Translate this page into:
A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: Phenotypic and genotypic characterization
⁎Corresponding authors. imoussa1@ksu.edu.sa (Ihab M. Moussa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The study reveals a picture for M. gallisepticum (MG) and M. synoviae (MS) in poultry industry of Egypt.
Methods
A total of 628 samples were collected from different species of diseased and apparently healthy poultry species from 15 Egyptian governorates. The isolates identified based on mgc2 and 16 s rRNA specific primers for MG and MS respectively. Sequencing for two isolates using mgc2 gene.
Results
Two hundred and five samples were microscopic fried egg colony. Using digitonin test, 160 samples were identified as genus Mycoplasma. Out of the 160 isolates, 86 were identified by PCR as MG, while 39 were identified as MS. Sequencing reveals that one isolate MGC2-EGY1 was related to the Egyptian strain MG-EIS6-T-10, while, the other isolate MGC2-EGY2 was related to the Indian strain MG-MGS-9B.
Conclusion
The study suggested that strictly applied programmes for the prevention and control of avian mycoplasmosis and confirmed the use of PCR on a large scale to help disease eradication programmes to limit economic losses in poultry farms.
Keywords
Mycoplasma gallisepticum
Mycoplasma synoviae
PCR
Sequencing
1 Introduction
Mycoplasmas are a big problem for all bird population. Mycoplasmas are very small bacteria that lack cell walls and belong to various genera within the class Mollicutes; these bacteria are able to cause serious and chronic diseases (Zakeri, 2016). Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS) are the most important avian pathogens, as they induce significant economic losses in the poultry industry. The rapid detection of Mycoplasma species is an essential step for adequate and fast control (Osman et al., 2009). Culturing of Mycoplasma can be quite difficult due to its fastidious nature, while serology is much faster. Non-specific reactions and cross-reactions, misinterpretations due to recent vaccination against Mycoplasma and cost are all disadvantages of the current diagnostic techniques (Feberwee et al., 2005; Erfan and Marouf, 2019). Therefore, a rapid, accurate, inexpensive and definitive diagnostic technique for mycoplasmas is of great importance. PCR methods are accepted, faster and less costly than culture methods (Yuan et al., 2003; Abdel Halium et al., 2019).
The present study give a picture for MG and MS in different Egyptian governorates from both apparently healthy and diseased birds based on different Mycoplasma diagnostic techniques.
2 Materials and methods
2.1 Animal ethics
All animal samples related procedures were approved ethically by institutional animal care and use committee (Vet.CU-IACUC) at Faculty of Veterinary Medicine, Cairo University. Approval document number is Vet.CU-10102019085.
2.2 Samples
A total of 628 samples were collected from chickens, turkeys, quails, ducks, parrots and pigeons during the period from January 2016 to December 2018 from fifteen different Egyptian governorates (Cairo, Giza, Fayoum, BaniSuef, Behera, Dakahlia, Gharbia, Monofia, Qualiobia, Sharkia, Alexandria, Ismaielia, Kafr El-Sheikh, El Minya, and Elwahat). The sample group included 278 apparently healthy and 350 diseased birds (coughing, nasal and ocular discharge, swelling in the infraorbital sinus, poor productivity, slow growth, leg problems, stunting, in-appetence, reduced hatchability and chick viability and abnormal feathers. Postmortem lesions were variable and included air sacculitis, pericarditis, perihepatitis, sinusitis and occasionally arthritis). Samples from chickens were taken from different breeds: 68 breeders (46 apparently healthy and 22 diseased), 100 layers (58 apparently healthy and 42 diseased) and 378 broiler (226 apparently healthy and 152 diseased). The collected samples included 190 tracheal swabs, 200 trachea, 168 lung tissues, 20 fertile eggs, 4 joints and 46 yolk sac samples of baby chicks. All samples were collected under complete hygienic conditions, transferred directly to the laboratory using transport media in an ice box and examined immediately (OIE, 2004). All previously data were listed in Tables 1 and 2.
