by preventing deforestation, using renewable energy as an alternative for fossil fuels and reusing the atmospheric CO2. Carbon capture and storage (CCS), Carbon capture and utilization (CCU) are the two technologies adapted to capture the atmospheric CO2, utilize it, and focus on permanent storage in the geological sites. Captured CO2 is used to produce many value added products such as polymers, biofuels, reactants etc. Plants and microorganisms act as a natural CO2 filter. Several biomolecules such as carbohydrates, proteins, and lipids are produced due to the biological carbon fixation process using photosynthesis. Six different photosynthetic pathways and some non-photosynthetic pathways to fix atmospheric CO2 have been reported in diverse species of plants and microbes such as bacteria, fungi, yeast, algae etc. Algae are the most potent microbe in CO2 utilization and biological carbon fixation compared to other microbes and used widely on a large industrial scale for biofuel production. Algal biofuel production using captured CO2 is the best productive method to recycle and reduce atmospheric CO2.
Keywords
Carbon dioxide
Health effects
Utilization
Storage
Sequestration
1 Introduction
Carbon dioxide is a gas present in the atmosphere at negligible amounts (0.03%). CO2 is significant for plants to synthesize carbohydrates (polysaccharides), proteins, and lipids through photosynthesis. It is a key component of the carbon cycle. The various ecosystems of the earth naturally balance the CO2 to O2 ratio in the atmosphere. CO2 plays a vital role in the cellular organization of living animals. The CO2 is incorporated into the cells in the form of organic carbon molecules. Photosynthesis plays an important role in the formation of biomass in plants and animals; it is transferred through the food chain; hence CO2 is directly involved in the energy budget of the biosphere. The CO2 is radioactive in nature due to the absorption of infrared rays from the solar radiation and thus plays an important role in maintaining the earth's temperature. For the past 100 years, the earth's temperature has been increased abruptly due to elevated CO2 level in the atmosphere. During the year 1800, less than 250 ppm of atmospheric CO2 has been reported. From 1900 CO2 had been gradually increased from 280 ppm to 360 ppm in 2005 and 419 ppm in 2019 has been reported. This elevated level of CO2 in the atmosphere is a key reason for global level climatic changes due to imbalanced atmospheric oxygen to carbon dioxide ratio. Since the carbon cycle is disturbed, it indirectly influences the biogeochemical cycles of various components that directly disturb the formation ozone in the oxygen cycle, thus chiefly affecting the ozone layer. The burning of fossil fuels has been reported as one of the principal causes of elevated emission of CO2 into the atmosphere. About 80–82% of greenhouse gas (GHG) has been reported to be the CO2. It has been predicted that the rise in global surface temperature will range from 1.5 °C to 5.9 °C for the next 25 years due to high atmospheric CO2. This directly upsets the balanced cycles of both the biotic and abiotic communities of the earth's various ecosystems. The CO2 that has been already emitted into the atmosphere should be removed and further emission of CO2 must be reduced. To achieve this goal, the excess CO2 present in the atmosphere should be captured, stored, reused to produce many Carbon-based products and further CO2 emission must be reduced by avoiding fossil fuel and replacing it with eco-friendly products such as biofuels. The best way to reuse the captured and stored CO2 is to utilize it as a feedstock to produce biofuels (Tokgoz, 2010).
2 Causes for CO2 emission
The emission of CO2 into the atmosphere occurs in both natural and artificial ways.
2.1 Natural emission of CO2
Natural emission of CO2 includes chiefly carbon cycle (soil, ocean, air), emission of ashes from volcanic eruption, removal of forest covers due to natural calamities such as forest fire, cyclone, hurricane, volcanic eruption, tectonic plate movements (Bradshaw and Bradshaw, 2005).
2.2 Artificial emission of CO2
The artificial source of CO2 emission due to human activities includes power plants, textiles, industries, plastic production, paper industry, metal factories, automobiles, waste disposal, incineration, deforestation, urbanization, burning of wood, burning of plastics waste, among these, burning of fossil fuels were the chief source for high emission CO2. From 1940 to 2005, the emission of CO2 has increased by more than 60% in addition to the natural emission of CO2 (Bazzaz, 1990). The power generation sector was the major source for release of CO2, contributing nearly 146% in the world level. About 121% of CO2 release from the automobile sector, 66% from industries, 45% due to urbanization, forest contributes 41%, deforestation and conversion of forest area into agricultural land 45%, 28% were reported from agriculture, 27% from the construction of buildings, due to power consumption during the construction of buildings which gives out around 76% of CO2 indirectly into the atmosphere. Due to an increased population, 61% of CO2 released based on their daily needs, which indirectly acts as a source of high CO2 into the air. It is estimated to be 111% of CO2 emission in 2030 due to utilization of fossil fuels alone. The gas used in the energy sector gives out 22% of CO2 and coal utilization releases 44% and 22% from the automobile (Colliver, 2012).
3 Effects of increased CO2 on earth
Increased emission of CO2 into the atmosphere is the key component for climatic changes over the years. The natural equilibrium has been upset due to high CO2 component in the air.
3.1 Impact of CO2 on plants
High CO2 has both beneficial as well as negative effects on plants. Under proper nutrient and water supply, the rate of photosynthesis may increase in plants. The negative effects of increased CO2 on plants follows: alteration in the rate of transpiration, stomatal conductance, decreased area of leaf, reduced leaf size, lowered nitrogen and phosphorous content their leaves, there is a huge alteration in flowering seasons and reproductive activities of plants depend on high CO2 stress, some species show decreased flowering duration, number of flowers, number of seedlings and time duration of seedling were found to be decreased, some species of plants produced thick capsule in fruit which prevents the entry of insects involved in pollination. Some plants showed positive effects like an increased number of flowers and seeds. However, the negative effects are larger than the positive effects.
3.2 Impact of CO2 on animals
The CO2 concentration greatly influences the plants, which act as the primary producers of the ecosystem. Seasonal changes in flowering and fruit production affect the insects, birds and other animals which consumes it and the reproductive rate of plant species decreases due to less access of pollination through insects and dispersal of seeds based on animals, particular plant population begins to decrease in number which directly affects the animals feeding on it leading to food scarcity which ultimately alters the food chain and food web causing an imbalance in predator to prey ratio, in severe cases the entire community may disappear. Ecological succession of new plant and animal species may occur due to a high regeneration rate on plants due to increased CO2. Molecular-level changes also take place due to high CO2 on animals. CO2 directly affects various metabolic activities of animals. Respiration becomes difficult. The acid-base balance is disturbed, bicarbonate buffer of body fluid is highly altered, difficulties while urinating due high Ca deposits in kidney, pH of becoming acidic, calcium metabolism alteration with increased CO2 uptake with bones which result in increased bicarbonate with loss of bone Ca and P, increased bone Ca and P with decreased carbonates, increase in age directly accelerate the rate of CO2 binding with the bones along with the elimination of H2O. Alteration in RBC electrolytes was reported with increased plasma Na with decreased K in RBC, increased gastric acidity, kidney damage due to electrolyte changes, the primary target organs are lungs, kidney, bones, and it affects the activity of parathyroid glands.
3.3 Impact of CO2 on humans
The normal level CO2 that a human body can tolerate is around 0.5% or 5000 parts per million for about less than 8.5 h. Hypercapnia is a clinical condition that occurs due to increased acidity in the blood due to inhaling a high level of CO2 results in acidosis. Fall in tissue pH results in the following conditions such as abnormal activities in respiration, blood circulation to the heart, and neural damage in the central nervous system (shock, headache, hyperventilation, visual impairment, and CNS impairment) high CO2 exposure can affect the attentiveness and problem associated memory and capacity to learn, headaches, giddiness tachycardia, difficulty breathing, etc. Short duration CO2 exposures under a range of 1–5% CO2 produces altered lung activities including reduced duration of breath, alveolar damage, rise in blood acidity, elevate blood pressure, brain shock, impaired vision, changes in the proportion of chemical components of urine and blood, degeneration and decalcification of bones, increased renal calcification, unpredictable behaviour, panic attack, altered mitosis, and altered enzyme metabolism (Schaefer et al., 1963; Colasanti et al., 2008; Abolhassani et al., 2009). Around 40,000 ppm of CO2 for nearly ½ h is hazardous and 50,000 ppm will be intoxicating, 70,000 causes catalepsy/unconsciousness, and acute toxic effect of CO2 is a lethal dose of about 9% or 90,000 ppm for less than 6 min and infantile death have been reported under 80,000 ppm. The following clinical conditions have been recorded and reported from patients exposed to high CO2 concentration for varying time periods, including fall in the number of neurons, decreased sensitivity of neurons to neuroreceptors, altered sleep cycle, and psychological alterations emotional irritation.
4 Methods to control the release of high CO2 into the atmosphere
Combined strategies are required to reduce atmospheric CO2 release (Alain Goeppert et al., 2012), which involves the following steps:
-
The amount of CO2 being released into the atmosphere must be reduced
-
Removal/elimination of high level of CO2 already present in the air.
-
Utilization of atmospheric CO2 to produce commercial products
5 Carbon dioxide capture
A new technique was widely implemented for the reuse and recycling of atmospheric CO2. Natural and artificial methods are too used sequester carbon dioxide. To achieve this, the CO2, present in the atmosphere should be captured, stored and utilized as a raw material for producing various carbon-based products. CCS- Carbon Capture and Storage technology is a perfect method that can be used to remove atmospheric CO2.
5.1 Artificial method to capture CO2
The CCS technology has 3 stages, including capturing the atmospheric CO2, separating it from other gases, sealing and transporting it for storage and final stages is the storage. Stage-1 includes identifying proper CO2 emission sources and adopting suitable methods to separate CO2 from other gases and impure substances. The major sources that release higher of CO2 into the atmosphere are the power generation sector, the energy sector (thermal energy), industries, some factories, and transport, including several CO2 emission sources e.g. land, water and air. To capture the CO2, two methods can be used, the direct capture of CO2 from emission sources using various materials like absorbent, adsorbent or membrane or metal catalyst to filter the flue gas for other sources including vehicles, and the second method involves capturing atmospheric air to separate CO2 by launching CCS plants in highly emitting sources or closer to that regions. Capturing CO2 from sources with nearly 20% has become indispensable, which leads to the implantation of materials to filter and prevent CO2 from being released in the flue gas.
5.2 Natural method to capture CO2
Biomass is the product of atmospheric CO2 through a series of biochemical reactions inside the photosynthetic organisms. Currently, 12% of global energy is provided by biomass. Plants act as an infinite natural resource to obtain energy by replenishing it with new plantation. Biomass has a benefit over other renewable energy that it locks energy within biochemical bonds. Biomass serves in various such wood, agricultural residues, biofuel and natural gas. Biomass is an alternate pathway to store the captured CO2. Various species are involved in converting CO2 into biomass ranging from plants to microorganisms like bacteria, fungi, yeast and algae. Food crops included in the first-generation biofuel have a drawback of food scarcity, land availability, water, manure, and difficulty handling with a limited amount of resources. Plants with higher conversion potency, rapid growth rate, with minimal nutrients and pesticide utilization and such crops must be grown to restore wasteland. A large number of resources, such as are required to produce biomass and energy from plants. The scope of algal cultivation on small ponds, tanks, lakes, sea, etc. has widened and unlocked a major pathway to producing bioenergy. Algae are the high potential micro-sized factories to manufacture biomass and energy with maximum yield.