Samples
Apparently healthy
Diseased
Total
Sources of samples
chickens
148
320
468
One day old chicks
68
10
78
Egg
14
6
20
Turkey
18
4
22
Quail
14
2
16
Duck
6
2
8
Parrot
2
4
6
pigeon
8
2
10
Total
278
350
628
Type of samples
Tracheal swabs
122
68
190
trachea
54
146
200
lung
48
120
168
Fertile Egg
14
6
20
yolk sac
40
6
46
Joint
4
4
Total
278
350
628
Governorates
Total examined No
Positive culture
PCR results
NO
%
MG
%
MS
%
1-Cairo
112
52
46.4%
35
31.2%
10
8.9%
2-Giza
80
32
40%
18
22.5%
7
8.7%
3-Al fayoum
24
5
20.8%
0
0%
0
0%
4-Beni seuf
20
8
40%
0
0%
0
0%
5-Behera
40
18
45%
3
7.5%
0
0%
6- AL Dakahlia
110
40
36.3%
16
14.5%
0
0%
7-AL Garbia
16
2
12.5%
2
12.5%
6
37.5%
8-AL Monofia
46
6
13%
0
0%
0
0%
9-AL Qaliobia
16
8
50%
3
18.7%
2
12.5%
10- AL Sharkia
20
8
40%
0
0%
2
10%
11-Alexandria
16
1
6.25%
1
6.25%
1
6.25%
12-Ismaileia
18
6
33.3%
2
11.1%
3
16.60%
13- Kafr EL Shiekh
48
8
16.6%
0
0%
0
0%
14- EL Menia
22
1
4.5%
0
0%
0
0%
15- EL Wahat
40
10
25%
6
15%
6
15%
Total
628
205
32.6%
86
53.7%
39
24.3%
2.3 Microbiological analysis of Mycoplasma spp.
All samples were inoculated into Frey’s broth medium and incubated at 37 °C with 5–10% CO2 in a humid atmosphere for 5–7 days. Any observable growth (yellow or straw-coloured broth changes) was subcultured on solid medium immediately and incubated at the same previously mentioned condition (OIE, 2004). The plates were examined daily for fried egg colonies for up to 20 days. The suspected colonies were subjected to a digitonin sensitivity test as described by Erno and Stipkovits (1973). The suspected positive digitonin sensitivity tests were serologically identified by growth inhibition (GI) tests using specific mycoplasmal antisera for MG and MS (Clyde, 1964).
2.4 Molecular identification
DNA of suspected isolates were extracted by QIAamp DNA Mini Kit was carried out by PCR using primers targeting the mgc2 and 16SrRNA genes for the detection of MG and MS, respectively. The sequence of mgc2 primer (F:CGC-AAT-TTG-GTC-CTA-ATC-CCC-AACA, R: TAA-ACC-CAC-CTC-CAG-CTT-TAT-TTC) Producing amplified product of 300 bp (Lynsyansky et al., 2005) with a cycling condition of (primary denaturation at 94 C/5 min followed by 94 C/30 s, then annealing step at 58/30 s followed by extension step at 72 C/30 s) for 35 cycles and finalized by final extension at 72 C/7 min. The sequence of 16S rRNA primer (F:GAG-AAG-CAA-AAT-AGT-GAT-ATC-A, R: CAG-TCG-TCT-CCG-AAG-TTA-ACA-A Producing amplified product of 210 bp (OIE, 2008) with a cycling condition of (primary denaturation at 94 C/5 min followed by 94 C/30 s, then annealing step at 55/30 s followed by extension step at 72 C/30 s) for 35 cycles and finalized by final extension at 72 C/7 min.
2.5 Sequencing and phylogenetic analysis
Two isolates were confirmed by mgc2 sequencing using Big dye Terminator V3.1 cycle sequencing kit.(Perkin-Elmer, Foster city, CA) cat-number 4336817. Nucleotide sequence alignment was carried out using BioEdit software and dnastar, Lasergene, megaalign. Sequence distance carried out using BioEdit software and dnastar, Lasergene, megaalign. The phylogenetic relationship was performed using MEGA5 software.
3 Results and discussion
3.1 Microbiological analysis
In the present study, a total of 205 samples revealed characteristic fried egg appearance of F. Mycoplasmataceae with an incidence of 32.6%. The recovery rate of Mycoplasma isolates (digitonin-sensitive) was 78% (160/205), resembling 25.4% of the total collected samples (160/628). The nearly results were documented by Mohamed et al. (1987), Levisohn and Kleven (2000), Nicholas and Ayling (2003), Feberwee et al. (2009) and Raviv and Ley (2013). The rate of culture results was higher than detected by Bibak et al. (2013) (10.66%) and Senthilnathan et al. (2015) (15.5%) and lower than that reported by Heleili et al. (2011) of 60.33%; however, this rate closely agreed with that obtained by Pourbakhsh et al. (2010). The obtained results indicate that fried egg colony-producing isolates are highly prevalent in poultry respiratory diseases, which agrees with the results of Ammar et al. (2016) and Pflaum et al.(2016).