6 Carbon dioxide sequestration
The natural method to sequester carbon involves plants and microorganisms, including bacteria, algae, fungi and yeast (Nitin Mistry et al., 2018) through two pathways, chiefly the photosynthesis the other is non-photosynthetic pathways. Autotrophic and heterotrophic organism incorporates CO2 into various organic carbon products like cellulose, lignocellulose, chitin, hemicellulose, lignin etc (Sundquist et al., 2008; Cole et al., 2007). Eco-friendly CO2 sequestration can be achieved through the proper utilization of these organisms for CCU. These organisms have different pathways, conversion mechanisms and the ability to produce biomass/bioenergy (MacDowell et al., 2010).
6.1 Plants
The atmospheric CO2 is integrated into the plant body through photosynthesis. Chlorophyll is the factory where CO2 is converted into a biomolecule by absorbing radiant energy from the sun, based on which dark and light reactions takes place. The light reaction involves solar radiation to produce energy molecules such as NADPH and ATP from NADP + and ADP. The dark reaction involves Calvin–Benson cycle along with energy molecules from the light reaction. Plants are classified into C3, C4, CAM, based on the adaptation to photosynthesis and various mechanism involved major to lower the rate of photorespiration (Berry and Björkman, 1980; Zelitch, 1992). In C4 plants, photorespiration is reduced by increased CO2 at RuBisCO activation site that inhibits oxygenase function and CO2 are incorporated with the help of cells in the bundle sheath and mesophyll present in the leaf (Lara and Andreo, 2011; Ghannoum et al., 2011) the conversion efficiency of C3 plants is lower than C4 plants. Plants with Crassulacean Acid Metabolism (CAM) pathway as an adaptation for efficient C sequestration are termed as CAM plants. This adaptation helps plants to survive in the dry ecosystem and drought season.
6.2 Microbe
Most microbes potentially fix CO2 from the air through various mechanisms and pathways (Table. 1). Photosynthetic and non-photosynthetic pathways are the microbial mechanism involved to sequester atmospheric CO2 into biomass and energy. The benefits of CO2 captured by microbes include the following; high production maximum rate of bio fixation, high ability to bioremediate atmospheric CO2, the capabilities to produce several additives are extreme, no difficulties in genetic augmentations, capable of being used in bioprocessing at industries, rapid growth and continuous culture in bioreactors, no competition and food scarcity. It includes bacteria, fungi, yeast and algae etc.
Input
Pathways
Enzymes
Organism
CO2
Calvin-Benson-Bassham cycle (CCB) or Reductive pentose phosphate cycle
RuBisCO
Plants, algae, cyanobacteria, proteobacteria, mycobacteria
CO2
Reductive tricarbocylic Acid cycle (rTCA)/Reductive citric acid cycle/Reverse Krebs cycle/Arnon Buchanon cycle
PEP carboxylase 2-Oxogluteratesynthase Isocitrate Dehydrogenase Pyruvate synthase
Proteobacteria, green,sulfur, bacteria, quaficae bacteria
CO2
Wood-Ljungdahl pathway (W-L) or Reductive acetyl-CoA pathway
Formate dehydrogenase Carbon monoxide dehydrogenase (CODH) Formylmethanofuran Dehydrogenase (FMFD)
Euryarchaeota, proteobacteria, plantomycetes, spirochaetes
CO2
3-Hydroxypropionate 4- hydroxybutyrate cycle (3HP-4HB)
Acetyl-CoA/PropionylCoA carboxylase
Aerobic crenarcheota
CO2
Dicarboxylate 4- hydroxybutyrate cycle (DC-4HB)
Pyruvate synthase PEP carboxylase
Anaerobic crenarcheota
CO2
3-Hydroxypropionate bi-cycle (3-HP)/Fuchs-Holo cycle
Acetyl-CoA carboxylase Propionyl-CoA carboxylase
Green non-sulfur bacteria
6.2.1 Bacteria
Bacteria are unicellular, a microscopic organism with 19 groups. Most of them fall under the following groups: Actinomycetes, Mycoplasma, Rickettsia’s, Archaebacteria, Cyanobacteria and Eubacteria etc. Among these six groups, the Archaebacteria, Cyanobacteria, and Eubacteria are autotrophic, which fixes CO2 for organic carbon production.
-
Clostridium
Anaerobic gram-negative bacteria were playing a substantial role in degrading organic carbon materials, acid synthesis and carbon cycle (Migliardini et al., 2014). Various species are chiefly involved in the bio-fixation of CO2 such as Clostridium autoethanogenum, Clostridium pasteurianum, C. formicoaceticum, Acetobacterium woodi, Clostridium thermoaceticum incorporates atmospheric carbon dioxide into acetyl-CoA through Wood–Ljungdahl pathway/Reductive acetyl CoA pathway which is the direct and most effective thermodynamic pathways (Tracy et al., 2012). The energy required for the fixation of CO2 is obtained from the hydrogen molecules. Carbon monoxide dehydrogenase and acetyl CoA synthetase are the two enzymes that play a chief role in acetyl-CoA synthesis by converting carbon monoxide into carbon dioxide (Liew et al., 2016; Fast and Papoutsakis, 2012; Ezeji et al., 2007; Ni and Sun, 2009). This bacterial sp. cannot survive atmospheric O2, a major disadvantage (Qureshi et al., 2007). Clostridium thermoaceticum was the first model used to study this pathway.
-
Proterobacterium
Proteobacteria are large phyla which are capable of incorporating atmospheric carbon dioxide via various biological mechanism such as the calvin cycle/reductive pentose phosphate cycle, TCA cycle/Krebs cycle and also directly in the cytoplasm (Paoli and Tabita, 1998; Ding and Yokota, 2004; Hügler et al., 2003). Calvin cycle is used by the following species Oligotropha carboxidovorans, Rhodobacter sphaeroides, Xanthobacter flavus, Rhodobacter capsulatus, Beta proteobacter, Ralstonia eutropha, Herbaspirillum autotrophicum, and Gama proteobacter, Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, Hydrogenovibrio marinus etc. (Yoshizawa et al., 2004; Wang et al., 2008; Brigham et al., 2011; Albuquerque et al., 2011). Some of the bacterial spp. are used to synthesize commercial products such as biopolymers and medicine (Hügler et al., 2005; Willey et al., 2008). In the cytoplasm of R. eutropha, polyhydroxy alkanes are produced by utilizing CO2. Sulphur reducing bacteria such as Desulfobacter hydrogenophilus (delta-proteobacteria) and Thiomicrospira denitrificans (epsilon-proteobacteria) utilizes CO2 and H2O to produce organic materials through the TCA cycle in which the ATP-citrate lyase splits acetyl-coA and oxaloacetate.
6.2.2 Archaea
Archaea is a unicellular, prokaryotic microbe termed as extremophiles and surviving under intense ecological extremities such as high fluctuating temperature, pH, and absence of O2. Archaea has three different groups, halophiles (ability to withstand higher salt), thermoacidophiles and methanogens. The methanogens produce biofuel-methane under an anaerobic environment by utilizing CO2 as raw material and energy obtained from hydrogen for the bioconversion process (Rittmann et al., 2015; Demirel and Scherer, 2008). Two types of methanogens are commercially used to produce methane; they are the acetoclastic and hydrogenotrophic methanogens. These methanogens potentially bioremediate wastewater and capture CO2; hence they can be used to generate methane from the sludge through the utilization of CO2 as well as to bioremediate wastewater for recycling and it includes Methanobacteriaceae, Methanospirrillaceae, and Methanosarcinaceae spp (Mohd Yasin et al., 2013, 2015). The 3-hydroxypropionate-4-hydroxybutyrate cycle pathway is used to fix atmospheric CO2 by Cenarchaeum, Archaeoglobus, and Metallosphaera and Sulfolobus, sp (Berg et al., 2007). Two acetyl-CoA and one bicarbonate molecule are used to produce succinyl-CoA, which undergoes 4-hydroxybutyrate pathway to generate two molecules of acetyl-CoA. In this pathway, Acetyl-CoA/Propionyl-CoA carboxylase is the enzyme that actively fixes atmospheric CO2 into biomolecules. Thermophilic methanogens produce carbonic anhydrase enzyme to produce methane, which can be used in large scale industries (Smith and Ferry, 2000; Henstra et al., 2007).
6.2.3 Cyanobacteria
Cyanobacteria are prokaryotic, blue-green bacteria or algae serving as a connecting link between bacteria and green plants. They are photoautotrophic gram negative bacteria present in both microscopic and macroscopic forms. They possess carboxysomes in their cytoplasm for carbon fixation. Some of the microorganisms, such as photoautotrophs and chemoautotrophs, have carboxysomes e.g., Bacteria and all cyanobacteria, resulting from an adaptation called the Carbon dioxide Concentration Mechanism (CCM). It is involved in Calvin -Benson cycle. The two enzymes RuBisCO and carbonic anhydrase are enclosed in bacterial microcompartments called carboxysomes located in the cytosol. There are two types of carboxysomes, the alpha carboxysomes with RuBisCO form IA and beta carboxysomes with RuBisCO IB. The alpha carboxysomes exist in all alpha-cyanobacteria, most of the chemoautotrophs and in some purple bacteria, but the beta carboxysomes are only seen in beta-cyanobacteria.
6.2.4 Algae
Algae is the most efficient photosynthetic biofactories that incorporate CO2 into biomass and energy. They range from macro to micro size. Macroalgae produce high lipid content; hence they are directly used to extract for biodiesel production. Micro-sized algae include cyanobacteria, diatoms, euglenoids, green, blue, red, brown, golden, yellow coloured algal species and they have huge potential to fix CO2 enzymatically by using RuBisCO in the Calvin–Benson cycle.eg. About 1 kg of micro-sized algae fixes 1.84 kg of atmospheric carbon dioxide. The carbon dioxide fixed by anabaena is 1.46 g/L/d and 6.24 g/L/d by Chlorella vulgaris. They utilize CO2 to produce biomass and bioenergy (Chen et al., 2009). Cultivating these algae very close to the emitting sources can chiefly reduce elevated CO2 levels and produce huge quantities of biomass and bioenergy (Cheah et al., 2015). Selection of algal species, optimal growth parameters and proper feedstock are the factors that should be taken into considerations before cultivation practices to obtain the maximum yield (Ghorbani et al., 2014). Perfect algal species should possess the following characters to achieve maximum CO2 bio fixation and yield. The ability to capture carbon and rate of incorporation should be extreme. It should tolerate maximum CO2 stress, proper utilization of limited nutrients, and tolerance to fluctuation in thermal and H+, OH− parameters (Rahaman et al., 2011). Algae, which are ideal for maximum carbon capture and fuel production like Botryococcus braunii, Scenedesmus obliquus, Nannochloropsis oculate, Chlorella vulgaris (Pires et al., 2012; Brilman et al., 2013; Singh and Ahluwalia, 2013). The algae Scenedesmus dimorphus was found to be tolerating up to 20% (v/v) of CO2 stress even though its optimal level to biofix CO2 was only 2%(v/v). This indicates that the amount of CO2 to which the algae were exposed would significantly reflect the bioconversion rate and yield. This clearly showed that the tolerance to high CO2 content by the Chlorella sp is remarkably extreme (Jiang et al., 2013). They show extreme tolerance to CO2 content, which is up to 40–41% under the temperature and pH of 30 °C and 5–6, respectively (Chen et al., 2014). Nannochloropsis spp. were found to be grown at a rate of 58% with a CO2 stress of 15% (v/v) (Jiang et al., 2011). It has been reported that a high concentration of carbon dioxide can significantly endorse the rate of photosynthetic CO2 bio fixation in a short duration and more than 5% (v/v) becomes toxic. Also, the Continuous injection of an elevated level of CO2 in the culture media inhibits the algal growth (Lee et al., 2000). Calvin Benson pathways are used for carbon fixation in algae. Production of 3-phosphoglycerate takes places through the carboxylation of ribulose 1,5-biphosphate by the catalytic activity of RuBisCO enzyme there by two molecules from which one enters the central metabolism and the other is exploited to continue the cycle.