3.2 Serotyping records
Serotyping of digitonin-sensitive isolates revealed that MG and MS were detected in 56.2% and 31.2%, respectively. The rates of MG and MS among different types of birds and the collected samples are illustrated in Table 3. MG was higher than the previously recorded rate of 47.7% by Abd El-Ghany (2008) and lower than in Abd El-Gawad and Rania (2005) in the Dokki Layer breed (67.5%) and Rauf et al. (2013) (79.8%). The serological identification of MS in this study was higher than that in Eissa et al. (2000) and Tawfik et al. (2016), which were 13.33% and 10%, respectively.
Sources and Types of samples
Digitonin sensitive
PCR
No. of samples
Positive
MGPositive
MSNo. of samples
Positive
MGPositive
MS
Sources
Chickens & fertile eggs
149
85
(57%)49
(32.8%)149
84
(56.3%)38
(25.5%)
Turkeys
3
2
(66.6%)1
(33.3%)3
2
(66.6%)1
(33.3%)
Quails
2
2
(100%)0
(0%)2
0
(0%)0
(0%)
Ducks
2
0
(0%)0
(0%)2
0
(0%)0
(0%)
Parrots
2
1
(50%)0
(0%)2
0
(0%)0
(0%)
Pigeons
2
0
(0%)0
(0%)2
0
(0%)0
(0%)
Total
160
90
(56.2%)
50
(31.2%)
160
86
(53.7%)
39
(24.3%)
Types
Tracheal swabs
28
10
(35.7%)3
(10.7%)28
9
(32.1%)1
(3.5%)
Trachea
63
46
(73%)13
(20.6%)63
44
(69.8%)10
(15.8%)
Lungs
54
28
(51.8%)23
(42.5%)54
27
(50%)20
(37%)
Fertile Eggs
6
2
(33.3%)4
(66.6%)6
2
(33.3%)3
(50%)
Joints
2
0
(0%)1
(50%)2
0
(0%)1
(50%)
Yolk sac
7
4
(57.1%)6
(85.7%)7
4
(57.1%)4
(57.1%)
Total
160
90
(56.2%)
50
(31.2%)
160
86
(53.7%)
39
(24.3%)
3.3 Molecular detection, sequencing and phylogenetic analysis
One hundred and sixty isolates was performed via PCR using mgc2 (300 bp) supported by Lynsyansky et al. (2005) and 16S rRNA genes (210 bp) according to OIE (2008) for the detection of MG and MS respectively. The investigation revealed 86 samples were positive for MG (53.7%) (Fig. 1) and 39 samples were positive for MS (24.3%) (Fig. 2). These results closely agreed with that reported by Gondal et al. (2015) and Elbehiry et al. (2016), 50% and 51.92%, respectively; however, these results disagree with those of Davinder et al. (2013) and Kamble et al. (2015), 35.3% and 66.4%, respectively.
Amplified PCR product of molecular size of 300 bp using primers targeting mgc2 gene of M. gallisepticum. Lane 1 showing 100 base pair ladder.

Amplified PCR product of molecular size 210 bp using primers targeting 16SrRNA gene of M. synoviae.