Microalgae have an adaptation to increase the CO2 concentration in the surrounding of the enzyme RuBisCO to fix more inorganic carbon and such adaption involves the Carbon Concentration Mechanism CCM (Atomi, 2002). This mechanism is majorly based on a key reaction in which carbon dioxide and bicarbonates' transportation takes place actively there by separating the RuBisCO through single to the multi-layered membrane. This adaptation is seen in all algae and cyanobacteria (Parry et al., 2003). In Cyanobacteria, it involves the transportation of carbon dioxide or bicarbonate inside the functional units of a chloroplast or cell membrane (Kaplan and Reinhold, 1999). Bicarbonate molecules are transported into the cytoplasm cytosol through various carrier molecules, taking place irrespectively to the periplasmic exclusion of dissolved inorganic carbon (DIC). This is reaction is followed by the diffusion of bicarbonates into carboxysomes where the carbonic anhydrase CA enzyme actively takes place in the cytosol (Raven and Beardall, 2003). This mechanism facilitates increased carboxylation activity of rubisco over the oxygenation reaction resulting in photorespiration (Lane and Morel, 2000). In this mechanism, four subsequent reactions are involved and they are the transportation and utilization of carbon dioxide and bicarbonates, separation and concentration of RuBisCO in specific micro-chamber and confined CA activity (Price et al., 2002; Wigmosta et al., 2011; Venteris et al., 2013, 2014). Algal bio-refineries should be located on wastewater bodies as recycling and restoration (Gong and You, 2014).
The following species are the most promising algae used for lipid-based biofuel production (Chisti, 2008; Damiani et al., 2010; Mandal and Mallick, 2009) including Botryococcus, Neochloris oleoabundans, Dunaliella, Chlorella, Scenedesmus spp, Haematococcus pluvialis, Scenedesmus obliquus, Chlorella sorokiniana, Chlamydomonas reinhardtii, Botryococcus braunii and diatoms such as Phaeodactylum tricornutum, Navicula pellicusa, Chaetoceros eg. Chaetoceros muelleri, Chaetoceros gracilis, Cyclotella cryptica, Navicula saprophila were noted for their high lipid content (Dayananda et al., 2006; Hu et al., 2008; Pratiwi et al., 2009; Mata et al., 2010).
Macroalgae or seaweed also utilizes a high amount of atmospheric CO2 to produce biomass and energy. They produce high amounts of carbohydrates with low lipid content; hence they are used as feedstock for biofuel based on fermentation process such as bioethanol, methanol, and isobutanol etc. Laminaria sp were found to produce carbohydrates over 65%, including Laminaria hyperborea, Sacchorhiza polyschides, Laminaria digitata, Alaria esculenta and Saccharina latissimi. Among different macroalgal species, the Ulva and Laminaria were found to be the most potent bioenergy producers Ulva lactuca, Enteromorpha intestinalis, and Catenella repens, Sargassam wightii, Kappaphycus alverezzi, Ulva lactuca, Gracilariopsis longissima, Chaetomorpha linum contains a high amount of lipids (Bastianoni et al., 2008; Muralidhar et al., 2010). As a result of CO2 utilization, algae ranks first compared to any other microorganism in carbon sequestration (Fig. 1).
Microalgal carbon sequestration and its byproducts.
6.2.5 Fungi
Fungi are the eukaryotic, multicellular organism with a rigid cell wall. They are heterotrophic and serve as primary decomposers and chief organisms to capture carbon in the terrestrial ecosystem. Based on decomposing organic matter, they are categorized into two groups, saprophytic and mycorrhizal fungi. Saprophytic fungi produce enzymes to decompose substances such as cellulose, hemicellulose, pectin and lignin, etc which are responsible for mineralization and carbon cycle and the decomposition by mycorrhizal fungi is low due to the lack of enzyme (Ahmed et al., 2019). Mycorrhizal fungi form a symbiotic association with plants and exist in three forms, including ectomycorrhizal, arbuscular mycorrhizal form and ericoid mycorrhizas only found in plants belonging to the order Ericales.
6.2.5.1 Mechanism
Fungi facilitate carbon sequestration in soil by forming organic humus and maintain the carbon balance, thereby contributing CCS largely in the terrestrial ecosystem when compared to bacteria (Fig. 2); three methods involved in soil carbon storage, including recalcitrant biomass and their secondary products, producing soil aggregate and incorporation of atmospheric CO2 into fungal biomass. The mycelium acts as a storage site for the carbon. Thus a large amount of biomass is produced by efficiently incorporating CO2 at a higher percentage.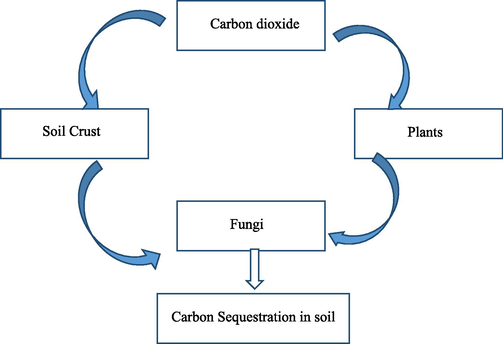
Carbon Sequestration of fungi in soil.