The nucleotide and amino acid sequences of the MG mgc2 gene for two pathogenic strains were deposited into GenBank under the accession number MG742314 for isolate EGY1-2017 and MG742315 for isolate EGY2-2017. The distance between the mgc2 genes of the MG isolates indicated that the percent identity of both the nucleotide and amino acid sequences was between 85% and 95.4%. The phylogenetic analysis of the nucleotides of the mgc2 genes of the two isolates and selected samples from Gen Bank revealed two main clusters. MGC2-EGY1 was in one cluster close to HQ591357.1MG-EIS6-T-10, which was related to an Egyptian strain isolated from a local turkey breed in 2011. The other isolate, MGC2-EGY2, was in another cluster close to KP279742.1 MG-MGS-9B (Fig. 3), which is related to an Indian strain isolated from chickens in 2014 (Loolmani et al., 2014).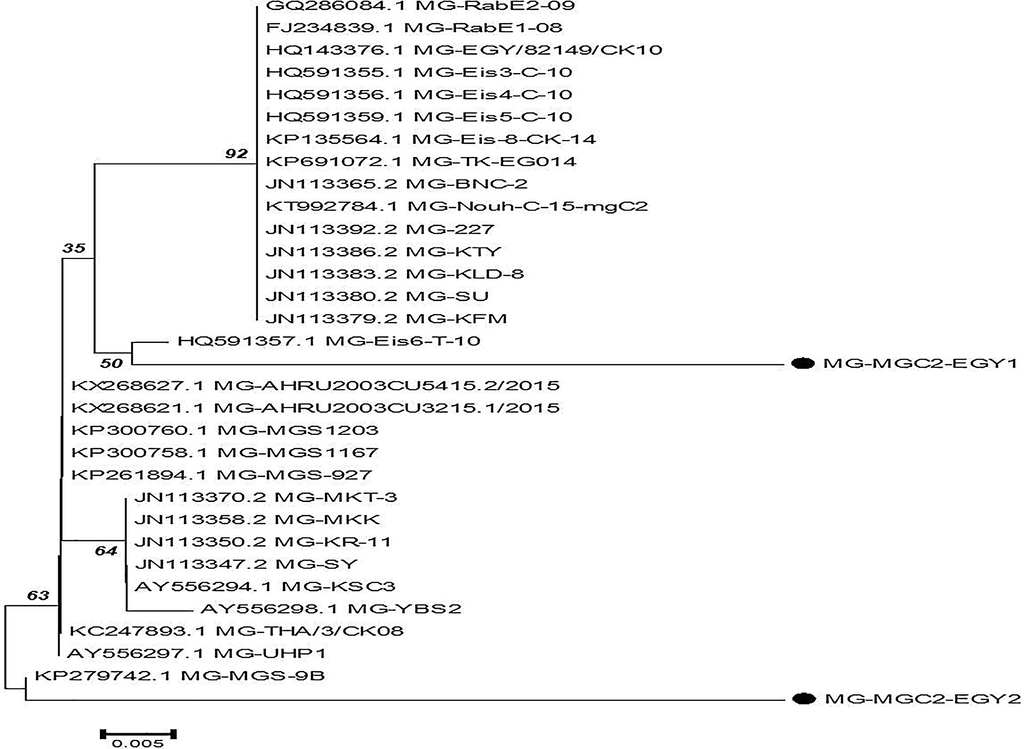
Phylogenetic tree of mgc2 gene sequence for 2 isolated field M. gallisepticum strains with other reference strain.
3.4 Incidence of MG and MS among the samples
Eighty-four positive samples of MG were isolated from chickens and fertile eggs, and 2 samples were isolated from turkey, while quail, duck, parrot and pigeon samples were negative for mgc2 gene of MG (Table 3). The highest percent of PCR detection for MG was from tracheal samples (69.8%). According to the different types of chicken, breeder, layer and broiler, the incidence of MG was 52.5% (21/40), 57.6% (15/26) and 60% (46/77), respectively.
A total of 39 samples were positive for MS with a recovery rate of 24.3%. Thirty-eight samples from chickens and eggs and one sample from a turkey were positive, while quail, duck, parrot and pigeon samples were negative for MS. Among the different samples of selected avian species, yolk sac samples showed the highest incidence of MS (57.1%) (Table 3). According to the different types of chickens, the incidence of MS was 27.5% (11/40), 30.7% (8/26) and 19.4% (15/27) from breeder, layer and broiler, respectively.
The isolation of MG from different samples agreed with Davinder et al. (2013) and Gondal et al. (2015) who concluded that tracheal, lung and air sac tissues may be used for the rapid screening of MG in poultry. MS was identified with the highest rate in yolk sac samples, followed by egg samples, which agreed with the findings of Dijkman et al. (2016).
From the obtained results, it was concluded that the highest incidence of MG was in broilers as recorded by Kapetanov et al. (2010), Heleili et al. (2011) and Feiziet al. (2013) in commercial broiler flocks, indicating that these farms do not consider biosecurity and hygienic conditions. Additionally, Muhammad et al. (2018) analysed 181 samples from broiler, layer and breeder chickens for mycoplasmal infection and detected 152 positive Mycoplasma by PCR. The agreement value between the tests was 67%, thus confirming that PCR is the most sensitive and reliable tool for the diagnosis of avian mycoplasmosis in field samples.