6.2.5.2 Role of mycelia in carbon capture
The vegetative tissue produced in fungi, the so-called mycelia, has a rapid growth rate and spreads much faster in the soil by facilitating access to nutrients and H2O. Fungal contribution to sequester carbon from CO2 is achieved through biomass formation and their secondary products and degradation of their necromass. The rate of carbon sequestered will depend upon the type of fungal species present in the soil and its biomass produced- i,e the higher amount of mycelia produced, the greater the percentage of carbon is incorporated, thus the amount of fungal biomass produced will directly reflect on the percentage of CO2 utilized. The mycelia serve as carbon sink in the soil. Fungal necromass acts as a resource for humus production up to 71% compared to bacteria, with only 26%. The degradation of fungal necromass is directly affected by the concentration of soil nutrients such as nitrogen and chitin; the mycelial necromass is decomposed within 8 days when the nitrogen and chitin are concentrated in the soil. Simultaneously, the decomposition of mycelia is resisted in cells filled with melanin pigment. Therefore, the carbon is locked up within the cells for a prolonged period depending upon the melanin concentration present in it. Therefore, the amount of necromass protected is directly correlated to the amount of carbon sequestered (Fernandez and Koide, 2014). Even soil aggregates also possess fully functional fungal necromass contributing in soil carbon storage.
6.2.5.3 Role of glomalin protein and soil aggregate in carbon capture
The mycorrhizal fungi produce a glomalin protein, secreted from the fungal hyphae and spores as a thermal adaptation. The glomalin is thick, sticky and acts as a recalcitrant to resist decomposition. Glomalin promotes carbon capture via two processes, making the fungal hyphae resist decomposition and endorsing soil aggregation. Thus, the carbon is ceased within the hyphae and soil aggregate for a prolonged period. Glomalin is found to be the principal substance responsible for the formation of stable soil clumps along with permeability to H2O and air (Pal and Pandey, 2014). Its hydrophobic nature protects the soil clump from microbial degradation and erosion. Hence, the organic carbon and nutrients located within the clump remain protected for more than 50 years. Thus, glomalin act as a carbon sequester in the terrestrial carbon pool and promotes soil quality. Fungal species present in the following genera secretes glomalin protein; they are Entrophospora, Gigaspora, Acaulospora, Scutellospora, Glomus, etc. (Pal and Pandey, 2014).
6.2.6 Yeast
Yeast is a unicellular microorganism widely used for fermentation in the large-scale industries to obtain various commercial products. Saccharomyces cerevisiae is the yeast strain widely used for the fermentation process. During fermentation, carbon dioxide is liberated as exhaust gas due to microbial respiration and a large amount of O2 is utilized. The mechanism observed in yeast was Glyoxylate and Krebs cycle. Yeast absorb carbon from a variety of carbon-based components such as sucrose, maltose, galactose, lactate, glycerol, ethanol, acetate, and oleate etc, e.g., carbon from glycerol is utilized with cytoplasmic kinase to form glycerol-3-phosphate before reaching the mitochondria. It is further converted into dihydroacetone phosphate in the mitochondria by FAD-dependent glycerol-3-phosphate dehydrogenase enzyme, which enters the glycolytic pathway.
6.3 CO2 sequestration by the genetically modified microbe
The genetically engineered microorganism for effective CO2 fixation involves few yeast strains, E. coli bacteria, and some micro and macroalgae.
6.3.1 Yeast
Few strains of yeast other than Saccharomyces cerevisiae were found to be the understudy for genetic and metabolic modifications to improve CO2 bio fixation. Methylotrophic yeast Pichia pastoris is a widely used yeast strain for protein and nucleic acid production; it utilizes methanol as a substrate and the mechanism involved is xylulose-5-phosphate pathway. This pathway occurs in peroxisome and very analogous to the Calvin Benson cycle. The xylulose-5-phosphate pathway has been genetically modified to improvise the production of biomass through CO2 fixation by recombing it to the CO2 fixation pathway. The modification includes the deletion of three genes responsible for methanol utilization and the integration of eight new genes to produce enzymes such as RuBisCO and PRK (phosphoribulokinase) involved in CBB pathway. Two genes, the DAS1 and DAS2 were removed to block the methanol utilization metabolism. As a result, the methanol would serve as an electron donor during the oxidation of formaldehyde. The yeast plasmid was genetically modified and the resulting strain showed expected results positively through efficient CO2 fixation. As a result, the nutrition of Pichia pastoris was modified into chemoorganoautrophic from its original chemoheterotrophic mode.
6.3.2 Bacteria
Gong and You (2014) experimented with quantifying the amount of CO2 bio fixated by the bacteria E. coli. For effective CO2 fixation, the bacteria E. coli was genetically modified. Heterotrophic nutrition is modified by integrating genes, resulting in a CO2 fixing bypass pathway in the carbon central metabolic pathway, after incorporating genes responsible for producing the enzymes RuBisCO and PRK involved in Calvin Benson cycle. After incorporation of the metabolic flux, recombinant plasmid into E. coli strain, the CO2 fixation was reported to be improved at a rate of 13% compared to the central metabolism. After integrating recombinant plasmid with carbonic anhydrase enzyme of CCM mechanism, the resultant E. coli strain showed improved CO2 fixation at a rate of 17%. The resultant amount of CO2 fixed by this E. coli was reported to be 19.6 mg CO2 L−1h−1, which is equal to CO2 fixated naturally by autotrophic algae and cyanobacteria.
6.3.3 Algae
Some of the autotrophic algae are recently understudies for genetic modification to improve biomass production via improved CO2 bio fixation. The following algal species genome has been sequenced and some of them are under the process of recombinant studies to improve biomass production. It includes Chlamydomonas reinhardtii, Phaeodactylum tricornutum, Thalassiosira pseudonana, Cyanidioschyzon merolae, Ostreococcus lucimarinus, Ostreococcus tauri, Micromonas pusilla. Fragilariopsis cylindrus, Pseudo nitzschia, Thalassiosira rotula, Botryococcus braunii, Chlorella vulgaris, Dunaliella salina, Micromonas pusilla, Galdieria sulphuraria, Porphyra purpurea, Volvox carteri, and Aureococcus anophageferrens are still under research.