The investigation found that MS is the predominant Mycoplasma spp. in layer flocks; these findings disagreed with Bayatzadeh et al. (2011) and Michiels et al. (2016), who recorded MS infections was higher in broiler chickens. Additionally, regarding the MG and MS positive yolk sac samples, only one isolate was positive for both mycoplasmas, indicating the possibility of both infections at the same time.
The incidence of both MG and MS in the governorates examined in this study are shown in Table 2.
4 Conclusion
The obtained findings suggested that strictly applied programmes for the prevention and control of avian mycoplasmosis, including chemotherapy and/or vaccination, are urgently needed to reduce the economic losses of mycoplasmal infection in Egyptian poultry flocks. Additionally, molecular methods for the accurate diagnosis of avian mycoplasmosis (MG and/or MS) should be applied on a large scale to help disease eradication programmes to limit economic losses in poultry farms
Acknowledgement
This work was supported by the Deanship of Scientific Research at King Saud University through the research group project No.: RG-162.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Detection of Mycoplasma Infection in Native Layers by using Recent Techniques. M. V. Sc., Microbiology Dept., Fac. of Vet. Med., KafrEL-Sheikh, Tanta Univ; 2005.
- Diagnostic investigation on Mycoplasma gallisepticum infections in different Egyptian breeder and broiler chicken flocks. J. Egypt. Vet. Med. Assoc.. 2008;68(3):29-45.
- [Google Scholar]
- Isolation and molecular characterization of Mycoplasma spp. in sheep and goats in Egypt. Vet. World. 2019;12(5):664-670.
- [Google Scholar]
- Mutations of domain V in 23S ribosomal RNA of macrolide resistant Mycoplasma gallisepticum isolates in Egypt. J. Infect. Develop. Countries. 2016;10(8):807-813.
- [Google Scholar]
- Application of culture and polymerase chain reaction (PCR) methods for isolation and identification of Mycoplasma synoviaeon broiler chicken farms. Arch. Razi Inst.. 2011;66(2):87-94.
- [Google Scholar]
- Isolation of Mycoplasma spp. from broiler flocks with respiratory syndrome in Mashhad, Iran. Iran. J. Vet. Sci. Technol.. 2013;5(1):11-18.
- [Google Scholar]
- Growth Inhibition Test. Method in Mycopla-smology. Vol vol. 1 405. New York: Academic Press; 1964.
- Davinder, Singh, Mahajan, N.K., Aman, Kumar, Sushila, Maan, Pawan, Kumar, 2013. Detection of Mycoplasma gallisepticumfrom field samples of poultry using conventional PCR. (Special Issue: Veterinarians approaches for safeguarding animal health and production.). Adv. Anim. Vet. Sci. 1(1S), 11–13.
- Development and evaluation of a multi-locus sequence typing scheme for Mycoplasma synoviae. Avian Pathol.. 2016;45(4):426-442.
- [Google Scholar]
- Application of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE) for identification of Mycoplasma in turkeys with special reference to treatment. Vet. Med. J.. 2000;48(2):197-206.
- [Google Scholar]
- Serological, rapid molecular characterization and antibiotic resistance for field isolates of Mycoplasma gallisepticum in chicken in Saudi Arabia. Alexandria J. Vet. Sci.. 2016;49(2):70-79.
- [Google Scholar]
- Cinnamon oil downregulates virulence genes of poultry respiratory bacterial agents and revealed significant bacterial inhibition: an in vitro perspective. Vet. World. 2019;12(11):1707-1715.
- [Google Scholar]
- Bovine mycoplasma cultural and biochemical studies. Acta Vet. Scand.. 1973;14:450-463.
- [Google Scholar]
- An experimental model to quantify horizontal transmission of Mycoplasma gallisepticum. Avian Pathol.. 2005;34:355-361.
- [Google Scholar]
- Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies. Avian Pathol.. 2009;38(1):77-85.
- [CrossRef] [Google Scholar]
- Study on clinical signs and gross lesions of Mycoplasma gallisepticum in broiler breeder farms. Eur. J. Exp. Biol.. 2013;3(2):387-390.