7 Conclusion
Biological carbon sequestration is important to be considered for the improvement in the condition of climate change. Among the various carbon sequestration microalgal fixation methods, CO2 has many advantages over others as the biomass can be utilized to produce energy, which is considered beneficial due to economic gains. This method is also considered to be a permanent sequestration technique. Hence we are concluding the microalgal CO2 is economical and eco-friendly for biological carbon sequestration from point sources.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the Project no. (IFKSURP-1435-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carbon dioxide inhalation causes pulmonary inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2009;296:L657-L665.
- [Google Scholar]
- Microbial Inoculants for Improving Carbon Sequestration in Agroecosystems to Mitigate Climate Change. In: Leal Filho W., ed. Handbook of Climate Change Resilience. Cham: Springer; 2019.
- [Google Scholar]
- Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Ener. Environ. Sci.. 2012;5:7833.
- [Google Scholar]
- Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol.. 2011;151:66-76.
- [Google Scholar]
- Microbial enzymes involved in carbon dioxide fixation. J. Biosci. Bioeng.. 2002;94:497-505.
- [Google Scholar]
- Biofuel potential production from the Orbetello lagoon macroalgae: a comparison with sunflower feedstock. Biomass. Bioener.. 2008;32:619-628.
- [Google Scholar]
- The Response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Eml. Sys.. 1990;21:167-196.
- [Google Scholar]
- A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Sci.. 2007;318:1782-1786.
- [Google Scholar]
- Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant. Physiol.. 1980;31:491-543.
- [Google Scholar]
- Bacterial carbon storage to value added products. J. Microbial. Biochem. Technol.. 2011;83:S3-002.
- [Google Scholar]
- Capturing atmospheric CO2 using supported amine sorbents for microalgae cultivation. Biomass. Bioener.. 2013;53:39-47.
- [Google Scholar]
- Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol.. 2015;184:190-201.
- [Google Scholar]
- Review of biological and engineering aspects of algae to fuels approach. Int. J. Agr. Biol. Eng.. 2009;2(4):1-30.
- [Google Scholar]
- Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour. Technol.. 2014;169:258-264.
- [Google Scholar]
- Carbon Dioxide induced emotion and respiratory symptoms in healthy volunteers. Neuro. Psycho. Pharmacol.. 2008;33:3103-3110.
- [Google Scholar]
- Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosys. 2007;10:172-184.
- [Google Scholar]
- Low-emission Technology Series: Introduction to Carbon Capture and Storage. Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the Global CCS Institute; 2012.
- Lipid analysis in Haematococcus pluvialis to assess its potential use as biodiesel feedstock. Bioresour. Technol.. 2010;101:3801-3807.
- [Google Scholar]
- Isolation and characterization of hydrocarbon producing green alga Botryococcus braunii from Indian freshwater bodies. Elec. J. Biotechnol.. 2006;10:78-91.
- [Google Scholar]
- The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev. Environ. Sci. Technol.. 2008;7:173-190.
- [Google Scholar]
- Proposals of Curvibacter gracilis gen. nov., sp. nov. and Herbaspirillum putei sp. nov. for bacterial strains isolated from well water and reclassification of [Pseudomonas] huttiensis,[Pseudomonas] lanceolata,[Aquaspirillum] delicatum and [Aquaspirillum] autotrophicum as Herbaspirillum huttiense comb. nov., Curvibacter lanceolatuscomb. nov., Curvibacter delicatus comb. nov. and Herbaspirillum autotrophicum comb, Nov. Int. J. System. Evol. Microbiol.. 2004;54:2223-2230.
- [Google Scholar]
- Butanol production from agricultural residues: impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol. Bioeng.. 2007;97:1460-1469.
- [Google Scholar]
- Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr. Opin. Chem. Eng.. 2012;1:380-395.
- [Google Scholar]
- Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol. Biochem.. 2014;77:150-157.
- [Google Scholar]
- Nitrogen and water use efficiency of C4 plants. In: Raghavendra A.S., Sage R.S., eds. C4 Photosynthesis and Related CO2 Concentrating Mechanisms. New York, NY: Springer; 2011. p. :129-146.
- [Google Scholar]
- A review of carbon capture and sequestration in Iran: microalgal biofixation potential in Iran. Renewable Sustainable Energy Rev.. 2014;35:73-100.
- [Google Scholar]
- Global optimization for sustainable design and synthesis of algae processing network for CO2 mitigation and biofuel production using life cycle optimization. AIChE J.. 2014;60:3195-3210.
- [Google Scholar]
- Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol.. 2007;18:200-206.
- [Google Scholar]
- Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J.. 2008;54:621-639.
- [Google Scholar]
- Autotrophic CO2 fixation pathways in archaea (Crenarchaeota) Archiv. Microbiol.. 2003;179:160-173.
- [Google Scholar]
- Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the ε subdivision of Proteobacteria. J. Bacteriol.. 2005;187:3020-3027.
- [Google Scholar]
- Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl. Energy. 2011;88:3336-3341.
- [Google Scholar]
- Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol.. 2013;128:359-364.
- [Google Scholar]
- John Bradshaw., John Bradshaw., 2005. Sources of CO2., IPCC Special Report on Carbon dioxide Capture and Storage. 2, 75-101.
- Concentrating mechanisms in photosynthetic microorganisms. Ann. Rev. Plant. Physiol. Plant. Mol. Biol.. 1999;50:539-559.
- [Google Scholar]
- Regulation of carbonic anhydrase expression by zinc, cobalt and carbon dioxide in the marine diatom Thalassiosira weissflogii. Plant Physiol.. 2000;123:345-352.
- [Google Scholar]
- C4 plants adaptation to high levels of co2 and to drought environments. In: Shanker A., ed. Abiotic Stress in Plants - Mechanisms and Adaptations. Croatia: InTech; 2011. p. :415-428. Chapter 18
- [Google Scholar]
- Methods to enhance tolerances of chlorella kr-1 to toxic compounds in flue gas. Appl. Biochem. Biotechnol. 2000:329-342.
- [Google Scholar]
- Insights into CO2 fixation pathway of Clostridium autoethanogenum by targeted mutagenesis. mBio.. 2016;7:e00427-e516.
- [Google Scholar]
- Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol.. 2009;84:281-291.
- [Google Scholar]
- Microalgae for biodiesel production and other applications: a review. Renewable Sustainable Energy Rev.. 2010;4:217-232.
- [Google Scholar]
- Biomimetic CO2 capture using a highly thermostable bacterial α-carbonic anhydrase immobilized on a polyurethane foam. J. Enz. Inhib. Med. Chem.. 2014;29:146-150.
- [Google Scholar]
- Biohydrogen production from oil palm frond juice and sewage sludge by a metabolically engineered Escherichia coli strain. Int. J. Hyd. Energy. 2013;38:10277-10283.
- [Google Scholar]
- CO2 sequestration by methanogens in activated sludge for methane production. Appl. Energy. 2015;142:426-434.
- [Google Scholar]
- Comparative studies on fatty acid composition of three marine macroalgae collected from Mandapam region: South east coast of India. World. Appl. Sci. J.. 2010;11:958-965.
- [Google Scholar]
- Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl. Microbiol. Biotechnol.. 2009;83:415-423.
- [Google Scholar]
- A Review on Biological Systems for CO2 Sequestration: Organisms and Their Pathways, Environmental Progress & Sustainable Energy. Wiley Online Library; 2018. (wileyonlinelibrary.com)
- Role of glomalin in improving soil fertility: a review. Int. J. Plant. Soil. Sci.. 2014;3(9):1112-1129.
- [Google Scholar]
- Aerobic chemolithoautotrophic growth and RubisCO function in Rhodobacter capsulatus and a spontaneous gain of function mutant of Rhodobacter sphaeroides. Archiv. Microbiol.. 1998;170:8-17.
- [Google Scholar]
- Manipulation of Rubisco: the amount, activity, function and regulation. J. Exp. Bot.. 2003;54:1321-1333.
- [Google Scholar]
- Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renewable Sustainable Energy Rev.. 2012;16:3043-3053.
- [Google Scholar]
- Fatty acid synthesis by Indonesian marine diatom Chaetoceros gracilis. Hayati J. Biosci.. 2009;16:151-156.
- [Google Scholar]
- Modes of active inorganic carbon uptake in the cyanobacterium Synechococcus sp. PCC7942. Func. Plant. Biol.. 2002;29:131-149.
- [Google Scholar]
- Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Biopro. Biosys. Eng.. 2007;30:419-427.
- [Google Scholar]
- A review of carbon dioxide capture and utilization by membrane integrated microalgal cultivation processes. Renewable Sustainable Energy Rev.. 2011;15:4002-4012.
- [Google Scholar]
- Carbon acquisition mechanisms in algae: carbon dioxide diffusion and carbon dioxide concentrating mechanisms. In: Larkum A.W.D., Douglas S.E., Raven J.A., eds. Photosynthesis in Algae. Dordrecht, the Netherlands: Kluwer; 2003. p. :225-244.
- [Google Scholar]
- Essential prerequisites for successful bioprocess development of biological CH4 production from CO2 and H2. Cri. Rev. Biotechnol.. 2015;35:141-151.
- [Google Scholar]
- Respiratory acclimatization to carbon dioxide. J. Appl. Physiol.. 1963;18:1071-1078.
- [Google Scholar]
- Microalgae: a promising tool for carbon sequestration. Miti. Adap. Strat. Global. Change. 2013;18:73-95.
- [Google Scholar]
- Sundquist, E., Burruss, R.., Faulkner, S., 2008. Carbon sequestration to mitigate climate change. U.S. Geological Survey, Fact Sheet, 3097.
- Numerical analysis of worldwide CO2 emissions and effects on atmospheric warming in Turkey. Energy Source Part A.. 2010;32:769-783.
- [Google Scholar]
- Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr. Opin. Biotechnol.. 2012;23:364-381.
- [Google Scholar]
- Siting algae cultivation facilities for biofuel production in the united states: trade-offs between growth rate, site constructability, water availability, and infrastructure. Environ. Sci. Technol.. 2014;48:3559-3566.
- [Google Scholar]
- A GIS cost model to assess the availability of freshwater, seawater, and saline groundwater for algal biofuel production in the United States. Environ. Sci. Technol.. 2013;47:4840-4849.
- [Google Scholar]
- Regulation of CO2 fixation gene expression in Acidithiobacillus ferrooxidans ATCC 23270 by Lix984n Shock. J. Microbiol. Biotechnol.. 2008;18:1747-1754.
- [Google Scholar]
- National microalgae biofuel production potential and resource demand. Water Resour. Res.. 2011;47:W00H04.
- [Google Scholar]
- Metabolism: The Use of Energy in Biosynthesis, Prescott, Harley, and Klein’s microbiology (seventh ed.). New York, NY: McGraw-Hill Higher Education; 2008. p. :225-246. Chapter 10
- CO2-responsive expression and gene organization of three Ribulose-1, 5-Bisphosphate Carboxylase/Oxygenase enzymes and carboxysomes in Hydrogenovibrio marinus Strain MH-110. J. Bacteriol.. 2004;186:5685-5691.
- [Google Scholar]
- Control of plant productivity by regulation of photorespiration. Bioscience. 1992;42:510-517.
- [Google Scholar]