- [Google Scholar]
- Characterization of Mycoplasma gallisepticum isolated from commercial poultry flocks. J. Anim. Plant Sci.. 2015;25(1):108-113.
- [Google Scholar]
- Incidence of avian mycoplasmosis in the region of Batna, Eastern Algeria. Vet. World. 2011;4(3):101-105.
- [Google Scholar]
- Kamble, S.Y., Gandge, R.S., Majee, S.B., 2015. Diagnosis of poultry mycoplasmosis by cultural isolation and PCR. Indian J. Anim. Sci. 85(10), 1073–1076.
- Kapetanov, M., Orlic, D., Potkonjak, D., Velhner, M., Stojanov, I., Milanov, D., Stojanovic, D., 2010. Mycoplasma in poultry flocks in the year 2009 compared to the year 2000 and significance of the control measures. Med. Vet. 43(1), 249–253.
- Avian Mycoplasmosis (Mycoplasma gallisepticum) Rev. Sci. Technol.. 2000;19:425-442.
- [Google Scholar]
- Phylogenetic analysis of mgc2 gene of Mycoplasma gallisepticum isolates from broiler breeder flocks in Tehran province, Iran. Eur. J. Zool. Res.. 2014;3(2):37-42.
- [Google Scholar]
- Use of mgc2-Polymerase chain reaction-Restriction fragment length polymorphism for rapid differentiation between field isolates and vaccine strains of Mycoplasma gallisepticum in Israel. Avian Dis.. 2005;49:238-245.
- [Google Scholar]
- Prevalence of Mycoplasma gallisepticum and Mycoplasma synoviae in commercial poultry, racing pigeons and wild birds in Belgium. Avian Pathol.. 2016;45(2):244-252.
- [Google Scholar]
- Economic impact of MG and MS in commercial layer flocks. Avian Dis.. 1987;31:477-489.
- [Google Scholar]
- Diagnosis of avian mycoplasmas: a comparison between PCR and culture technique. Arch. Razi Inst.. 2018;73(3):239-244.
- [Google Scholar]
- Mycoplasma bovis: diseases, diagnosis and control. Res. Vet. Sci.. 2003;74(2):105-112.
- [Google Scholar]
- OIE, 2004. Office International Des Epizooties (OIE), Avian Mycoplasmosis (Mycoplasma gallisepticum), Chapter 2.7.3., Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, fifth ed.
- OIE, 2008. Avain mycoplasmosis in Manual of diagnostic tests and vaccines for terrasterial animals. sixth ed. (chapter 2.3.5).
- Mycoplasma gallisepticum: an emerging challenge to the poultry industry in Egypt. Rev. Sci. Technol.. 2009;28(3):1015-1023.
- [Google Scholar]
- Global changes in Mycoplasma gallisepticumphase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Infect. Immun.. 2016;84(1):351-355.
- [Google Scholar]
- Detection of Mycoplasma synoviae infection in broiler breeder farms of Tehran province using PCR and culture methods. Arch. Razi Inst.. 2010;65(2):75-81.
- [Google Scholar]
- Identification of Mycoplasma gallisepticum by polymerase chain reaction and conventional diagnostics from white leghorn layer flocks. J. Anim. Plant Sci.. 2013;23(2):393-397.
- [Google Scholar]
- Raviv, Z., Ley, D.H., 2013. Mycoplasma gallisepticum infection. In: Swayne, D.E., (Ed.). Diseases of Poultry. 13th ed. Iowa State University Press, USA, Ames, IA. pp. 877–893.
- Isolation and molecular confirmation of Mycoplasma synoviaeinfection from broiler breeder farms in Tamil Nadu. Indian J. Anim. Res.. 2015;49(1):91-94.
- [Google Scholar]
- Mycoplasma synoviaeand other associated bacteria causing arthritis in chicken. Alexandria J. Vet. Sci.. 2016;49(2):163-169.
- [Google Scholar]
- Yuan, X., Egan, W., Chang, A., Webber, K., 2003. Mycoplasma in-process and lot release testing: to PCR or not to PCR. Proceedings of the WCBP CMC Strategy Forum.
- Polymerase chain reaction of mgc2 and 16S rRNA genes for detection of Mycoplasma gallisepticum. Iran. J. Appl. Anim. Sci.. 2016;6(1):61-66.
- [Google Scholar]







